Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Valérie Maraval | -- | 2204 | 2024-03-13 14:06:38 | | | |
| 2 | Camila Xu | Meta information modification | 2204 | 2024-03-14 02:11:42 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Maraval, V.; Caminade, A. Two-Photon Absorbing Dendrimers. Encyclopedia. Available online: https://encyclopedia.pub/entry/56221 (accessed on 04 March 2026).
Maraval V, Caminade A. Two-Photon Absorbing Dendrimers. Encyclopedia. Available at: https://encyclopedia.pub/entry/56221. Accessed March 04, 2026.
Maraval, Valérie, Anne-Marie Caminade. "Two-Photon Absorbing Dendrimers" Encyclopedia, https://encyclopedia.pub/entry/56221 (accessed March 04, 2026).
Maraval, V., & Caminade, A. (2024, March 13). Two-Photon Absorbing Dendrimers. In Encyclopedia. https://encyclopedia.pub/entry/56221
Maraval, Valérie and Anne-Marie Caminade. "Two-Photon Absorbing Dendrimers." Encyclopedia. Web. 13 March, 2024.
Copy Citation
Dendrimers, arborescent macromolecules exhibiting a large number of functional groups at their surface, appeared as naturally attractive targets to consider as TPA chromophores. Indeed, dendrimers are a special kind of perfectly defined hyperbranched polymers constructed stepwise from a multifunctional core at the periphery of which can be grafted a large density and variety of chromophores.
dendrimer
two-photon absorption
optical power limitation
photopolymerization
1. Introduction
After the theoretical prediction of the two-photon absorption (TPA) process by M. Göppert Mayer in 1931 [1], and the first experimental evidence of the phenomenon 30 years later [2], this third-order non-linear optical property has received considerable attention. Indeed, the TPA process is involved in a wide range of applications such as three-dimensional microfabrication [3], optical limitation [4], photodynamic therapy [5], or optical data storage [6]. Depending on the targeted application, TPA chromophores have to exhibit specific characteristics such as high fluorescence and/or solubility in aqueous media, for example. Many structure activity relationship studies were reported, and a large number of new organic dyes were designed and synthesized with the objective of reaching high TPA efficiencies [7]. The efficiency of a TPA chromophore is given by its cross-section value (σTPA), expressed in Göppert-Mayer (1 GM = 10−50 cm4 s photon−1).
In the design of chromophores for two-photon absorption, dipolar structures D-π-A, in which a donor group D is separated from an acceptor group A by a π-conjugated bridge, were first investigated [8], followed by quadrupolar molecules D-π-D or A-π-A [9]. These studies evidenced that D-π-D or D-π-A-π-D chromophores are generally more efficient than A-π-A or A-π-D-π-A counterparts [10]. Later, octupolar branched molecules were investigated and were shown to be efficient TPA chromophores [11].
Dendrimers, macromolecules exhibiting a large number of functional groups at their surface, appeared as the natural next targets to investigate as TPA chromophores. Indeed, dendrimers are a special kind of perfectly defined hyperbranched polymers constructed stepwise from a multifunctional core [12]. Their synthesis is most of the time performed through a divergent method and involves the use of a branched monomer. The repetition of a two-step sequence of reactions allows the progressive growth of the branches of the dendrimer and an increase in the number of terminal functions. A new generation (Gn + 1) of the dendrimer is created each time the number of functional groups is multiplied, generally by two [13] (up to five in some cases) [14], thanks to the use of the branched monomer. Dendrimers are thus defined by their core, their branches, their generation (a new generation being created each time a branching point is introduced through the use of the branched monomer) and their peripheral (or surface) functions. In the last decades, dendrimers were reported to possess a large variety of properties, depending on their structure, size, and functionalization at the surface or core, and to open up prospects for many applications in the fields of nanomaterials, catalysis, or biology, for example [15].
2. Two-Photon Absorbing Dendrimers
The first report of a TPA cross-section of a dendrimer was published by M. G. Humphrey in 1999 [16]. This pioneer study was performed on organometallic alkynylruthenium dendrimers and showed that the TPA cross-section value measured for 1-G1 (σTPA = 4800 ± 500 GM) was much higher than the value obtained for the smaller dendrimer 1-G0 (σTPA = 700 ± 120 GM) (Figure 1). Interestingly, the value for 1-G1 was higher than the one calculated by the addition of the values measured for each constitutive motif of the molecule. This non-linear increase of the TPA cross-section values from 1-G0 to 1-G1 was the first observation of a dendritic effect, or multivalence effect, on the TPA property. Such dendritic effects, though not clearly explained, have been observed in different domains, such as for example in catalysis and in TPA properties [17].
