
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Michel MOLIERE | -- | 1716 | 2024-03-14 02:23:49 | | | |
| 2 | Catherine Yang | -1 word(s) | 1715 | 2024-03-14 02:28:32 | | |
Video Upload Options
Land-based gas turbines (GTs) are continuous-flow engines that run with permanent flames once started and at stationary pressure, temperature, and flows at stabilized load. Combustors operate without any moving parts and their substantial air excess enables complete combustion. These features provide significant space for designing efficient and versatile combustion systems. In particular, as heavy-duty gas turbines have moderate compression ratios and ample stall margins, they can burn not only high- and medium-BTU fuels but also low-BTU ones. Hydrogen is an energy carrier and not a primary energy as there are very scarce natural sources thereof; the rare reservoirs of hydrogen originate from chemical reactions inside the earth crust and are sometimes referred to as “natural H2”or “white H2”.
1. Introduction
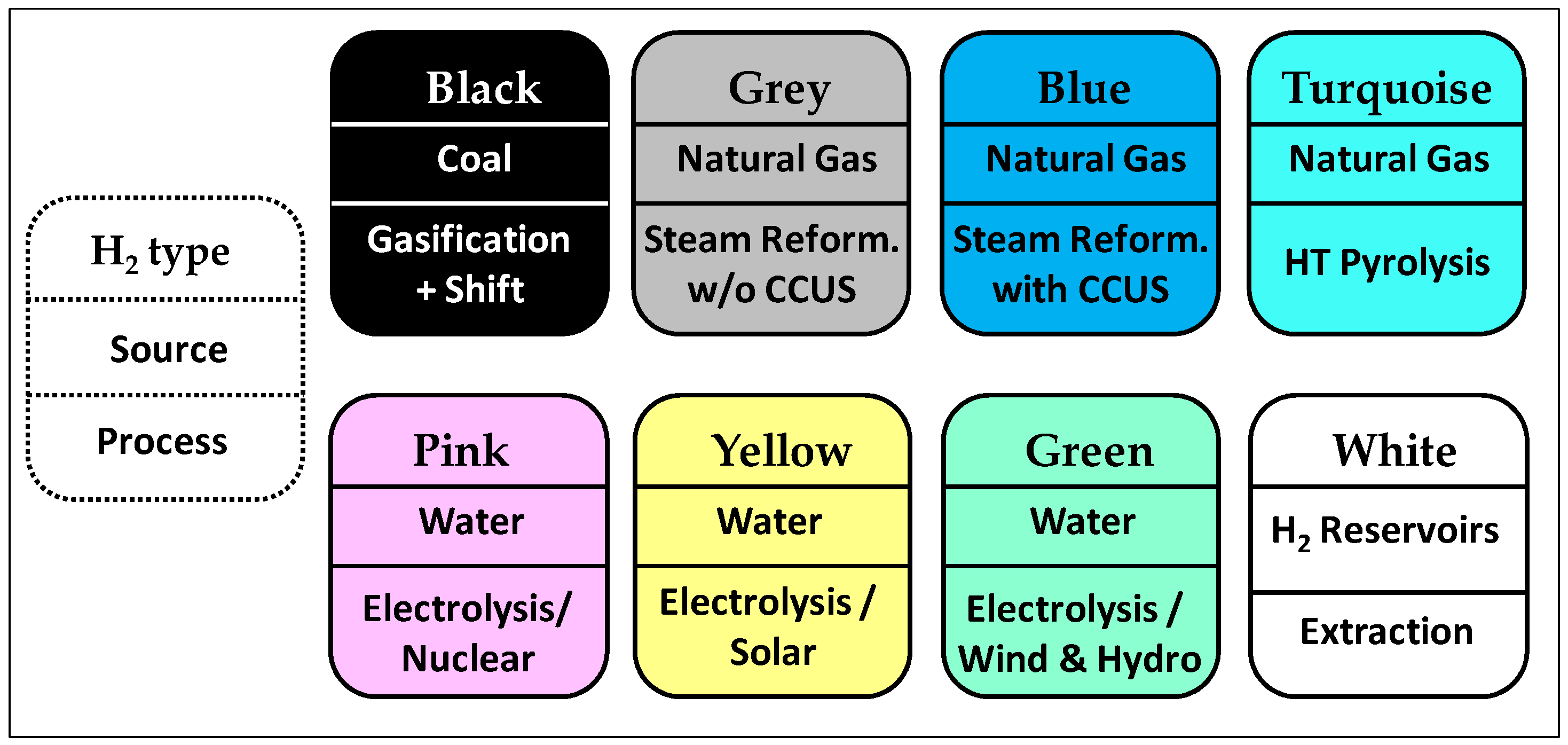
2. The “Hydrogen Rainbow”
| DATA − FUEL |
No of Molecules of Combustion Products | LHV Mass Volume MJ/kg MJ/Nm3 |
Diffusion Coefficient 1 atm; 25 °C − 10−6 m2s−1 |
LFL – UFL Low/Up. Flam. Lim. − % vol |
Lamin. Flame Speed − cm.s−1 |
Min. Ignition Energy − mJ |
|---|---|---|---|---|---|---|
| H2 | 1 (H2O) | 120 10.7 | 7.9 | 4.0 - 75 | 265 | 0.018 |
| CH4 | 3 (CO2 + 2 H2O) | 50 35.8 | 0.2 | 5.0 - 15 | 33 | 0.033 |
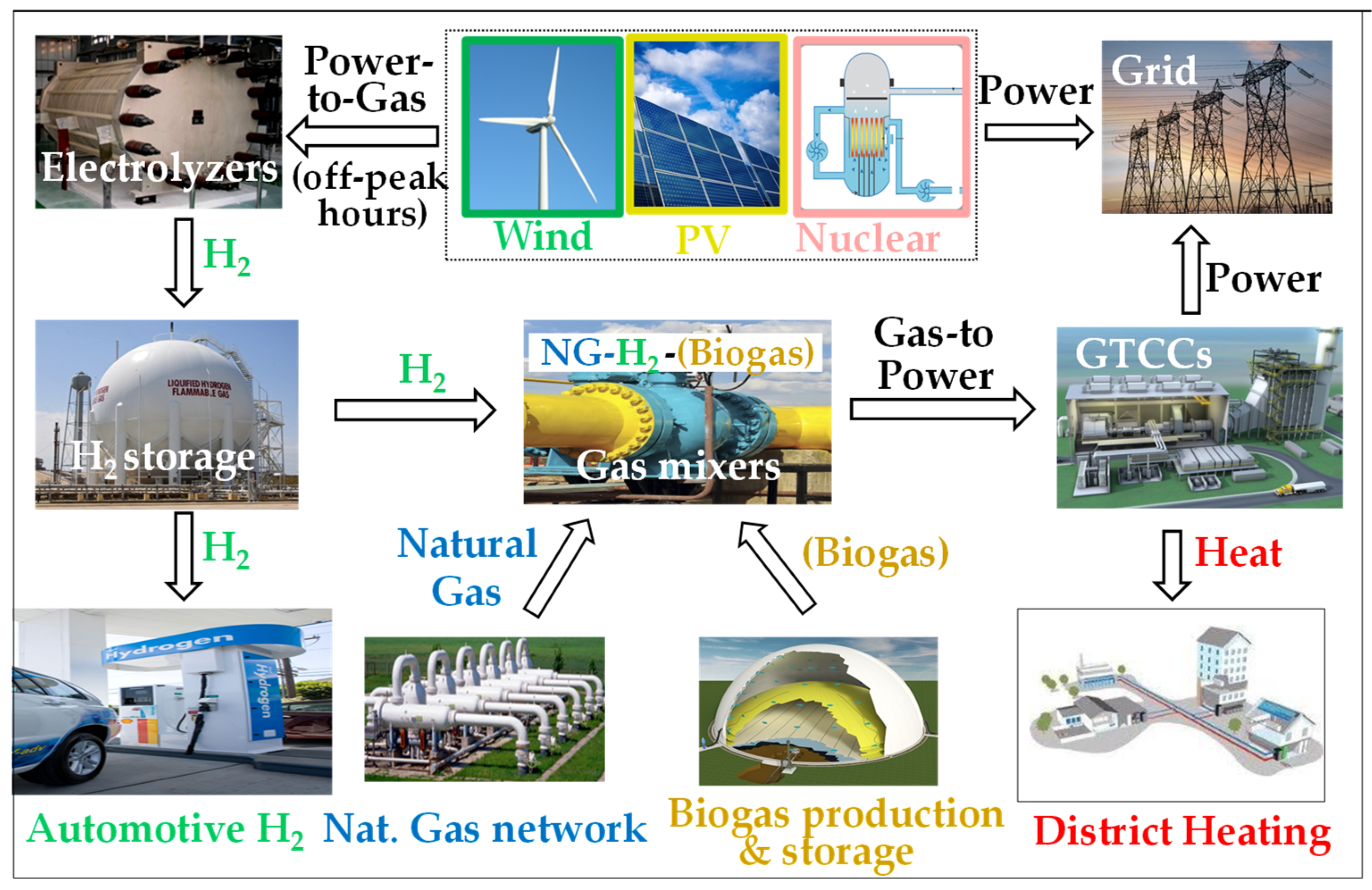
