
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ronald B. Brown | -- | 986 | 2024-03-13 14:03:49 | | | |
| 2 | Peter Tang | Meta information modification | 986 | 2024-03-14 03:44:04 | | |
Video Upload Options
A diet high in phosphorus fed to mice deficient in klotho, a cofactor that regulates phosphate metabolism, accelerates aging, sarcopenia, general organ atrophy, kyphosis, and osteoporosis. Similar effects are seen in phenotypes of mutant p53 mice that overexpress the p53 tumor suppressor gene. Although mutant p53 mice do not develop tumors compared to wild-type mice, mutant p53 mice have shorter mean lifespans. Furthermore, tumorigenesis is associated with the sequestration of excessive inorganic phosphate, and dangerous levels of phosphate are released into circulation during tumor lysis syndrome. In total, this evidence implies that tumorigenesis may be a compensatory mechanism that provides protective effects against systemic exposure to dysregulated phosphate metabolism and phosphate toxicity related to cachexia in cancer.
1. Introduction
2. Klotho Knockout Mice and p53 Mutant Mice
2.1. Klotho Knockout Mice
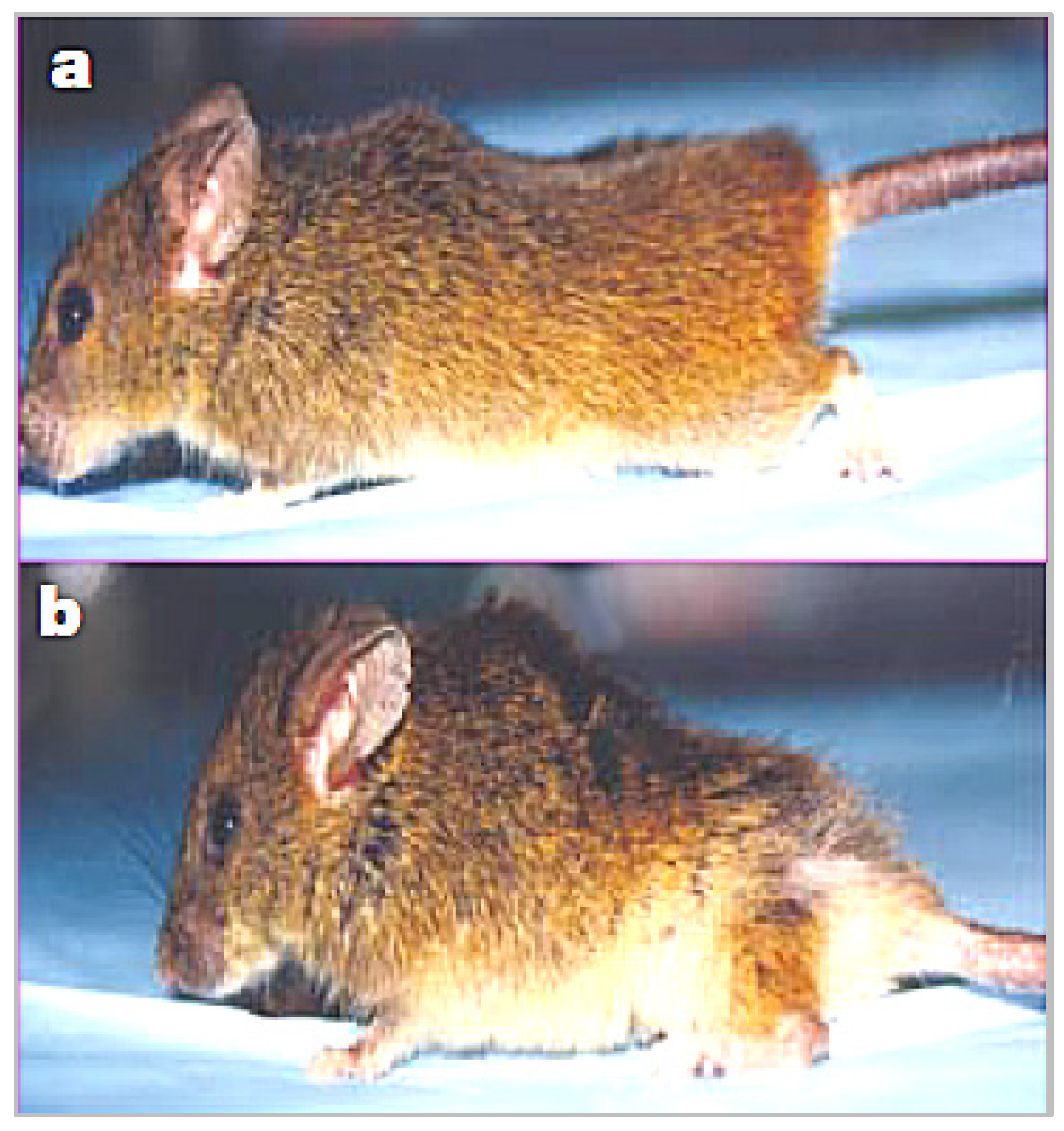
“Klotho knockout mice (klotho−/−) have a short life span and show numerous physical, biochemical, and morphological features consistent with premature aging, including kyphosis, uncoordinated movement, hypogonadism, infertility, severe skeletal muscle wasting, emphysema, and osteopenia, as well as generalized atrophy of the skin, intestine, thymus, and spleen”.[6]

2.2. p53 Mutant Mice
“Skinned, older p53+/m mice exhibit clear reductions in body mass, adipose tissue deposition, muscle mass and a pronounced lordokyphosis”.[15]
References
- Brown, R.B.; Razzaque, M.S. Chapter 31—Endocrine Regulation of Phosphate Homeostasis. In Textbook of Nephro-Endocrinology, 2nd ed.; Singh, A.K., Williams, G.H., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 539–548.