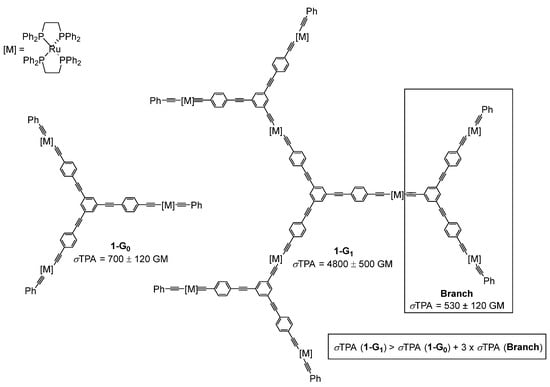
Figure 1. First example of TPA cross-section measurements of organometallic dendrimers.
In 2004, the TPA properties of the same kind of alkynylruthenium dendrimers bearing acceptor nitrophenyl groups at the periphery were investigated by the same authors [18]. No TPA process was observed with this nitro-substituted dendrimer upon irradiation at 800 nm. The combination of experimental data and theoretical calculations showed that at this wavelength, there is absorption saturation, and that the TPA process can only occur at a higher wavelength (around 1200 nm). These dendrimers were also shown to exhibit electrochemically induced switching of their NLO properties, in particular their nonlinear refraction and nonlinear absorption [19]. Later, the synthesis of a second generation of this type of alkynyl-metal dendrimer was reported, along with an investigation of the effect of the generation on nonlinearities [20]. Comparisons of the TPA properties relative to the number of ruthenium complexes, the molecular weight, or the number of delocalizable electrons all provided the same conclusions, i.e., that the NLO response increases with the generation, and that this increase is not only due to the increase of the molecular size, thus indicating a dendritic effect. In 2016, the same authors reported on the measurement of three- and four-photon absorption cross-section values for a ruthenium alkynyl dendrimer with nine ruthenium complexes in its structure. The σ(4PA) of 2100.10−110 cm8 s3, measured for this dendrimer upon irradiation at 1600 nm, was a record value at that time [21]. In 1999, the same year as the first report on the TPA properties of organometallic dendrimers [16], a preliminary evaluation of the TPA efficiency of the organic dendrimer 2-G0 based on bis-(diphenylamino)stilbene units was published [22]. These preliminary data were later confirmed and completed with measurements performed on the corresponding first- and second-generation dendrimers 2-G1 and 2-G2 (Figure 2) [23]. A cooperative effect was later demonstrated by comparison of the TPA efficiencies of 2-G0 with the monomer 3 and the related dendrimer 4-G0 constructed from a trifunctional core.
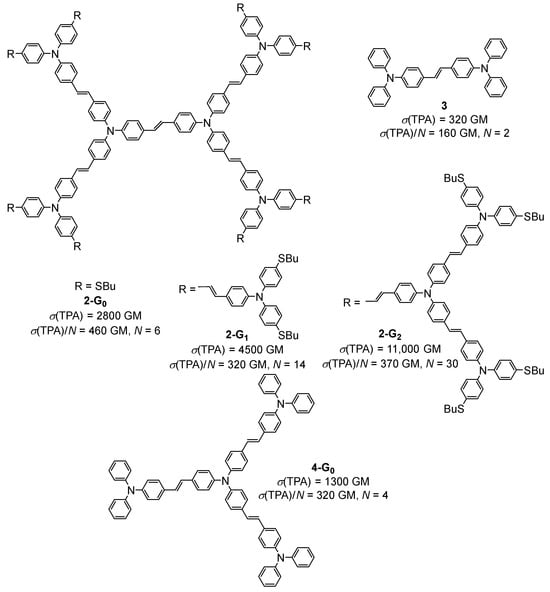
Figure 2. Structures and TPA cross-section values of bis-(diphenylamino)stilbene-based dendrimers.
In this study, the TPA cross-section values were expressed as a function of the number of chromophore units in the molecules, namely the number of triphenylamine moieties, thus evidencing that 2-G0 is the most efficient TPA chromophore of the series and that the σ(TPA)/N values of the three generations of dendrimers 2-Gn (n = 0–2) are almost similar. This latter observation was explained by a phenomenon of saturation when the dendrimers become larger [24].
Several theoretical studies were performed on this family of bis-diphenylamino)stilbene-based dendrimers of different generations and with different types of cores [25][26][27]. The TPA cross-section values were theoretically calculated, showing that these dendrimers exhibit very large TPA cross-sections and have a good transparency, making them promising materials for optical power limitation. Their TPA efficiency was calculated to increase linearly with the number of stilbene motifs in their structure, giving theoretical values up to 100,000 GM for 2-G1, this theoretical value being largely overestimated as compared to the experimental value of 4500 GM.
