
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ronald B. Brown | -- | 1782 | 2024-03-13 13:38:55 | | | |
| 2 | Peter Tang | Meta information modification | 1782 | 2024-03-14 03:24:48 | | |
Video Upload Options
Phosphate toxicity, the accumulation of excess phosphate in the body from dysregulated phosphate metabolism, is associated with tumorigenesis. High levels of hormones that regulate phosphate metabolism, such as parathyroid hormone and fibroblast growth factor 23, are also associated with obesity, providing a potential link between obesity and phosphate toxicity. Increased dietary intake of inorganic phosphate is linked to excessive consumption of foods processed with phosphate additives, and consumption of ultra-processed foods is associated with an increase in the incidence of obesity.
1. Introduction
2. Phosphate Metabolism
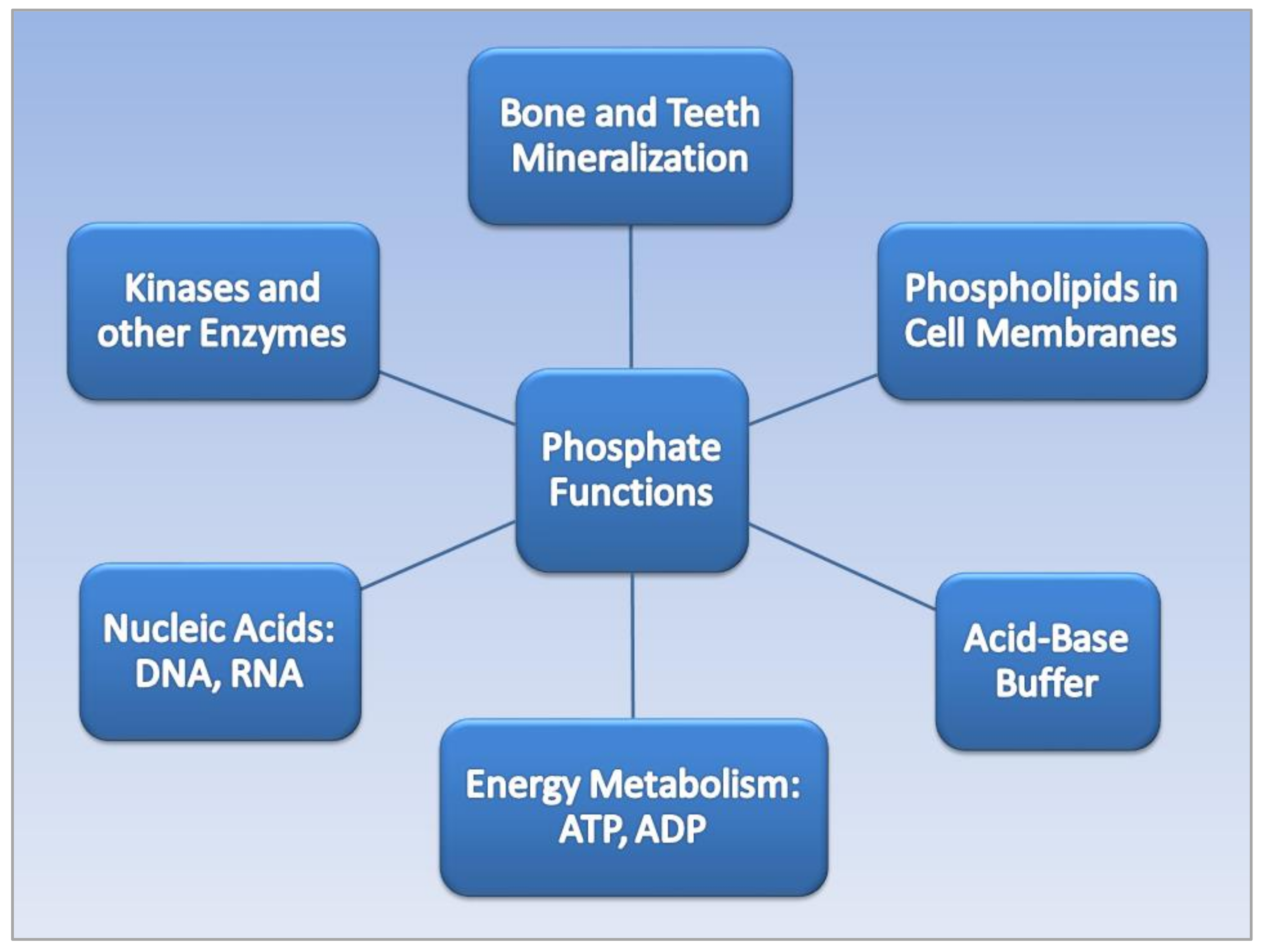
2.1. Phosphate Functions
2.2. Phosphate Regulation and Dysregulation
|
Bone |
Kidney |
Parathyroid |
Intestine |
|---|---|---|---|
|
FGF23 inhibits serum Pi reabsorption in kidneys. |
Regulates serum Pi reabsorption and Pi urinary excretion. |
PTH increases serum calcium through bone resorption. |
Pi absorption from dietary sources. |
|
Increases Pi urinary excretion. |
Biosynthesis of calcitriol from vitamin D3 increases Pi intestinal absorption. |
Increases Pi urinary excretion with FGF23. |
Type II sodium-dependent phosphate cotransporters. |
3. Phosphate Toxicity and Tumorigenesis
4. Phosphate Toxicity and Obesity
References
- who.int. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 28 November 2021).