3. The Possible Role of Gas Turbines
- -
-
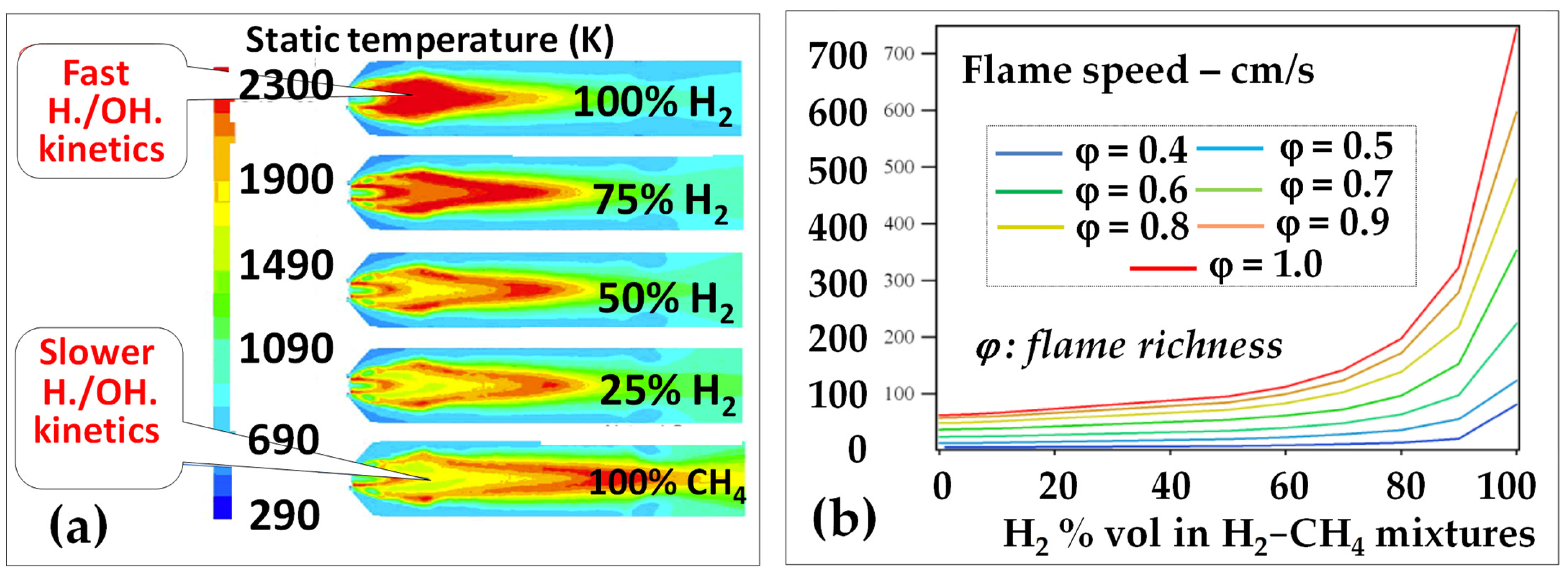
Hydrogen flames are very hot (Figure 3a) [14]. Indeed, one molecule of H2 generates three times less molecules of combustion products than does CH4; therefore, its stoichiometric combustion temperature (at the flame front) is much higher (2120 °C versus 1950 °C, in E-class GT conditions) since its combustion heat is transferred to one molecule instead of three. This is why hydrogen generates much higher NOx in diffusion flames than methane;
- -
-
Conventional Dry Low NOx systems based on the current fuel/air premix devices are defeated due to the very fast flame speed (Figure 3b) [15] that results in very short flames (Figure 3a) but causes very difficult flashback issues.