In 2000, one of the first reports on the use of dendrimers as TPA chromophores described the convergent synthesis of first-, second-, and third-generation dendrons 5-G1, 5-G2, and 5-G3 with a phenol core and the TPA chromophore 5-M, embedding the bis-diphenylamino)stilbene motive in its structure at the periphery (Figure 3) [28].
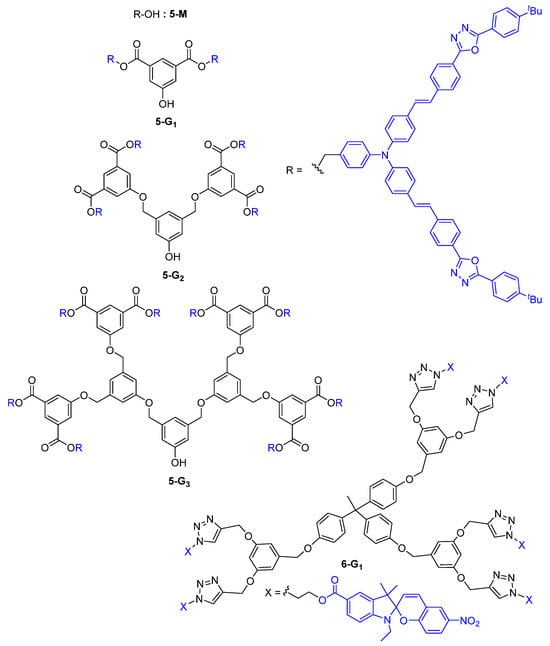
Figure 3. Non-conjugated dendrimers envisaged as TPA chromophores.
The relative TPA cross-section of the monomer 5-M and dendrons 5-Gn was found to double each time the number of chromophores was multiplied by two, namely from 5-M to 5-G0, then from 5-G0 to 5-G1, and so on. No dendritic effect was observed in this case, since there was a linear correlation between the number of peripheral chromophores and the TPA cross-section values. The dendrimer 6-G1, which has the same type of branches substituted by photochromic motifs, was built from a trifunctional core and was shown to exhibit a modest TPA efficiency with a σ(TPA) = 27 GM [29].
The effect of using dibenzofurane or dibenzothiophene as the cores of first- and second-generation bis-(diphenylamino)stilbene-based dendrimers 7-Gn and 8-Gn (n = 0, 1) on the TPA properties was reported in 2009 (Figure 4) [30][31]. Later, the properties of the same kind of bis-(diphenylamino)stilbene-based dendrimers 9-Gn (n = 0–2) with an anthracene core were investigated up to the second generation (Figure 4) [32]. These studies showed that the effect of the modification of the core of the dendrimer on its TPA cross-section is more important than the effect of the increase in generation. In particular, the TPA cross-section of 9-G0 (407 GM) with an anthracene core is more than 20 times higher than that of the benzofurane-cored analog 7-G0 (19 GM). In the case of the anthracene dendrimer 9-Gn, a dendritic effect was observed upon increasing the generation, as evidenced by the values of TPA cross-sections divided by the molecular weights.
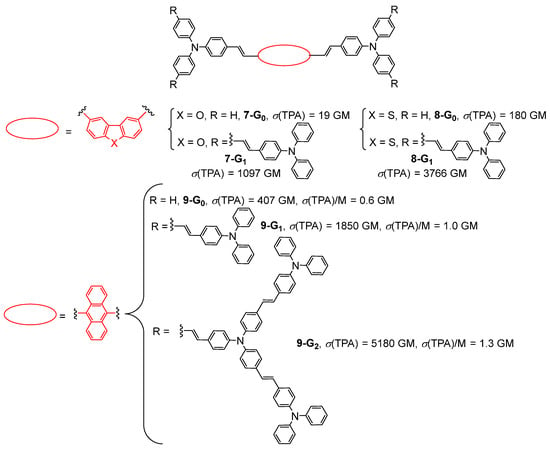
Figure 4. Bis-(diphenylamino)stilbene-based dendrimers with various cores.
Four first-generation dendrimers with a tetrapyrrolic core, either a porphyrin core bearing four diphenylaminostilbene branches 10-G1 and the metalated analog 10-G1-Zn, or a phthalocyanine core and eight branches 11a,b-G1, were investigated for their TPA properties (Figure 5) [33]. While the phthalocyanine dendrimers 11a,b-G1 showed almost no cooperative effect, their TPA efficiency corresponding to that of their branches, the porphyrin counterparts 10-G1 and 10-G1-Zn were found to exhibit a very strong cooperative effect. For example, the TPA cross-section of 10-G1 is nine times larger than the sum of those of its constituents. This difference between the two cores was explained by the less efficient conjugation along the tetrapyrrolic macrocycle in the case of the phthalocyanine core.
