Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ronald B. Brown | -- | 2076 | 2024-03-13 14:04:36 | | | |
| 2 | Peter Tang | Meta information modification | 2076 | 2024-03-14 03:47:36 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Brown, R.B. Multiple Sclerosis and Sodium Toxicity. Encyclopedia. Available online: https://encyclopedia.pub/entry/56220 (accessed on 07 February 2026).
Brown RB. Multiple Sclerosis and Sodium Toxicity. Encyclopedia. Available at: https://encyclopedia.pub/entry/56220. Accessed February 07, 2026.
Brown, Ronald B.. "Multiple Sclerosis and Sodium Toxicity" Encyclopedia, https://encyclopedia.pub/entry/56220 (accessed February 07, 2026).
Brown, R.B. (2024, March 13). Multiple Sclerosis and Sodium Toxicity. In Encyclopedia. https://encyclopedia.pub/entry/56220
Brown, Ronald B.. "Multiple Sclerosis and Sodium Toxicity." Encyclopedia. Web. 13 March, 2024.
Copy Citation
Salt intake is associated with multiple sclerosis; however, controversial findings that challenge this association rely primarily on methods that do not measure total sodium storage within the body, such as food surveys and urinary sodium excretion. In contrast, tissue sodium concentrations measured with sodium MRI confirm high sodium levels in multiple sclerosis, suggesting a role for sodium toxicity as a risk factor for the disease.
multiple sclerosis
sodium toxicity
low-salt diet
high-salt diet
demyelination
myelin phase transition
tissue sodium concentration
urinary sodium excretion
1. Introduction
Multiple sclerosis (MS) is a chronic neurodegenerative disease associated with progressive demyelination of the central nervous system (CNS) [1]. A 2020 global epidemiological study reported 2.8 million people living with MS, with rising disease prevalence since 2013 [2]. The reported mean age of MS diagnosis is 32 years, and twice as many females as males live with the disease. MS is the leading cause of non-traumatic disability among young adults, and initial symptoms mostly affect people between 20 to 40 years of age [3]. MS symptoms commonly include muscle weakness, numbness and tingling, loss of vision, bladder problems, incoordination and imbalance, and gait impairment [4]. Among subtypes of MS, relapsing remitting MS (RRMS) is the most common type, in which approximately 85% of patients experience alternating periods of neurological symptoms and complete or partial symptom remission [5]. By about 19 years from the onset of RRMS, most untreated patients will have developed more severe neurological symptoms in secondary progressive MS (SPMS), but with continued periods of relapse and remission. In primary progressive MS (PPMS), onset and progressive severity of neurological symptoms occur without relapse and remission [5].
MS etiology is not completely understood, and more research is needed to investigate modifiable risk factors in the pathogenesis of the disease [2]. Current research supports the hypothesis that the risk of developing MS increases with global changes toward a ‘Western-type lifestyle’, which includes a high level of dietary salt intake that is hypothesized to be a factor in the pathogenesis of MS [6]. For example, using 24 h urine collection to estimate sodium intake, Farez et al. observed a positive association of dietary sodium with exacerbation of symptoms in patients with RRMS [7]. Additionally, a recent systematic review found emerging evidence that dietary sodium is a risk factor for autoimmune responses and inflammation in the progression of MS, and sodium is a potential risk factor in the onset of the disease [8].
2. Sodium Toxicity and Demyelination
Axon degeneration in the CNS can be caused by injury, toxins, and genetic defects [9]. Bechtold and Smith [10] reviewed early studies of inflammatory demyelinating disease and reported that sodium ions can accumulate in axons, disturb axonal sodium ion homeostasis, and cause axonal degeneration. The authors described how the magnitude of degeneration in axons in patients with MS is correlated with the degree of neuroinflammation in demyelinating axonal lesions, which the authors attributed to potentially harmful concentrations of sodium.
The Nav 1.6 voltage-gated sodium channel is associated with increased neuroinflammation and axonal degeneration in genetically altered C57BL/6 mice in the EAE model of MS [11]. Additionally, neuroinflammation and axonal degeneration were reduced in an EAE model when dark agouti male rats were treated with the sodium channel blocker flecainide [12]. However, subsequent EAE studies found that treatment withdrawal of the sodium channel blockers phenytoin and carbamazepine in diseased C57BL/6 mice exacerbated symptoms and increased risk of death compared with healthy controls [13]. Another study found that tailored withdrawal of phenytoin in the EAE model prevented deaths in diseased C57BL/6 mice, but symptoms neared non-treatment levels [14].