- Cancerresearchuk.Org. When could overweight and obesity overtake smoking as the biggest casue of cancer in the UK? Available online: https://www.cancerresearchuk.org/sites/default/files/obesity_tobacco_cross_over_report_final.pdf (accessed on 19 December 2021).
- Renehan, A.G.; Lloyd, K.; Renehan, I. Awareness of the link between obesity and cancer in UK school curricula. Lancet 2019, 393, 1591–1592.
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K. Body Fatness and Cancer-Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016, 375, 794–798.
- Lee, D.H.; Giovannucci, E.L. The Obesity Paradox in Cancer: Epidemiologic Insights and Perspectives. Curr. Nutr. Rep. 2019, 8, 175–181.
- Stone, T.W.; McPherson, M.; Gail Darlington, L. Obesity and Cancer: Existing and New Hypotheses for a Causal Connection. EBioMedicine 2018, 30, 14–28.
- Shu, Y.; Hassan, F.; Ostrowski, M.C.; Mehta, K.D. Role of hepatic PKCβ in nutritional regulation of hepatic glycogen synthesis. JCI Insight 2021, 6, e149023.
- Rivera, M.J.; Contreras, A.; Nguyen, L.T.; Eldon, E.D.; Klig, L.S. Regulated inositol synthesis is critical for balanced metabolism and development in Drosophila melanogaster. Biol. Open 2021, 10, bio058833.
- IOM. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride; Institute of Medicine Standing Committee on the Scientific Evaluation of Dietary Reference Intakes; National Academy of Sciences: Washington, DC, USA, 1997.
- Qadeer, H.A.; Bashir, K. Physiology, Phosphate; StatPearls Publishing: Treasure Island, FL, USA, 2021.
- Brown, R.B. Stress, inflammation, depression, and dementia associated with phosphate toxicity. Mol. Biol. Rep. 2020, 47, 9921–9929.
- Saki, F.; Kassaee, S.R.; Salehifar, A.; Omrani, G.H.R. Interaction between serum FGF-23 and PTH in renal phosphate excretion, a case-control study in hypoparathyroid patients. BMC Nephrol. 2020, 21, 176.
- Brown, R.B.; Razzaque, M.S. Dysregulation of phosphate metabolism and conditions associated with phosphate toxicity. BoneKEy Rep. 2015, 4, 705.
- Brown, R.B.; Razzaque, M.S. Phosphate toxicity and tumorigenesis. Biochim. Et Biophys. Acta (BBA)-Rev. Cancer 2018, 1869, 303–309.
- Ward, D.N.; Griffin, A.C. Phosphorus incorporation into nucleic acids and proteins of liver nuclei of normal and azo dye-fed rats. Cancer Res. 1955, 15, 456–461.
- Jin, H.; Xu, C.-X.; Lim, H.-T.; Park, S.-J.; Shin, J.-Y.; Chung, Y.-S.; Park, S.-C.; Chang, S.-H.; Youn, H.-J.; Lee, K.-H. High dietary inorganic phosphate increases lung tumorigenesis and alters Akt signaling. Am. J. Respir. Crit. Care Med. 2009, 179, 59–68.
- Lin, Y.; McKinnon, K.E.; Ha, S.W.; Beck, G.R., Jr. Inorganic phosphate induces cancer cell mediated angiogenesis dependent on forkhead box protein C2 (FOXC2) regulated osteopontin expression. Mol. Carcinog. 2015, 54, 926–934.
