Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alima Abilkassymova | -- | 3655 | 2024-03-13 12:31:01 | | | |
| 2 | Jason Zhu | Meta information modification | 3655 | 2024-03-26 02:33:12 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Abilkassymova, A.; Turgumbayeva, A.; Sarsenova, L.; Tastambek, K.; Altynbay, N.; Ziyaeva, G.; Blatov, R.; Altynbayeva, G.; Bekesheva, K.; Abdieva, G.; et al. Four Atraphaxis Species. Encyclopedia. Available online: https://encyclopedia.pub/entry/56206 (accessed on 07 February 2026).
Abilkassymova A, Turgumbayeva A, Sarsenova L, Tastambek K, Altynbay N, Ziyaeva G, et al. Four Atraphaxis Species. Encyclopedia. Available at: https://encyclopedia.pub/entry/56206. Accessed February 07, 2026.
Abilkassymova, Alima, Aknur Turgumbayeva, Lazzat Sarsenova, Kuanysh Tastambek, Nazym Altynbay, Gulnar Ziyaeva, Ravil Blatov, Gulmira Altynbayeva, Kuralay Bekesheva, Gulzhamal Abdieva, et al. "Four Atraphaxis Species" Encyclopedia, https://encyclopedia.pub/entry/56206 (accessed February 07, 2026).
Abilkassymova, A., Turgumbayeva, A., Sarsenova, L., Tastambek, K., Altynbay, N., Ziyaeva, G., Blatov, R., Altynbayeva, G., Bekesheva, K., Abdieva, G., Ualieva, P., Shynykul, Z., & Kalykova, A. (2024, March 13). Four Atraphaxis Species. In Encyclopedia. https://encyclopedia.pub/entry/56206
Abilkassymova, Alima, et al. "Four Atraphaxis Species." Encyclopedia. Web. 13 March, 2024.
Copy Citation
Atraphaxis is a genus of flowering plants in the family Polygonaceae, with approximately 60 species. Species of Atraphaxis are much-branched woody plants, forming shrubs or shrubby tufts, primarily inhabiting arid zones across the temperate steppe and desert regions of Central Asia, America, and Australia. Atraphaxis species have been used by diverse groups of people all over the world for the treatment of various diseases.
Atraphaxis
Polygonaceae
Atraphaxis laetevirens
Atraphaxis frutescens
Atraphaxis spinosa L.
1. Distribution of Four Atraphaxis Species
Four Atraphaxis species exhibit diverse distributions across Central Asia, spanning from Afghanistan, Kazakhstan, and Kirgizstan to regions within Russia, Mongolia, parts of the Middle East, and China, showcasing their adaptability to varied geographical landscapes (Figure 1) [1]. A. pyrifolia spans Afghanistan, Kazakhstan, Kirgizstan, Pakistan, Tadzhikistan, Uzbekistan, and Xinjiang. A. spinosa L. extends across diverse territories, including Afghanistan, East European Russia, Egypt, Iran, Kazakhstan, Kirgizstan, Lebanon-Syria, Mongolia, North Caucasus, Pakistan, Palestine, Saudi Arabia, Sinai, Tadzhikistan, Transcaucasia, Turkey, Turkmenistan, Uzbekistan, West Siberia, and Xinjiang. A. laetevirens predominantly inhabits Altay, Kazakhstan, Krasnoyarsk, Mongolia, Tuva, Uzbekistan, and Xinjiang. Lastly, A. frutescens occupies regions such as Altay, North-Central China, East European Russia, Inner Mongolia, Kazakhstan, Krasnoyarsk, Mongolia, Qinghai, South European Russia, Turkmenistan, Tuva, Uzbekistan, West Siberia, and Xinjiang. These distributions highlight the wide-ranging presence of Atraphaxis species across Central Asia, parts of Europe, and portions of the Middle East, demonstrating their adaptability to diverse geographical and climatic conditions [2][3].
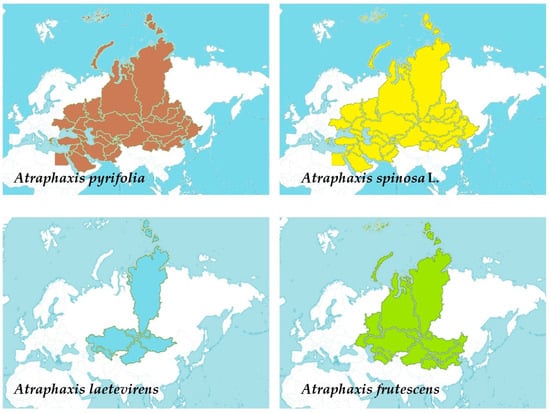
Figure 1. The distribution of four different Atraphaxis species using shaded colors: Atraphaxis pyrifolia represented in brown, Atraphaxis spinosa L. in yellow, Atraphaxis laetevirens in light blue, and Atraphaxis frutescens in green.