Related to this, osmotic demyelination syndrome in humans, which affects the central pontine and extrapontine regions of the brain, is caused by rapid saline infusion in the clinical correction of hyponatremia [15]. Moreover, an early study of MRI revealed that demyelination of the central pontine occurs in several conditions, including MS [16], further implying an association between MS and sodium toxicity.
Recent research has discovered novel roles for lipid phase properties of myelin in the etiology and recovery from MS [17]. Beck and Shaharabani described the structure and function of the myelin sheath as a multilamellar complex composed of different types of proteins and lipids which circumscribe axons and preserve nerve conduction integrity by providing a layer of insulation [17]. The researchers also described how myelin’s structure is undermined and its function is impaired in MS.
Biological membrane characteristics are determined by the distinctive organization of proteins and/or lipids, which can transition between a lamellar lipid phase, with cylinder shapes that add no curvature strain within the membrane, and hexagonal lipid phases including inverted-cone shapes (HII phase) that induce a positive curvature strain within the membrane [18]. Beck and Shaharabani reported pathological phase transitions in the multiple membrane (multilamellar) stacked structure of myelin sheaths in MS, correlated with small variations in myelin lipid composition and reduced adhesiveness in myelin basic protein (MBP) [17]. Figure 1 shows in vitro and in vivo examples of normal myelin and myelin with structural instabilities caused by lipid phase transitions. The researchers noted that ion-specific structural instabilities are increased by elevated salinity (saltiness) and temperature, which change normal lamellar stacks to a disrupted inverted hexagonal phase.
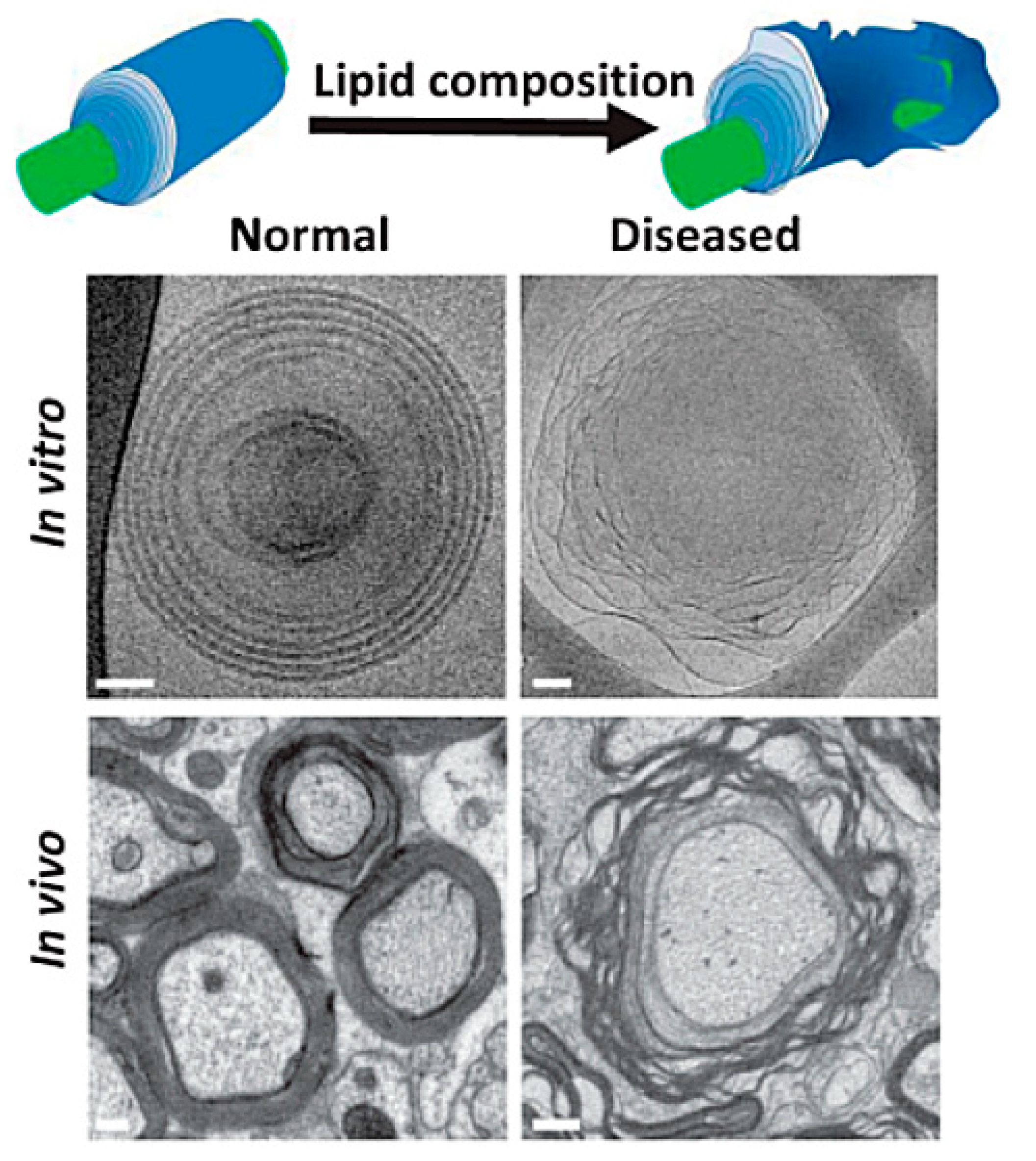
Figure 1. Normal myelin sheath membrane, in vitro and in vivo, and myelin with structural instabilities due to lipid phase transition, in vitro and in vivo [19].
3. Salt-Related Inflammatory and Immune Responses in MS