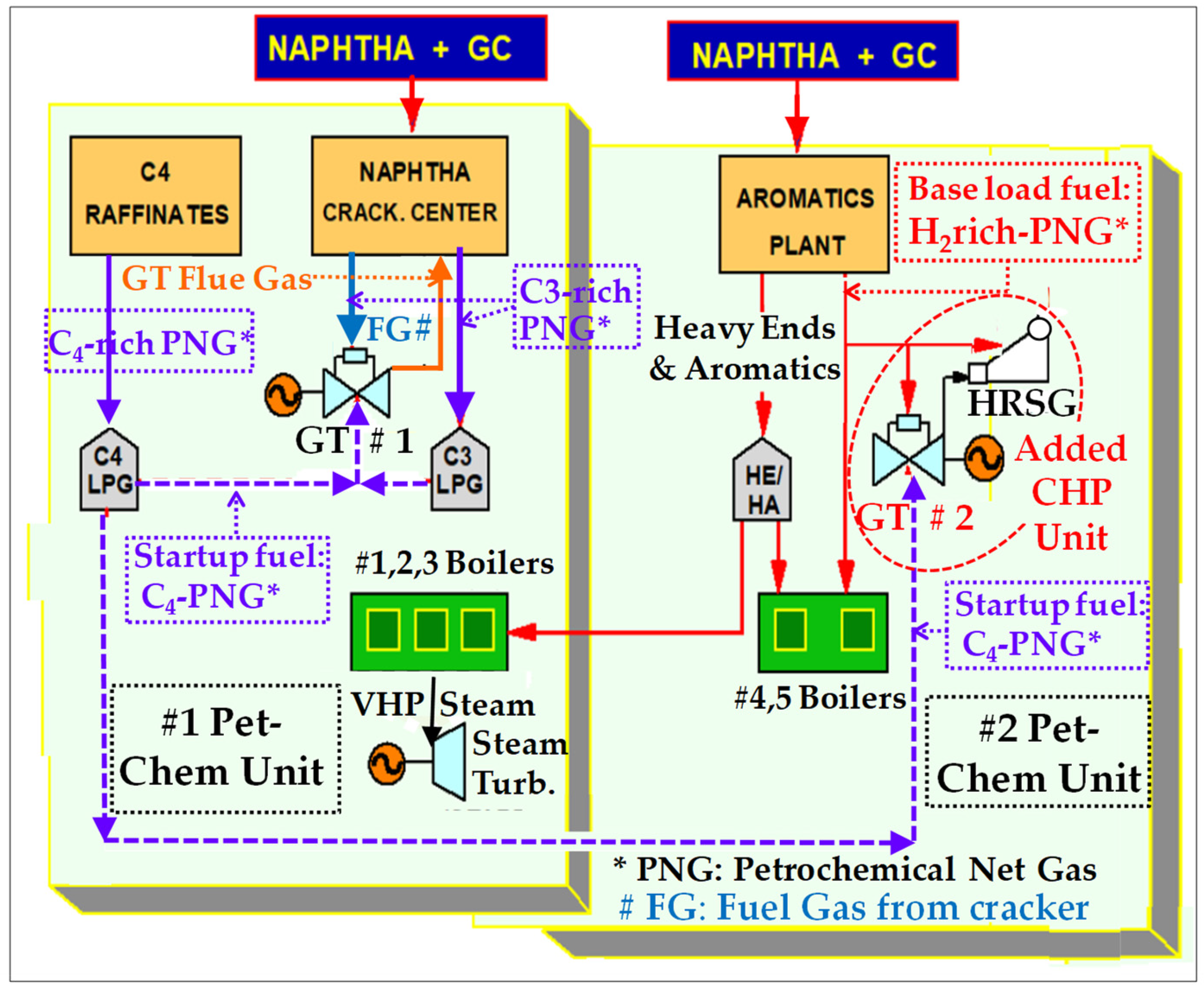
4. Exploiting the Experience Gained with H2-rich fuels
References
- Hainsch, K.; Hainsch, K.; Löffler, K.; Burandt, T.; Auer, H.; del Granado, P.C.; Pisciella, P.; Zwickl, S. Energy transition scenarios: What policies, societal attitudes, and technology developments will realize the EU Green Deal? Energy 2022, 239, 122067.
- IEA On-Line Report, World Energy Outlook 2022. Available online: https://iea.blob.core.windows.net/assets/830fe099-5530-48f2-a7c1-11f35d510983/WorldEnergyOutlook2022.pdf (accessed on 17 March 2023).
- Farhat, H.; Farhat, H.; Salvini, C. Novel Gas Turbine Challenges to Support the Clean Energy Transition. Energies 2022, 15, 5474.
- IEA. Power Systems in Transition, Section: Maintaining Reliability; IEA Report; IEA: Paris, France, 2020; pp. 25–27.
- Capurso, T.; Stefanizzi, M.; Torresi, M.; Camporeale, S.M. Perspective of the role of hydrogen in the 21st century energy transition. Energy Convers. Manag. 2022, 251, 114898.
- ClimaTalk Association On-Line Publication. Available online: https://climatalk.org/2022/04/18/the-hydrogen-rainbow/ (accessed on 17 March 2023).