2. Botany of Some Atraphaxis Species
Atraphaxis species display leaves that are diverse in size and shape, reflecting their adaptability to different habitats. The leaves are typically arranged alternately along the stems. These leaves can be linear, lanceolate, or elliptical, depending on the species. One distinctive aspect of Atraphaxis leaves is their grayish-green coloration, which is often associated with xerophytic adaptations. The leaf surfaces may be smooth or covered with fine hairs, a characteristic known as pubescence. This pubescence can serve as a protective mechanism, reducing water loss by limiting transpiration. Atraphaxis species can be found in the form of compact or towering shrubs, occasionally taking the form of small subshrubs, with heights ranging from 20 cm to 300 cm. Annual shoots for Atraphaxis species are elongated and constricted. In the case of the majority of Atraphaxis species, the leaf margin typically displays a finely crenulate, undulate, flat, or slightly revolute pattern. Additionally, the ochrea in thyrses takes the form of an oblique, funnel-shaped structure, featuring a reduced leaf blade or a keel, and measuring between 2 to 7 mm in length. The position of thyrses in Atraphaxis species can be either terminal or lateral. The shape of the perianth in Atraphaxis species during the fruiting stage can be described as campanulate, with segments that are either of equal size or with inner segments significantly enlarged, tightly encasing the achene. The length of the perianth in Atraphaxis species typically falls within the range of 6.5 to 14.5 mm [4][5]. The segments in Atraphaxis species can vary in size, with some being equal, subequal, or unequal. Furthermore, the shape of these segments may be described as rotundate, reniform, broadly elliptical, broadly ovate, obtuse, flat, undulate, and occasionally, but rarely, concave. The consistence of the segments in Atraphaxis species is described as petaloid, indicating that they have a petal-like texture or appearance. The surface of the perianth in Atraphaxis species is typically smooth (glabrous), with the rare occurrence of slight papillate features at the base of the tube and segments. The edge of the segments in Atraphaxis species can either be papillate, meaning they have small, raised, nipple-like structures, or not papillate, having a smooth edge. Stomata on the segments of Atraphaxis species are either absent or very rarely found, and if present, they are typically located only at the base of the segments. The shape of the perianth tube in most species can be described as filiform, featuring a wedge-shaped or cup-shaped extension at the top. The length of the perianth tube in Atraphaxis species typically falls within the range of 0.5 to 7.5 mm. The length of the filiform part of the perianth tube in Atraphaxis species typically ranges from 0.5 to 7.0 mm [4][6]. The size of the achene in species typically measures between 2.5 to 5.5 mm in length and 1.5 to 5.0 mm in width. The shape of the achene in most species can be described as ovoid, triquetrous (having three distinct angles or faces), or lenticular (lens-shaped). The styles and stigma in Atraphaxis species can either be connate at the base (joined together) or free (not joined), and they may have a capitate (having a rounded, knob-like shape) or fimbriate (having fringed or filamentous structures) appearance. Moreover, the surface of the achene in species can be described as smooth, smooth-pitted (featuring small pits or depressions), minutely rugulate (with fine wrinkles or ridges), or tuberculate (having small tubercles or warty protrusions). The surface of the sporoderm in most species can be characterized as striae-perforate, with rare occurrences of being reticulato-perforate. This indicates that the surface has fine parallel lines or striations with perforations, although reticulate (net-like) perforations are occasionally present [4][7][8].
The stems of Atraphaxis plants can exhibit variability, with some appearing woody and others herbaceous, depending on the species and the environmental conditions. A striking feature of many Atraphaxis species is the presence of swollen nodes or joints along the stem. These nodes give rise to the common name “knotweed family”. These nodes serve various functions, including structural support and the storage of water and nutrients. They are often an important characteristic for identification and taxonomic classification within the genus. Atraphaxis species produce relatively small and often inconspicuous flowers that are typically greenish, pink, or white in color. These flowers are arranged in axillary clusters or spikes along the stems. Although the flowers may not be showy, they play a crucial role in the reproductive success of these plants. The coloration and arrangement of these flowers can vary among species, contributing to the diversity within the genus. The inflorescence structure varies among Atraphaxis species but is commonly a raceme or panicle. These arrangements are essential for the dispersion of flowers and the successful pollination of the plants. The diversity in inflorescence structures provides insights into the adaptation of different species to their specific environments and pollinators. The fruits of Atraphaxis plants are small and typically triangular to orbicular in shape. These fruits, known as achenes, each contain a single seed. One distinguishing feature of Atraphaxis fruits is their enclosure within the persistent calyx. This calyx, which surrounds the fruit, persists even after flowering, serving as a protective covering. It can also aid in seed dispersal, and it is an important trait for identifying species within the genus [4]. The presence or absence of pubescence is a notable characteristic in Atraphaxis species. Pubescence refers to the fine hairs or trichomes found on the surfaces of leaves, stems, and other plant parts. Some Atraphaxis species display pronounced pubescence, which contributes to their ability to reduce water loss by limiting transpiration. In contrast, others have a smoother, glabrous surface. This variability in pubescence is linked to their adaptation to specific environmental conditions [4][9].