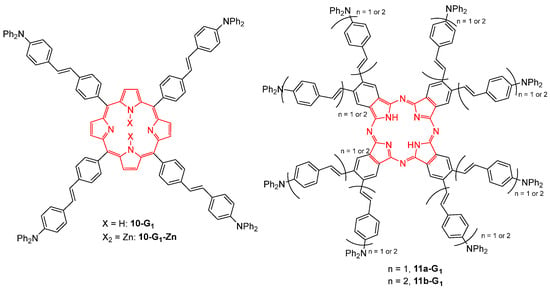
Figure 5. Dendritic TPA chromophores with tetrapyrrolic cores.
Dendrimers with a tetraphenylmethane core and phenothiazine fluorophores at the periphery were also considered as TPA chromophores and were shown to exhibit a moderate TPA cross-section of 89 GM for 12-G0 and 343 GM for 12-G1 (Figure 6) [34].
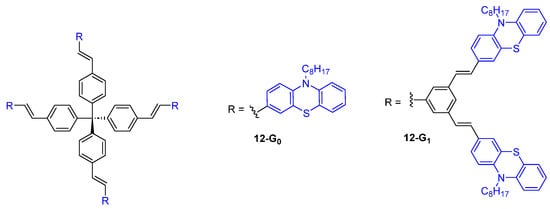
Figure 6. Dendrimers with a tetraphenylmethane core and phenothiazine substituents.
The structural modification of the bis-(diphenylamino)stilbene dendrimers was also envisaged by replacing the double bonds of 4-Gn (Figure 2) with triple bonds [35], thus rigidifying the structure, or by replacing the nitrogen atoms of 4-Gn with 1,3,5-substituted benzene rings [36], but without a significative effect on the obtained TPA cross-section values. Related triphenylamine-based dendrimers 13-Gn in which the triphenylamine moieties were linked by methylene bridges, thus rigidifying the structure a lot as compared to classical triphenylamine-based dendrimers such as 4-Gn (Figure 2), were also considered as TPA chromophores [37]. This enhancement of the rigidity and planarity of the molecules was shown to improve their TPA efficiency, reaching values up to 6100 GM for a first-generation dendrimer 13-G1 (Figure 7). Another rigidified small dendrimer 14-G1 with six chromophores at the periphery was also described as presenting quite a high TPA cross-section of 1400 GM [38].
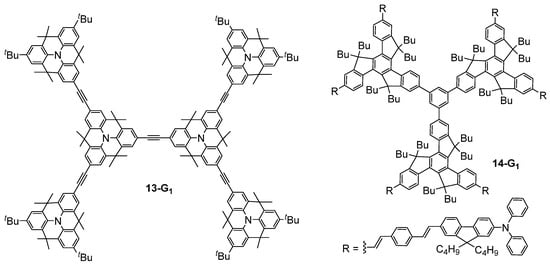
Figure 7. Structure of dendritic TPA chromophores with enhanced rigidity.
The TPA properties of all-thiophene dendrons 15d-G1–4 up to the fourth generation were investigated and a linear increase of the TPA cross-section values was observed upon increasing the dendrimer generation (Figure 8) [39]. All-thiophene dendrimers were also studied by entangled two-photon absorption [40][41][42], entangled photons being pairs of photons with a high degree of temporal and spatial correlation. In the entangled two-photon absorption process, the transition between the ground state and the two-photon excited state is accomplished in a single step by a pair of entangled photons [43]. The entangled TPA values of these dendrimers were found to follow the same trends of the values measured by the classical Two-Photon Excited Fluorescence (TPEF) method, although 10 orders of magnitude fewer photons are used.
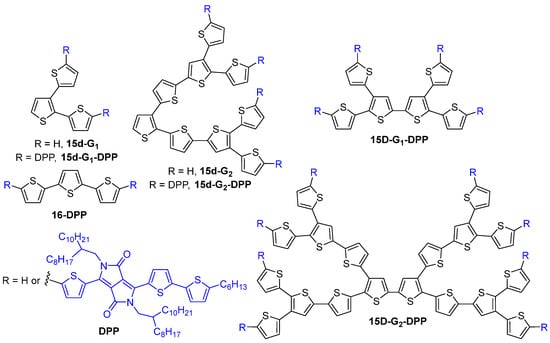
Figure 8. All-thiophene dendrons and dendrimers.