This section briefly reviews several inflammatory and immune responses associated with dietary sodium and MS. Proinflammatory cytokines released from activated T cells, especially interleukin 17 (IL-17) induced by Th17 cells, are associated with disease activity in patients with MS [20]. Of relevance, increased sodium chloride concentrations under physiological conditions significantly enhanced induction of Th17 cells in male C57BL/6J mice and in blood samples from healthy humans [21]. Demyelinated lesions in the CNS are also infiltrated with increased levels of macrophages and T cells in people with MS, and blood levels of monocytes are increased [8]. Related to this, infiltration of macrophages in the CNS and levels of monocytes in the blood of healthy people increased with exposure to a high-salt diet [22]. When sodium levels were lowered in healthy people, proinflammatory cytokines IL-6 and IL-23 were reduced, whereas levels of the anti-inflammatory cytokine IL-10 increased.
A high-salt diet in EAE-diseased C57BL/6 mice also elevated CNS levels of macrophages and proinflammatory cytokines, including IL-6, IL-12, IL-23, and tumor necrosis factor alpha (TNFα) [23]. Additionally, a high-salt diet in male NSG immunodeficient mice reduced immunosuppressive function of forkhead box protein 3 (FOXP3+) T regulatory cells (Tregs) and increased interferon gamma (IFNγ) [24], a cytokine associated with neuroinflammation in MS [25].
4. MS Comorbidities Potentially Mediated by Sodium Toxicity
4.1. Systemic Lupus Erythematosus and MS
Systemic lupus erythematosus (SLE) has rarely been reported to coexist in patients with MS, and differential diagnosis between the two diseases is often difficult [26]. Yet, both diseases have similar immune responses to salt intake [27], suggesting shared etiologies. For example, a high-salt diet in a murine model of SLE increased lupus nephritis progression and mortality, and a high-salt diet also upregulated Th17 cells in MRL/lpr mice [28].
4.2. Rheumatic Arthritis and MS
Self-reported rheumatic or rheumatoid arthritis (RA) was found to have a dose-dependent relationship with daily sodium intake in a case–control study in Spain [29]. Moreover, a nationwide retrospective cohort study in Taiwan found that patients diagnosed with MS had a higher subsequent diagnosis of RA compared to controls [30]. Patients with early RA were also found to have significantly higher sodium excretion than controls, even after controlling for nonsteroidal anti-inflammatory drugs, hypertension drugs, and smoking status [31]. Recently, a high urinary sodium-potassium ratio was associated with RA disease activity in patients, which the researchers suggested was linked to high sodium intake and low potassium intake [32]. Taken together, these findings provide supporting evidence that high sodium intake mediates the association of MS with RA.