- Brown, R.B.; Razzaque, M.S. Phosphate toxicity and tumorigenesis. Biochim. Biophys. Acta (BBA)—Rev. Cancer 2018, 1869, 303–309.
- Qiao, Y.; Liu, F.; Peng, Y.; Wang, P.; Ma, B.; Li, L.; Si, C.; Wang, X.; Zhang, M.; Song, F. Association of serum Klotho levels with cancer and cancer mortality: Evidence from National Health and Nutrition Examination Survey. Cancer Med. 2022, 00, 1–13.
- Wolf, I.; Levanon-Cohen, S.; Bose, S.; Ligumsky, H.; Sredni, B.; Kanety, H.; Kuro-o, M.; Karlan, B.; Kaufman, B.; Koeffler, H.P.; et al. Klotho: A tumor suppressor and a modulator of the IGF-1 and FGF pathways in human breast cancer. Oncogene 2008, 27, 7094–7105.
- Kuro-o, M.; Matsumura, Y.; Aizawa, H.; Kawaguchi, H.; Suga, T.; Utsugi, T.; Ohyama, Y.; Kurabayashi, M.; Kaname, T.; Kume, E.; et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997, 390, 45–51.
- Ohnishi, M.; Razzaque, M.S. Dietary and genetic evidence for phosphate toxicity accelerating mammalian aging. FASEB J. 2010, 24, 3562–3571.
- Kuro-o, M. Klotho and the aging process. Korean J. Intern. Med. 2011, 26, 113–122.
- Argilés, J.M.; Busquets, S.; Felipe, A.; López-Soriano, F.J. Muscle wasting in cancer and ageing: Cachexia versus sarcopenia. Adv. Gerontol. 2006, 18, 39–54.
- Bruggeman, A.R.; Kamal, A.H.; LeBlanc, T.W.; Ma, J.D.; Baracos, V.E.; Roeland, E.J. Cancer Cachexia: Beyond Weight Loss. J. Oncol. Pract. 2016, 12, 1163–1171.
- National Cancer Institute. Treating Cancer Cachexia: Progress Looks Possible. Available online: https://www.cancer.gov/about-cancer/treatment/research/cachexia (accessed on 18 November 2022).
- Advani, S.M.; Advani, P.G.; VonVille, H.M.; Jafri, S.H. Pharmacological management of cachexia in adult cancer patients: A systematic review of clinical trials. BMC Cancer 2018, 18, 1174.
- Del Fabbro, E. More is better: A multimodality approach to cancer cachexia. Oncologist 2010, 15, 119–121.
- Yamashita, K.; Yotsuyanagi, T.; Yamauchi, M.; Young, D.M. Klotho Mice: A Novel Wound Model of Aged Skin. Plast. Reconstr. Surg. Glob. Open 2014, 2, e101.
- Mijit, M.; Caracciolo, V.; Melillo, A.; Amicarelli, F.; Giordano, A. Role of p53 in the Regulation of Cellular Senescence. Biomolecules 2020, 10, 420.
- Tyner, S.D.; Venkatachalam, S.; Choi, J.; Jones, S.; Ghebranious, N.; Igelmann, H.; Lu, X.; Soron, G.; Cooper, B.; Brayton, C.; et al. p53 mutant mice that display early ageing-associated phenotypes. Nature 2002, 415, 45–53.