The same kind of all-thiophene dendrons of the first, second and third generations 15d-G1–3-DPP and the corresponding first- and second-generation dendrimers 15D-G1–2-DPP with diketopyrrolopyrrole groups at the surface were also described as efficient TPA chromophores (Figure 8) [44]. Their TPA efficiency was compared to that of a linear derivative 16-DPP. As compared to the linear molecules, the dendrons and dendrimers exhibited enhanced TPA cross-section values that could be assigned to the 3D structure of the dendritic compounds. Indeed, the value obtained for 15d-G1-DPP was 215% higher than the one obtained for 16-DPP, which has the same molecular weight but a linear instead of a branched structure. Increasing the number of branches, going from the first-generation dendron 15d-G1-DPP to the first-generation dendrimer 15D-G1-DPP, also increased the TPA cross-section value from 2499 GM to 6984 GM. However, it was shown that increasing the generation did not induce a further enhancement of the TPA efficiency, probably because of a less efficient orbital overlap due to steric hindrance.
The phosphorus dendrimers 17-Gn, developed by Anne-Marie Caminade and Jean-Pierre Majoral for more than 25 years [45], were also envisaged as TPA chromophores. More precisely, this family of arborescent molecules was proposed to be used as organic “nanodots”, alternatives to inorganic quantum dots that were shown to exhibit a very high TPA efficiency [46]. Various chromophores such as fluorene, stilbazole, and Nile Red derivatives were introduced either at the surface [47][48][49], at the core [50], or inside the branches of phosphorus dendrimers [51]. Phosphorus dendrimers with chromophore units both at the core and at their periphery were also envisaged (Figure 9) [52]. All these data were described in a recent review [53]. Some of these dendritic “nanodots” were shown to exhibit very high TPA cross-section values, outperforming those of quantum dots. The highest TPA cross-section value of 55,900 GM was measured for a fourth-generation dendrimer with 96 fluorene-based fluorophores at the periphery [47].
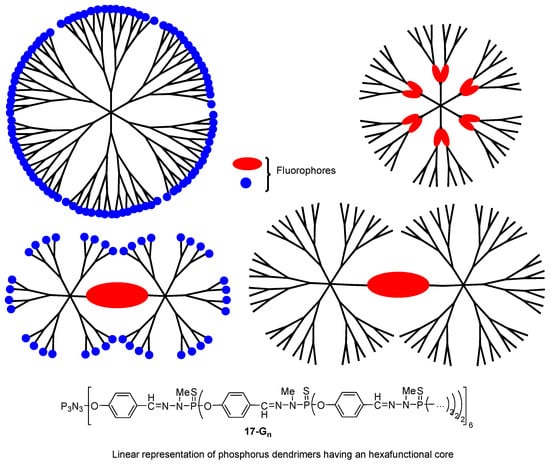
Figure 9. Schematic representations of the different types of phosphorous dendrimers embedding fluorophores in their structure.
The two-photon absorption ability of dendrimers has been largely studied, as illustrated above, but very often this property is targeted with a particular objective related to a prospect of application. The use of dendritic TPA chromophores for light harvesting, for photopolymerization, for optical power limitation, for cell imaging and for photodynamic therapy was particularly considered.
References
- Göppert-Mayer, M. Über Elementarakte mit zwei Quantensprüngen. Ann. Phys. 1931, 401, 273–294.
- Kaiser, W.; Garrett, C.G.B. Two-Photon Excitation in CaF2:Eu2+. Phys. Rev. Lett. 1961, 7, 229–231.
- Xing, J.-F.; Zheng, M.-L.; Duan, X.-M. Two-photon polymerization microfabrication of hydrogels: An advanced 3D printing technology for tissue engineering and drug delivery. Chem. Soc. Rev. 2015, 44, 5031–5039.
- Pascal, S.; David, S.; Andraud, C.; Maury, O. Near-infrared dyes for two-photon absorption in the short-wavelength infrared: Strategies towards optical power limiting. Chem. Soc. Rev. 2021, 50, 6613–6658.
- Ogawa, K.; Kobuke, Y. Two-Photon Photodynamic Therapy by Water-Soluble Self-Assembled Conjugated Porphyrins. BioMed Res. Int. 2013, 2013, 125658.
- Ogawa, K. Two-Photon Absorbing Molecules as Potential Materials for 3D Optical Memory. Appl. Sci. 2014, 4, 1–18.
- Myung Kim, H.; Cho, B.R. Two-photon materials with large two-photon cross sections. Structure–property relationship. Chem. Commun. 2009, 2009, 153–164.
- Rebane, A.; Drobizhev, M.; Makarov, N.S.; Beuerman, E.; Haley, J.E.; Krein, D.M.; Burke, A.R.; Flikkema, J.L.; Cooper, T.M. Relation between Two-Photon Absorption and Dipolar Properties in a Series of Fluorenyl-Based Chromophores with Electron Donating or Electron Withdrawing Substituents. J. Phys. Chem. A 2011, 115, 4255–4262.