4.3. Heart Failure and MS
A Danish cohort study found that patients with MS had an increased risk of heart failure compared to the general population [33], and an increased risk of heart failure associated with MS was confirmed in a recent systematic review and meta-analysis [34]. Coincidently, another recent study using 23Na MRI reported that patients with heart failure have extremely high levels of tissue sodium storage [35], inferring that increased risk of heart failure in patients with MS could be associated with high tissue sodium storage.
4.4. Inflammatory Bowel Disease and MS
Inflammatory bowel disease (IBD), including ulcerative colitis and Crohn’s disease, has been associated with a high-salt diet which induced gastrointestinal responses including “production of pro-inflammatory cytokines by intestinal mononuclear cells” [27] in eight-week-old female Balb/c mice [36] and eight-to-10-week-old female C57BL/6 mice [37]. Furthermore, a recently published systematic review and meta-analysis found that risk of IBD in patients with MS was higher than in controls [38], inferring that increased risk of IBD in MS could be associated with high salt consumption.
4.5. Ischemic Stroke and MS
A recent systematic review and meta-analysis found that people with MS have an increased risk of developing all types of stroke, especially ischemic stroke, compared to the general population [39]. A high-salt diet is a risk factor for acute ischemic disease [40], and salt restriction is an accepted lifestyle intervention in stroke prevention [41], implying that increased risk of ischemic stroke in MS is likely associated with high sodium concentrations.
4.6. Hypertension and MS
High blood pressure, or hypertension, is the most common risk factor for stroke [42], and hypertension’s close relationship with dietary salt intake is well established [43]. In a case–control study, U.S. patients with MS were 48% more likely to have had hypertension than a control group [44]. A retrospective cohort study found that higher systolic blood pressure was associated with progressively worse MS-related disabilities [45]. A large cross-sectional study that examined 37 million electronic health records from across the United States found that hypertension was 25% more common in the MS population compared to the rest of the population. Similar to MS, hypertension is also associated with inflammatory and immune responses to high dietary salt [46][47], further implying sodium toxicity as a common causative factor.
4.7. Migraine, Non-Specific Low Back Pain, and MS
The prevalence of primary headache among MS patients is higher than in the general population, according to a systematic review and meta-analysis of global studies [48]. Migraine headaches in particular are often comorbid with MS [49], and evidence suggests that sodium chloride intake is associated with migraine headache pain [50]. Sodium chloride intake is also associated with posterior lumbar subcutaneous edema in non-specific low back pain [51]. Coincidently, a recent study of the French MS population found that the prevalence of lower back pain is two to three times higher than in the general population [52].
4.8. Obstructive Sleep Apnea, Anxiety, and MS
Patients with MS have a higher predisposition for obstructive sleep apnea compared to patients without MS [53]. Obstructive sleep apnea is associated with sodium chloride intake as fluid overload is redistributed toward the upper body [54]. Salt also increases anxiety by triggering the action of angiotensin II which facilitates the release of “fight or flight” adrenal catecholamines from the sympathetic nervous system [54]. Of relevance, MS patients in a Canadian population have a 28.7% prevalence rate of anxiety disorders, self-rated with the Hospital Anxiety and Depression Scale, which is higher than the prevalence of anxiety reported in the general population [55].
4.9. Menstrual Disorders and MS
MS incidence is uncommon in people past ages 50 and 60 years [56]—the approximate ages of menopause and post menopause in women—and MS incidence is highest in females during childbearing years [57], although onset of the disease temporarily drops during the third trimester of pregnancy [58]. MS is also associated with a high prevalence of menstrual disorders [59][60], and menstrual disorders are associated with fluid overload [61], potentially related to increased interstitial sodium storage [62]. In the EAE model of MS, dietary salt worsened the disease only in female mice of the SJL/JCrHsd strain [63], suggesting involvement of sex-specific genetic factors. MS in human females is also associated with reproductive hormones [64] and with higher sodium sensitivity [62]. More research is needed to examine the relationship between menstrual disorders and greater exposure to sodium toxicity in MS.
Evidence implicating MS and comorbidities with sodium toxicity and other environmental factors challenges the prevailing concept of autoimmunity in MS [1] and in other related diseases including type I diabetes mellitus, Guillain-Barr syndrome, and myasthenia gravis [65]. Future directions in basic, epidemiological, and clinical research should continue to investigate etiological and epigenetic effects of sodium toxicity in MS and related comorbidities. Furthermore, lifestyle preventative measures should be investigated to modify dietary salt intake. Low-salt diet trials may show improvements in MS and comorbid conditions. However, separate trials should be conducted to directly link low salt as a causative factor in the pathogenesis of conditions comorbid with MS.