- Chudek, J.; Nagy, A.; Kokot, F.; Podwinski, A.; Wiecek, A.; Ritz, E.; Kovacs, G. Phosphatemia is related to chromosomal aberrations of parathyroid glands in patients with hyperparathyroidism. J. Nephrol. 2007, 20, 164–172.
- Bobko, A.A.; Eubank, T.D.; Driesschaert, B.; Dhimitruka, I.; Evans, J.; Mohammad, R.; Tchekneva, E.E.; Dikov, M.M.; Khramtsov, V.V. Interstitial inorganic phosphate as a tumor microenvironment marker for tumor progression. Sci. Rep. 2017, 7, 41233.
- Levan, K.; Mehryar, M.; Mateoiu, C.; Albertsson, P.; Bäck, T.; Sundfeldt, K. Immunohistochemical evaluation of epithelial ovarian carcinomas identifies three different expression patterns of the MX35 antigen, NaPi2b. BMC Cancer 2017, 17, 303.
- D’Arcangelo, M.; Brustugun, O.; Xiao, Y.; Choi, Y.; Behrens, C.; Solis, L.; Wang, Y.; Firestein, R.; Boyle, T.; Lund-Iversen, M. 194P prevalence and prognostic significance of sodium-dependent phosphate transporter 2B (NAPI2B) protein expression in non-small cell lung cancer (NSCLC). Ann. Oncol. 2014, 25, iv66–iv67.
- Elser, J.J.; Kyle, M.M.; Smith, M.S.; Nagy, J.D. Biological stoichiometry in human cancer. PLoS ONE 2007, 2, e1028.
- Camalier, C.E.; Young, M.R.; Bobe, G.; Perella, C.M.; Colburn, N.H.; Beck, G.R. Elevated phosphate activates N-ras and promotes cell transformation and skin tumorigenesis. Cancer Prev. Res. 2010, 3, 359–370.
- Papaloucas, C.; Papaloucas, M.; Kouloulias, V.; Neanidis, K.; Pistevou-Gompaki, K.; Kouvaris, J.; Zygogianni, A.; Mystakidou, K.; Papaloucas, A. Measurement of blood phosphorus: A quick and inexpensive method for detection of the existence of cancer in the body. Too good to be true, or forgotten knowledge of the past? Med. Hypotheses 2014, 82, 24–25.
- Wulaningsih, W.; Michaelsson, K.; Garmo, H.; Hammar, N.; Jungner, I.; Walldius, G.; Holmberg, L.; Van Hemelrijck, M. Inorganic phosphate and the risk of cancer in the Swedish AMORIS study. BMC Cancer 2013, 13, 257.
- Lacerda-Abreu, M.A.; Russo-Abrahão, T.; Cosentino-Gomes, D.; Nascimento, M.T.C.; Carvalho-Kelly, L.F.; Gomes, T.; Rodrigues, M.F.; König, S.; Rumjanek, F.D.; Monteiro, R.Q.; et al. H(+)-dependent inorganic phosphate transporter in breast cancer cells: Possible functions in the tumor microenvironment. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 2180–2188.
- Mansinho, A.; Ferreira, A.R.; Casimiro, S.; Alho, I.; Vendrell, I.; Costa, A.L.; Sousa, R.; Abreu, C.; Pulido, C.; Macedo, D.; et al. Levels of Circulating Fibroblast Growth Factor 23 (FGF23) and Prognosis in Cancer Patients with Bone Metastases. Int. J. Mol. Sci. 2019, 20, 695.
- Li, J.R.; Chiu, K.Y.; Ou, Y.C.; Wang, S.S.; Chen, C.S.; Yang, C.K.; Ho, H.C.; Cheng, C.L.; Yang, C.R.; Chen, C.C.; et al. Alteration in serum concentrations of FGF19, FGF21, and FGF23 in patients with urothelial carcinoma. Biofactors 2019, 45, 62–68.
- Kim, W.T.; Bang, W.J.; Seo, S.P.; Kang, H.W.; Byun, Y.J.; Piao, X.M.; Jeong, P.; Shin, K.S.; Choi, S.Y.; Lee, O.J.; et al. Parathyroid hormone is associated with prostate cancer. Prostate Int. 2020, 8, 116–120.
- Kato, H.; Ito, N.; Makita, N.; Nangaku, M.; Leung, A.M.; Inoue, K. Association of Serum Parathyroid Hormone Levels With All-Cause and Cause-Specific Mortality Among U.S. Adults. Endocr. Pract. 2022, 28, 70–76.