- Lee, S.K.; Yang, W.J.; Choi, J.J.; Kim, C.H.; Jeon, S.-J.; Cho, R.B. 2,6-Bisanthracene Derivatives with Large Two-Photon Cross Sections. Org. Lett. 2005, 7, 323–326.
- Mongin, O.; Porrès, L.; Charlot, M.; Katan, C.; Blanchard-Desce, M. Synthesis, Fluorescence, and Two-Photon Absorption of a Series of Elongated Rodlike and Banana-Shaped Quadrupolar Fluorophores: A Comprehensive Study of Structure–Property Relationships. Chem.-Eur. J. 2007, 13, 1481–1498.
- Ceymann, H.; Roopeintner, A.; Schreck, M.H.; Mützel, C.; Stoy, A.; Vauthey, E.; Lambert, C. Cooperative enhancement versus additivity of two-photon-absorption cross sections in linear and branched squaraine superchromophores. Phys. Chem. Chem. Phys. 2016, 18, 16404–16413.
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A New Class of Polymers: Starburst-Dendritic Macromolecules. Polym. J. 1985, 17, 117–132. Available online: https://www.nature.com/articles/pj198510 (accessed on 1 January 1985).
- Newkome, G.R.; Shreiner, C.D. Poly(amidoamine), polypropylenimine, and related dendrimers and dendrons possessing different 1→2 branching motifs: An overview of the divergent procedures. Polymer 2008, 49, 1–173.
- Maraval, V.; Caminade, A.-M.; Majoral, J.-P.; Blais, J.-C. Dendrimer Design: How to Circumvent the Dilemma of a Reduction of Steps or an Increase of Function Multiplicity? Angew. Chem. Int. Ed. 2003, 42, 1822–1826.
- Caminade, A.-M. Inorganic Dendrimers and Their Applications. In Smart Inorganic Polymers; Wiley: Weinheim, Germany, 2019; pp. 277–315.
- McDonagh, A.M.; Humphrey, M.G.; Samoc, M.; Luther-Davies, B. Organometallic Complexes for Nonlinear Optics. 17.1 Synthesis, Third-Order Optical Nonlinearities, and Two-Photon Absorption Cross Section of an Alkynylruthenium Dendrimer. Organometallics 1999, 18, 5195–5197.
- Caminade, A.-M.; Ouali, A.; Laurent, R.; Turrin, C.-O.; Majoral, J.-P. The dendritic effect illustrated with phosphorus dendrimers. Chem. Soc. Rev. 2015, 44, 3890–3899.
- Powell, C.E.; Morrall, J.P.; Ward, S.A.; Cifuentes, M.P.; Notaras, E.G.A.; Samoc, M.K.; Humphrey, M.G. Dispersion of the Third-Order Nonlinear Optical Properties of an Organometallic Dendrimer. J. Am. Chem. Soc. 2004, 126, 12234–12235.
- Cifuentes, M.P.; Powell, C.E.; Morrall, J.P.; McDonagh, A.M.; Lucas, N.T.; Humphrey, M.G.; Samoc, M.; Houbrechts, S.; Asselberghs, I.; Clays, K.; et al. Electrochemical, Spectroelectrochemical, and Molecular Quadratic and Cubic Nonlinear Optical Properties of Alkynylruthenium Dendrimers1. J. Am. Chem. Soc. 2006, 128, 10819–10832.
- Green, K.A.; Simpson, P.V.; Corkery, T.C.; Cifuentes, M.P.; Samoc, M.; Humphrey, M.G. Divergent Synthesis of Ruthenium Alkynyl Dendrimers and a Two-Photon Absorption Cross-Section Dendritic Effect. Macromol. Rapid Commun. 2012, 33, 573–578.
- Simpson, P.V.; Watson, L.A.; Barlow, A.; Wang, G.; Cifuentes, M.P.; Humphrey, M.G. Record Multiphoton Absorption Cross-Sections by Dendrimer Organometalation. Angew. Chem. Int. Ed. 2016, 55, 2387–2391.
- Elandaloussi, E.H.; Spangler, C.W.; Dirk, C.; Casstevens, M.; Kumar, D.; Burzynski, R. Design and Synthesis of Dendrimers with Enhanced Two-Photon Absorption. MRS Proc. 1999, 561, 63–68. Available online: https://link.springer.com/article/10.1557/PROC-561-63 (accessed on 10 February 2011).