References
- Chaudhuri, A. Multiple sclerosis is primarily a neurodegenerative disease. J. Neural Transm. 2013, 120, 1463–1466.
- Walton, C.; King, R.; Rechtman, L.; Kaye, W.; Leray, E.; Marrie, R.A.; Robertson, N.; La Rocca, N.; Uitdehaag, B.; van der Mei, I.; et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult. Scler. 2020, 26, 1816–1821.
- Tullman, M.J. Overview of the epidemiology, diagnosis, and disease progression associated with multiple sclerosis. Am. J. Manag. Care 2013, 19, S15–S20.
- Barkhane, Z.; Elmadi, J.; Satish Kumar, L.; Pugalenthi, L.S.; Ahmad, M.; Reddy, S. Multiple Sclerosis and Autoimmunity: A Veiled Relationship. Cureus 2022, 14, e24294.
- Klineova, S.; Lublin, F.D. Clinical Course of Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2018, 8, a028928.
- Pugliatti, M. People with MS should consume a low-salt diet—Commentary. Mult. Scler. 2016, 22, 1781–1782.
- Farez, M.F.; Fiol, M.P.; Gaitán, M.I.; Quintana, F.J.; Correale, J. Sodium intake is associated with increased disease activity in multiple sclerosis. J. Neurol Neurosurg. Psychiatry 2015, 86, 26–31.
- Probst, Y.; Mowbray, E.; Svensen, E.; Thompson, K. A Systematic Review of the Impact of Dietary Sodium on Autoimmunity and Inflammation Related to Multiple Sclerosis. Adv. Nutr. 2019, 10, 902–910.
- Herwerth, M.; Wyss, M.T. Axon degeneration: New actor in an old play. Neural Regen. Res. 2023, 18, 547–548.
- Bechtold, D.A.; Smith, K.J. Sodium-mediated axonal degeneration in inflammatory demyelinating disease. J. Neurol. Sci. 2005, 233, 27–35.
- Alrashdi, B.; Dawod, B.; Schampel, A.; Tacke, S.; Kuerten, S.; Marshall, J.S.; Côté, P.D. Nav1.6 promotes inflammation and neuronal degeneration in a mouse model of multiple sclerosis. J. Neuroinflammation 2019, 16, 215.
- Bechtold, D.A.; Kapoor, R.; Smith, K.J. Axonal protection using flecainide in experimental autoimmune encephalomyelitis. Ann. Neurol. 2004, 55, 607–616.
- Black, J.A.; Liu, S.; Carrithers, M.; Carrithers, L.M.; Waxman, S.G. Exacerbation of experimental autoimmune encephalomyelitis after withdrawal of phenytoin and carbamazepine. Ann. Neurol. 2007, 62, 21–33.
- Liu, S.; Zwinger, P.; Black, J.A.; Waxman, S.G. Tapered withdrawal of phenytoin removes protective effect in EAE without inflammatory rebound and mortality. J. Neurol. Sci. 2014, 341, 8–12.
- Gaillard, F.; Bell, D.; Sharma, R. Osmotic Demyelination Syndrome. Available online: https://radiopaedia.org/articles/osmotic-demyelination-syndrome?lang=us (accessed on 25 January 2023).
- Miller, G.M.; Baker, H.L., Jr.; Okazaki, H.; Whisnant, J.P. Central pontine myelinolysis and its imitators: MR findings. Radiology 1988, 168, 795–802.
- Beck, R.; Shaharabani, R. Physical insights on the self-assembly of myelin sheaths: What drives healthy lamellar stacks to disrupted inverted hexagonal phase. In Proceedings of the APS March Meeting Abstracts, Boston, Massachusetts, 1 January 2019; p. H30.003.
- Jouhet, J. Importance of the hexagonal lipid phase in biological membrane organization. Front. Plant Sci. 2013, 4, 494.
- Shaharabani, R.; Ram-On, M.; Avinery, R.; Aharoni, R.; Arnon, R.; Talmon, Y.; Beck, R. Structural Transition in Myelin Membrane as Initiator of Multiple Sclerosis. J. Am. Chem. Soc. 2016, 138, 12159–12165.