- Vautrin, A.; Bossmann, T.; Beaussant, O. METIS Study on Costs and Benefits of a Pan-European Hydrogen Infrastructure, On-Line Publication of the EU Commission, 2021. Available online: https://op.europa.eu/fr/publication-detail/-/publication/c50a12fc-5eeb-11ec-9c6c-01aa75ed71a1/ (accessed on 17 March 2023).
- Conrad, R.; Rheinardt, T. Hydrogen Strategy Enabling A Low-Carbon Economy, On-Line DOE Publication, 2020. Available online: https://netl.doe.gov/sites/default/files/2020-12/USDOE_FE_Hydrogen_Strategy_July2020.pdf (accessed on 17 March 2023).
- UK Government On-Line Publication, UK Hydrogen Strategy Update to the Market, 2022. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1123751/hydrogen-strategy-update-to-the-market-december-2022.pdf (accessed on 17 March 2023).
- Hord, J. Is Hydrogen a Safe Fuel? Int. J. Hydrogen Energy 1978, 3, 157–176.
- Rigas, F.; Amiotte, P. Hydrogen Properties Associated with Hazards. In Hydrogen Safety; CRC Press: Boca Raton, FL, USA, 2013; Chapter 3; pp. 19–25.
- Patel, M.; Roy, S.; Roskilly, A.P.; Smallbone, H. The techno-economics potential of hydrogen interconnectors for electrical energy transmission and storage. J. Clean. Prod. 2022, 335, 130045.
- Molière, M.; Hugonnet, H. Hydrogen-Fueled Gas Turbines: Experience and Prospects. In Proceedings of the PowerGen Asia Conference, Bangkok, Thailand, 22–26 November 2004.
- Tomczak, H.J.; Benelli, G. Investigation of Gas Turbine combustion system fired with mixtures of natural gas and hydrogen. IFRF Combust. J. 2002, 19, 200–207.
- Glaude, P.A.; Fournet, R.; Bounaceur, R.; Moliere, M. Kinetic study of the combustion of hydrogen under pressure. In Proceedings of the Conference at Ecole Nationale Supérieure des Industries Chimiques (ENSIC), Nancy, France, 5 October 2022.
- Ayed, A.H.; Kusterer, K.; Funke, H.H.-W.; Keinz, J.; Striegan, C.; Bohn, D. Experimental and numerical investigations of the dry-low-NOx hydrogen micromix combustion chamber of an industrial gas turbine. Propuls. Power Res. 2015, 4, 123–131.
- Funke, H.H.-W.; Beckmann, N.; Abanteriba, S. An overview on dry low NOx micromix combustor development for hydrogen-rich gas turbine applications. Int. J. Hydrogen Energy 2019, 44, 6978–6990.7.
- Noble, D.; Wu, D.; Emerson, B.; Sheppard, S.; Lieuwen, T.; Angello, L. Assessment of Current Capabilities and Near-Term Availability of Hydrogen-Fired Gas Turbines Considering a Low-Carbon Future. J. Eng. Gas Turbines Power 2021, 143, 041002.
- Kim, D. Review on the Development Trend of Hydrogen Gas Turbine Combustion Technology. J. Korean Soc. Combust. 2019, 24, 1–10.
- Nagumo, M. Fundamentals of Hydrogen Embrittlement; Springer: Berlin/Heidelberg, Germany, 2016.
- Olbes, A.; Pujol, M.; Moliere, M.; Colas, M. High Compatibility between Gas Turbine and Refinery Utilities. In Proceedings of the PowerGen Europe Conference, Madrid, Spain, 17–19 June 1997.
- Gonzales-Martin, J.M.; Mora, A.; Lafuente-Perez, J.M.; Moliere, M.; Martinez, M. The Achievements of the Frame 6B Gas Turbine in Refinery Cogenerations. In Proceedings of the PowerGen Europe Conference, Amsterdam, The Netherlands, 16–18 May 1995.
- Skinner, T.; Powell, D.; Molière, M.; Lasne, D.; Hugonnet, M. Heavy Duty Gas Turbines Give Impetus to Large-Scale Cogens: Fuel Flexibility as Added Value at the ESSO Fawley Plant, UK. In Proceedings of the PowerGen Asia Conference, Shenzhen, China, September 2000.
- Molière, M.; Lallemand, R.; Chun, M.K.; Son, H.K. Heavy-Duty Gas Turbines in Petrochemical Plants: Samsung’s Daesan Plant (Korea) Beats Fuel Flexibility Records with over 95% Hydrogen in Process Gas. In Proceedings of the PowerGen Asia Conference, Bangkok, Thailand, 21 September 1999.