- Drobizhev, M.; Karotki, A.; Rebane, A.; Spangler, C.W. Dendrimer molecules with record large two-photon absorption cross section. Opt. Lett. 2001, 26, 1081–1083.
- Drobizhev, M.; Karotki, A.; Kruk, M.; Dzenis, Y.; Rebane, A.; Meng, F.; Nickel, E.; Spangler, C.W. Strong two-photon absorption in new porphyrins with asymmetrical meso-substitution. Proc. SPIE 2003, 5211, 63–74.
- Zhou, X.; Ren, A.-M.; Feng, J.-K.; Liu, X.-J.; Zhang, G. Theoretical investigation on dendritic molecules with large two-photon absorption cross section. J. Mol. Struct. (Theochem.) 2004, 672, 175–182.
- Zhang, X.-B.; Feng, J.-K.; Ren, A.-M.; Zhao, X.-J.; Sun, C.-C. Theoretical study of two-photon absorption properties for triphenylamine (boron, aluminum)-cored dendritic compounds. J. Mol. Struct. (Theochem.) 2006, 764, 69–75.
- Mukhopadhyay, S.; Ramasesha, S. Study of linear and nonlinear optical properties of dendrimers using density matrix renormalization group method. J. Chem. Phys. 2009, 131, 074111. Available online: https://pubs.aip.org/jcp/article/131/7/074111/188135/Study-of-linear-and-nonlinear-optical-properties (accessed on 20 August 2009).
- Adronov, A.; Fréchet, J.M.J. Novel Two-Photon Absorbing Dendritic Structures. Chem. Mater. 2000, 12, 2838–2841. Available online: https://pubs.acs.org/doi/10.1021/cm000586o (accessed on 23 September 2000).
- Zhang, Q.; Ning, Z.; Yan, Y.; Qian, S.; Tian, H. Photochromic Spiropyran Dendrimers: ‘Click’ Syntheses, Characterization, and Optical Properties. Macromol. Rapid Commun. 2008, 29, 193–201.
- Wang, X.; Yang, P.; Li, B.; Jiang, W.; Huang, W.; Qian, S.; Tao, X.; Jiang, M. Two-photon absorption of new multibranched chromophore with dibenzothiophene core. Chem. Phys. Lett. 2006, 424, 333–339.
- Wang, D.; Qin, C.; Wang, X.; Jiang, W.; Fang, X.; Zhao, J.; Chen, G. Synthesis of branched-chromophores with enhanced two-photon absorption via core effect. Optics Mat. 2009, 31, 805–811.
- Xu, B.; Zhang, J.; Fang, H.; Ma, S.; Chen, Q.; Sun, H.; Im, C.; Tian, W. Aggregation induced enhanced emission of conjugated dendrimers with a large intrinsic two-photon absorption cross-section. Polym. Chem. 2014, 5, 479–488.
- Rebane, A.; Makarov, N.; Drobizhev, M.; Spangler, C.W.; Gong, A.; Meng, F. Broad bandwidth near-IR two-photon absorption in conjugated porphyrin-core dendrimers. Proc. SPIE 2007, 6653, 665307.
- Qiu, X.; Lu, R. Synthesis and two-photon properties of phenothiazine-based dendrimers with tetraphenylmethane cores. Optical Mat. 2021, 112, 110550.
- Varnavski, O.; Yan, X.; Mongin, O.; Blanchard-Desce, M.; Goodson, T., III. Strongly Interacting Organic Conjugated Dendrimers with Enhanced Two-Photon Absorption. J. Phys. Chem. C 2007, 111, 149–162.
- Mongin, O.; Brunel, J.; Porrès, L.; Blanchard-Desce, M. Synthesis and two-photon absorption of triphenylbenzene-cored dendritic chromophores. Tetrahedron Lett. 2003, 44, 2813–2816.
- Fang, Z.; Teo, T.-L.; Cai, L.; Lai, Y.-H.; Samoc, A.; Samoc, M. Bridged Triphenylamine-Based Dendrimers: Tuning Enhanced Two-Photon Absorption Performance with Locked Molecular Planarity. Org. Lett. 2009, 11, 1–4.
- Zheng, Q.; He, G.S.; Prasad, P.N. π-Conjugated Dendritic Nanosized Chromophore with Enhanced Two-Photon Absorption. Chem. Mater. 2005, 17, 6004–6011.
- Ramakrishna, G.; Bhaskar, A.; Bäuerle, P.; Goodson, T., III. Oligothiophene Dendrimers as New Building Blocks for Optical Applications. J. Phys. Chem. A 2008, 112, 2018–2026.
- Harpham, M.R.; Süzer, Ö.; Ma, C.-Q.; Bäuerle, P.; Goodson, T., III. Thiophene Dendrimers as Entangled Photon Sensor Materials. J. Am. Chem. Soc. 2009, 131, 973–979.