- van Langelaar, J.; van der Vuurst de Vries, R.M.; Janssen, M.; Wierenga-Wolf, A.F.; Spilt, I.M.; Siepman, T.A.; Dankers, W.; Verjans, G.M.G.M.; de Vries, H.E.; Lubberts, E.; et al. T helper 17.1 cells associate with multiple sclerosis disease activity: Perspectives for early intervention. Brain 2018, 141, 1334–1349.
- Kleinewietfeld, M.; Manzel, A.; Titze, J.; Kvakan, H.; Yosef, N.; Linker, R.A.; Muller, D.N.; Hafler, D.A. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 2013, 496, 518–522.
- Yi, B.; Titze, J.; Rykova, M.; Feuerecker, M.; Vassilieva, G.; Nichiporuk, I.; Schelling, G.; Morukov, B.; Choukèr, A. Effects of dietary salt levels on monocytic cells and immune responses in healthy human subjects: A longitudinal study. Transl. Res. 2015, 166, 103–110.
- Hucke, S.; Eschborn, M.; Liebmann, M.; Herold, M.; Freise, N.; Engbers, A.; Ehling, P.; Meuth, S.G.; Roth, J.; Kuhlmann, T.; et al. Sodium chloride promotes pro-inflammatory macrophage polarization thereby aggravating CNS autoimmunity. J. Autoimmun. 2016, 67, 90–101.
- Hernandez, A.L.; Kitz, A.; Wu, C.; Lowther, D.E.; Rodriguez, D.M.; Vudattu, N.; Deng, S.; Herold, K.C.; Kuchroo, V.K.; Kleinewietfeld, M.; et al. Sodium chloride inhibits the suppressive function of FOXP3+ regulatory T cells. J. Clin. Invest. 2015, 125, 4212–4222.
- Ottum, P.A.; Arellano, G.; Reyes, L.I.; Iruretagoyena, M.; Naves, R. Opposing Roles of Interferon-Gamma on Cells of the Central Nervous System in Autoimmune Neuroinflammation. Front. Immunol. 2015, 6, 539.
- Jácome Sánchez, E.C.; García Castillo, M.A.; González, V.P.; Guillén López, F.; Correa Díaz, E.P. Coexistence of systemic lupus erythematosus and multiple sclerosis. A case report and literature review. Mult. Scler. J. Exp. Transl. Clin. 2018, 4, 2055217318768330.
- Li, X.; Alu, A.; Wei, Y.; Wei, X.; Luo, M. The modulatory effect of high salt on immune cells and related diseases. Cell Prolif. 2022, 55, e13250.
- Yang, X.; Yao, G.; Chen, W.; Tang, X.; Feng, X.; Sun, L. Exacerbation of lupus nephritis by high sodium chloride related to activation of SGK1 pathway. Int. Immunopharmacol. 2015, 29, 568–573.
- Salgado, E.; Bes-Rastrollo, M.; de Irala, J.; Carmona, L.; Gómez-Reino, J.J. High Sodium Intake Is Associated With Self-Reported Rheumatoid Arthritis: A Cross Sectional and Case Control Analysis Within the SUN Cohort. Medicine 2015, 94, e0924.
- Tseng, C.-C.; Chang, S.-J.; Tsai, W.-C.; Ou, T.-T.; Wu, C.-C.; Sung, W.-Y.; Hsieh, M.-C.; Yen, J.-H. Increased incidence of rheumatoid arthritis in multiple sclerosis: A nationwide cohort study. Medicine 2016, 95, e3999.
- Marouen, S.; du Cailar, G.; Audo, R.; Lukas, C.; Vial, G.; Tournadre, A.; Barrat, E.; Ribstein, J.; Combe, B.; Morel, J.; et al. Sodium excretion is higher in patients with rheumatoid arthritis than in matched controls. PLoS ONE 2017, 12, e0186157.
- Minamino, H.; Katsushima, M.; Hashimoto, M.; Fujita, Y.; Yoshida, T.; Ikeda, K.; Isomura, N.; Oguri, Y.; Yamamoto, W.; Watanabe, R.; et al. Urinary sodium-to-potassium ratio associates with hypertension and current disease activity in patients with rheumatoid arthritis: A cross-sectional study. Arthritis Res. Ther. 2021, 23, 96.