- Palmieri, S.; Roggero, L.; Cairoli, E.; Morelli, V.; Scillitani, A.; Chiodini, I.; Eller-Vainicher, C. Occurrence of malignant neoplasia in patients with primary hyperparathyroidism. Eur. J. Intern. Med. 2017, 43, 77–82.
- Mirza, M.A.; Alsiö, J.; Hammarstedt, A.; Erben, R.G.; Michaëlsson, K.; Tivesten, A.; Marsell, R.; Orwoll, E.; Karlsson, M.K.; Ljunggren, O.; et al. Circulating fibroblast growth factor-23 is associated with fat mass and dyslipidemia in two independent cohorts of elderly individuals. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 219–227.
- Ministrini, S.; Ricci, M.A.; Daviddi, G.; Scavizzi, M.; De Vuono, S.; D’Abbondanza, M.; Paganelli, M.T.; Boni, M.; Roscini, A.R.; Scarponi, A.M.; et al. Determinants of High Parathyroid Hormone Levels in Patients With Severe Obesity and Their Relationship With the Cardiometabolic Risk Factors, Before and After a Laparoscopic Sleeve Gastrectomy Intervention. Obes. Surg. 2020, 30, 2225–2232.
- Coluzzi, I.; Raparelli, L.; Guarnacci, L.; Paone, E.; Del Genio, G.; le Roux, C.W.; Silecchia, G. Food Intake and Changes in Eating Behavior After Laparoscopic Sleeve Gastrectomy. Obes. Surg. 2016, 26, 2059–2067.
- Ha, J.; Jo, K.; Lim, D.J.; Lee, J.M.; Chang, S.A.; Kang, M.I.; Cha, B.Y.; Kim, M.H. Parathyroid hormone and vitamin D are associated with the risk of metabolic obesity in a middle-aged and older Korean population with preserved renal function: A cross-sectional study. PLoS ONE 2017, 12, e0175132.
- Jiang, S.; Ma, X.; Li, M.; Yan, S.; Zhao, H.; Pan, Y.; Wang, C.; Yao, Y.; Jin, L.; Li, B. Association between dietary mineral nutrient intake, body mass index, and waist circumference in U.S. adults using quantile regression analysis NHANES 2007-2014. PeerJ 2020, 8, e9127.
- Hartman, M.-L.; Groppo, F.; Ohnishi, M.; Goodson, J.M.; Hasturk, H.; Tavares, M.; Yaskell, T.; Floros, C.; Behbehani, K.; Razzaque, M.S. Can salivary phosphate levels be an early biomarker to monitor the evolvement of obesity. In Phosphate and Vitamin D in Chronic Kidney Disease; Karger Publishers: Basel, Switzerland, 2013; Volume 180, pp. 138–148.
- Håglin, L.; Törnkvist, B.; Bäckman, L. Obesity, smoking habits, and serum phosphate levels predicts mortality after life-style intervention. PLoS ONE 2020, 15, e0227692.
- Stoian, M.; Stoica, V. The role of disturbances of phosphate metabolism in metabolic syndrome. Maedica 2014, 9, 255–260.
- Engin, A. The Definition and Prevalence of Obesity and Metabolic Syndrome. Adv. Exp. Med. Biol. 2017, 960, 1–17.
- García-Estévez, L.; Cortés, J.; Pérez, S.; Calvo, I.; Gallegos, I.; Moreno-Bueno, G. Obesity and Breast Cancer: A Paradoxical and Controversial Relationship Influenced by Menopausal Status. Front. Oncol. 2021, 11, 705911.
- Piiroinen, O.; Kaihola, H.L. Uterine size measured by ultrasound during the menstrual cycle. Acta Obstet. Gynecol. Scand. 1975, 54, 247–250.
- Gorczyca, A.M.; Sjaarda, L.A.; Mitchell, E.M.; Perkins, N.J.; Schliep, K.C.; Wactawski-Wende, J.; Mumford, S.L. Changes in macronutrient, micronutrient, and food group intakes throughout the menstrual cycle in healthy, premenopausal women. Eur. J. Nutr. 2016, 55, 1181–1188.
- Shanmugalingam, T.; Crawley, D.; Bosco, C.; Melvin, J.; Rohrmann, S.; Chowdhury, S.; Holmberg, L.; Van Hemelrijck, M. Obesity and cancer: The role of vitamin D. BMC Cancer 2014, 14, 712.