- Kang, G.; Avanaki, K.N.; Mosquera, M.A.; Burdick, R.K.; Villabona-Monsalve, J.P.; Goodson, T., III; Schatz, G.C. Efficient Modeling of Organic Chromophores for Entangled Two-Photon Absorption. J. Am. Chem. Soc. 2020, 142, 10446–10458.
- Giri, S.J.; Schatz, G.C. Manipulating Two-Photon Absorption of Molecules through Efficient Optimization of Entangled Light. J. Phys. Chem. Lett. 2022, 13, 10140–10146.
- Javanainen, J.; Gould, P.L. Linear intensity dependence of a two-photon transition rate. Phys. Rev. A 1990, 41, 5088–5091.
- Gao, W.; Luo, Q.; Wang, J.; Lin, Y.; Tang, C.; Dou, J.; Tan, H.; Zheng, Q.; Ma, C.-Q.; Cui, Z. Peripherally diketopyrrolopyrrole-functionalized dendritic oligothiophenes—synthesis, molecular structure, properties and applications. Polym. Chem. 2017, 8, 1460–1476.
- Caminade, A.-M.; Turrin, C.-O.; Majoral, J.-P. Phosphorous Dendrimers in Biology and Nanomedecine—Synthesis, Characterization, and Properties, 1st ed.; Jenny Stanford Publishing Pte., Ltd.: New York, NY, USA, 2018.
- Larson, D.R.; Zipfel, W.R.; Williams, R.M.; Clark, S.W.; Bruchez, M.P.; Wise, F.W.; Webb, W.W. Water-Soluble Quantum Dots for Multiphoton Fluorescence Imaging in Vivo. Science 2003, 300, 1434–1436. Available online: https://www.science.org/doi/10.1126/science.1083780 (accessed on 30 May 2003).
- Mongin, O.; Krishna, T.R.; Wertz, M.H.V.; Caminade, A.-M.; Majoral, J.-P.; Blanchard-Desce, M. A modular approach to two-photon absorbing organic nanodots: Brilliant dendrimers as an alternative to semiconductor quantum dots? Chem. Commun. 2006, 2006, 915–917.
- Terenziani, F.; Parthasarathy, V.; Pla-Quintana, A.; Maishal, T.; Caminade, A.-M.; Majoral, J.-P.; Blanchard-Desce, M. Cooperative Two-Photon Absorption Enhancement by Through-Space Interactions in Multichromophoric Compounds. Angew. Chem. Int. Ed. 2009, 48, 8691–8694.
- Robin, A.-C.; Parthasarathy, V.; Pla-Quintana, A.; Mongin, O.; Terenziani, F.; Caminade, A.-M.; Majoral, J.-P.; Blanchard-Desce, M. Cooperative TPA enhancement via through-space interactions in organic nanodots built from dipolar chromophores. Proc. SPIE 2010, 7774, 77740N.
- Rouxel, C.; Charlot, M.; Mongin, O.; Krishna, T.R.; Caminade, A.-M.; Majoral, J.-P.; Blanchard-Desce, M. From Graftable Biphotonic Chromophores to Water-Soluble Organic Nanodots for Biophotonics: The Importance of Environmental Effects. Chem.-Eur. J. 2012, 18, 16450–16462.
- Mongin, O.; Rouxel, C.; Vabre, J.-M.; Mir, Y.; Pla-Quintana, A.; Wei, Y.; Caminade, A.-M.; Majoral, J.-P.; Blanchard-Desce, M. Customized multiphotonics nanotools for bioapplications: Soft organic nanodots as an eco-friendly alternative to quantum dots. Proc. SPIE 2009, 7403, 740303.
- Mongin, O.; Pla-Quintana, A.; Terenziani, F.; Drouin, D.; Le Droumaguet, C.; Caminade, A.-M.; Majoral, J.-P.; Blanchard-Desce, M. Organic nanodots for multiphotonics: Synthesis and photophysical studies. New J. Chem. 2007, 31, 1354–1367.
- Caminade, A.-M.; Zibarov, A.; Diaz, E.C.; Hameau, A.; Klausen, M.; Moineau-Chane Ching, K.; Majoral, J.-P.; Verlhac, J.-B.; Mongin, O.; Blanchard-Desce, M. Fluorescent phosphorus dendrimers excited by two photons: Synthesis, two-photon absorption properties and biological uses. Belstein J. Org. Chem. 2019, 15, 2287–2303.
More
Information
Subjects:
Chemistry, Organic
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
697
Revisions:
2 times
(View History)
Update Date:
14 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