- Christiansen, C.F.; Christensen, S.; Farkas, D.K.; Miret, M.; Sørensen, H.T.; Pedersen, L. Risk of arterial cardiovascular diseases in patients with multiple sclerosis: A population-based cohort study. Neuroepidemiology 2010, 35, 267–274.
- Rapp, D.; Michels, S.; Schöpe, J.; Schwingshackl, L.; Tumani, H.; Senel, M. Associations between multiple sclerosis and incidence of heart diseases: Systematic review and meta-analysis of observational studies. Mult. Scler. Relat. Disord. 2021, 56, 103279.
- Lemoine, S.; Salerno, F.R.; Akbari, A.; McKelvie, R.S.; McIntyre, C.W. Tissue Sodium Storage in Patients With Heart Failure: A New Therapeutic Target? Circ. Cardiovasc. Imaging 2021, 14, e012910.
- Monteleone, I.; Marafini, I.; Dinallo, V.; Di Fusco, D.; Troncone, E.; Zorzi, F.; Laudisi, F.; Monteleone, G. Sodium chloride–enriched Diet Enhanced Inflammatory Cytokine Production and Exacerbated Experimental Colitis in Mice. J. Crohn’s Colitis 2016, 11, 237–245.
- Guo, H.X.; Ye, N.; Yan, P.; Qiu, M.Y.; Zhang, J.; Shen, Z.G.; He, H.Y.; Tian, Z.Q.; Li, H.L.; Li, J.T. Sodium chloride exacerbates dextran sulfate sodium-induced colitis by tuning proinflammatory and antiinflammatory lamina propria mononuclear cells through p38/MAPK pathway in mice. World J. Gastroenterol. 2018, 24, 1779–1794.
- Wang, X.; Wan, J.; Wang, M.; Zhang, Y.; Wu, K.; Yang, F. Multiple sclerosis and inflammatory bowel disease: A systematic review and meta-analysis. Ann. Clin. Transl. Neurol. 2022, 9, 132–140.
- Hong, Y.; Tang, H.R.; Ma, M.; Chen, N.; Xie, X.; He, L. Multiple sclerosis and stroke: A systematic review and meta-analysis. BMC Neurol. 2019, 19, 139.
- Gardener, H.; Rundek, T.; Wright, C.B.; Elkind, M.S.; Sacco, R.L. Dietary sodium and risk of stroke in the Northern Manhattan study. Stroke 2012, 43, 1200–1205.
- He, F.J.; MacGregor, G.A. Role of salt intake in prevention of cardiovascular disease: Controversies and challenges. Nat. Rev. Cardiol. 2018, 15, 371–377.
- Wajngarten, M.; Silva, G.S. Hypertension and Stroke: Update on Treatment. Eur. Cardiol. 2019, 14, 111–115.
- Grillo, A.; Salvi, L.; Coruzzi, P.; Salvi, P.; Parati, G. Sodium intake and hypertension. Nutrients 2019, 11, 1970.
- Saroufim, P.; Zweig, S.A.; Conway, D.S.; Briggs, F.B.S. Cardiovascular conditions in persons with multiple sclerosis, neuromyelitis optica and transverse myelitis. Mult. Scler. Relat. Disord. 2018, 25, 21–25.
- Goldman, M.D.; Min, S.; Lobo, J.M.; Sohn, M.-W. Retrospective cohort study of the relationship between systolic blood pressure variability and multiple sclerosis disability. BMJ Open 2020, 10, e034355.
- Wenzel, U.O.; Bode, M.; Kurts, C.; Ehmke, H. Salt, inflammation, IL-17 and hypertension. Br. J. Pharm. 2019, 176, 1853–1863.
- Rucker, A.J.; Rudemiller, N.P.; Crowley, S.D. Salt, Hypertension, and Immunity. Annu. Rev. Physiol. 2018, 80, 283–307.
- Wang, L.; Zhang, J.; Deng, Z.R.; Zu, M.D.; Wang, Y. The epidemiology of primary headaches in patients with multiple sclerosis. Brain Behav. 2021, 11, e01830.
- Gebhardt, M.; Kropp, P.; Hoffmann, F.; Zettl, U.K. Headache in Multiple Sclerosis—Pharmacological Aspects. Curr. Pharm. Des. 2022, 28, 445–453.
- Brown, R.B. Sodium Chloride, Migraine and Salt Withdrawal: Controversy and Insights. Med. Sci. 2021, 9, 67.
- Brown, R.B. Non-Specific Low Back Pain, Dietary Salt Intake, and Posterior Lumbar Subcutaneous Edema. Int. J. Environ. Res. Public Health 2022, 19, 9158.
- Massot, C.; Donze, C.; Guyot, M.A.; Leteneur, S. Low back pain in patients with multiple sclerosis: A systematic review and the prevalence in a French multiple sclerosis population. Rev. Neurol. (Paris) 2021, 177, 349–358.
- Braley, T.J.; Segal, B.M.; Chervin, R.D. Sleep-disordered breathing in multiple sclerosis. Neurology 2012, 79, 929–936.
- Brown, R.B. Hypertension, Anxiety and Obstructive Sleep Apnea in Cardiovascular Disease and COVID-19: Mediation by Dietary Salt. Diseases 2022, 10, 89.
- Pham, T.; Jetté, N.; Bulloch, A.G.M.; Burton, J.M.; Wiebe, S.; Patten, S.B. The prevalence of anxiety and associated factors in persons with multiple sclerosis. Mult. Scler. Relat. Disord. 2018, 19, 35–39.
- Roohani, P.; Emiru, T.; Carpenter, A.; Luzzio, C.; Freeman, J.; Scarberry, S.; Beaver, G.; Davidson, L.; Parry, G. Late onset multiple sclerosis: Is it really late onset? Mult. Scler. Relat. Disord. 2014, 3, 444–449.
- Houtchens, M.K.; Edwards, N.C.; Schneider, G.; Stern, K.; Phillips, A.L. Pregnancy rates and outcomes in women with and without MS in the United States. Neurology 2018, 91, e1559–e1569.
- Hughes, S.E.; Spelman, T.; Gray, O.M.; Boz, C.; Trojano, M.; Lugaresi, A.; Izquierdo, G.; Duquette, P.; Girard, M.; Grand’Maison, F.; et al. Predictors and dynamics of postpartum relapses in women with multiple sclerosis. Mult. Scler. J. 2014, 20, 739–746.
- Zarabadipour, S.; Amini, L.; Nabavi, S.M.; Haghani, H. Menstrual disorders and related factors in women with multiple sclerosis. Iran. J. Obstet. Gynecol. Infertil. 2018, 21, 43–52.
- Mirmosayyeb, O.; Badihian, S.; Manouchehri, N.; Basiri, A.K.; Barzegar, M.; Esmaeil, N.; Fayyazi, E.; Shaygannejad, V. The interplay of multiple sclerosis and menstrual cycle: Which one affects the other one? Mult. Scler. Relat. Disord. 2018, 21, 46–50.
- White, C.P.; Hitchcock, C.L.; Vigna, Y.M.; Prior, J.C. Fluid Retention over the Menstrual Cycle: 1-Year Data from the Prospective Ovulation Cohort. Obs. Gynecol. Int. 2011, 2011, 138451.
- Olde Engberink, R.H.G.; Selvarajah, V.; Vogt, L. Clinical impact of tissue sodium storage. Pediatr. Nephrol. 2020, 35, 1373–1380.
- Krementsov, D.N.; Case, L.K.; Hickey, W.F.; Teuscher, C. Exacerbation of autoimmune neuroinflammation by dietary sodium is genetically controlled and sex specific. Faseb. J. 2015, 29, 3446–3457.
- Foroughipour, A.; Norbakhsh, V.; Najafabadi, S.H.; Meamar, R. Evaluating sex hormone levels in reproductive age women with multiple sclerosis and their relationship with disease severity. J. Res. Med. Sci. 2012, 17, 882–885.
- Wenstedt, E.F.; Verberk, S.G.; Kroon, J.; Neele, A.E.; Baardman, J.; Claessen, N.; Pasaoglu, Ö.T.; Rademaker, E.; Schrooten, E.M.; Wouda, R.D.; et al. Salt increases monocyte CCR2 expression and inflammatory responses in humans. JCI Insight 2019, 4, e130508.
More
Information
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
654
Revisions:
2 times
(View History)
Update Date:
14 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