Overall, the genus Atraphaxis stands as a testament to nature’s capacity for adaptation and resilience in the face of challenging environmental conditions. Its morphological characteristics, such as leaves, stems, flowers, inflorescence, fruits, growth forms, and pubescence, are essential for species identification, providing insights into their ecological roles. These plants have evolved to thrive in arid and semi-arid regions, contributing to the intricate tapestry of life in these often-harsh environments.
A. pyrifolia displays spinescent branches, obovate rhomboid leaves with rounded-obtuse apices and slightly revolute margins [10][11]. A. binaludensis manifests spinescent branches with obovate-rhomboid leaves having rounded-obtuse apices and slightly revolute margins. A. intricata, A. seravschanica, and A. radkanensis differ further in specific leaf sizes, shapes, petiole lengths, flower sizes, pedicel lengths, and fruit lengths, contributing to their distinct morphological identities within the genus [10][11][12]. These species’ morphological variations, including leaf shape, apices, margins, and branch types, contribute significantly to their taxonomic classification and ecological adaptations. Studying these morphological differences aids in better understanding species diversity, evolutionary relationships, and ecological roles within their respective habitats.
3. Traditional Use
Traditional medical usage of Atraphaxis species has a long history, especially in Asia and the Middle East. Traditional medical systems have used these plants because of their possible medicinal benefits. Atraphaxis species have been utilized to treat gastrointestinal issues like diarrhea, indigestion, and stomachaches in several traditional medical systems. To treat these symptoms, the plants can be consumed as herbal decoctions or infusions. Some Atraphaxis species have been employed for their ability to reduce inflammation. To treat illnesses like arthritis, joint discomfort, or skin inflammations, they can be administered topically or taken internally, as herbal treatments. Atraphaxis species have been utilized by traditional healers in some areas to promote wound healing [13]. To speed up the healing process, the plants can be made into poultices or ointments and applied to cuts and wounds. There is some ethnopharmacological data pointing to the possibility of antidiabetic effects in Atraphaxis species. Traditional medicine has employed these plants’ extracts to control blood sugar levels. Atraphaxis species have been used to cure respiratory conditions such as asthma and coughing in several cultures. To treat respiratory ailments, infusions or extracts from these plants are consumed orally or by steam inhalation. Traditional medicine may make use of Atraphaxis species due to their antioxidant capabilities, which can assist the body in fighting oxidative stress [14].
4. Bioactive Compounds from Four Atraphaxis Species
Phytochemical studies of Atraphaxis species have revealed the presence of various bioactive compounds, although the specific phytochemical composition can vary among different species and even within the same species. Some of the common phytochemicals that have been identified in Atraphaxis species include polyphenols, triterpenoids, alkaloids, essential oils, phenolic acids, saponins, and lignans. Atraphaxis species are known to contain various polyphenolic compounds, such as flavonoids and tannins. These compounds have antioxidant properties and may contribute to the plant’s ability to protect itself from oxidative stress. Triterpenoids are a class of compounds that have been found in some Atraphaxis species. These compounds have various biological activities and are known for their potential anti-inflammatory and cytotoxic properties. Some Atraphaxis species have been reported to contain alkaloids. Alkaloids are nitrogen-containing compounds with a wide range of pharmacological activities. The presence of alkaloids in Atraphaxis species may contribute to their medicinal properties. Certain Atraphaxis species produce essential oils that contain volatile compounds responsible for the characteristic aroma of the plant. These essential oils may have various uses, including in traditional medicine and as flavoring agents [15]. Phenolic acids, such as caffeic acid and ferulic acid, have been detected in Atraphaxis species. These compounds have antioxidant and anti-inflammatory properties and are commonly found in many plant species. Saponins are glycosides found in some Atraphaxis species. They are known for their foaming properties and have been studied for their potential health benefits, including as anticancer agents and immune system modulators. Lignans are polyphenolic compounds with antioxidant properties. Some Atraphaxis species have been reported to contain lignans, which may contribute to their medicinal potential. It is important to note that the phytochemical composition of Atraphaxis species can vary depending on factors such as species, geographical location, and environmental conditions. Additionally, the presence of specific phytochemicals may have implications for the traditional medicinal uses of these plants in various cultures. Phytochemical studies of Atraphaxis species are ongoing, and researchers continue to explore the potential pharmacological properties and applications of the compounds found in these plants. These studies may lead to the development of new drugs or natural products for various purposes, including medicine, agriculture, and industry [16].
A. frutescens and A. spinosa L. are distinguished from other Atraphaxis species due to their rich assortment of chemical components. A study on A. frutescens revealed the presence of several flavonoids featuring pyrogallol B-ring structures, which are renowned for their antioxidative qualities. A. frutescens has yielded fisetinidol, a compound known for its potential biological activities, including its antioxidant and anti-inflammatory properties. Another compound discovered in A. frutescens is catechin, which is recognized for its antioxidant properties and potential health benefits. Furthermore, the aerial parts of A. frutescens yielded quercetin and butin, both of which are flavonoids with antioxidative and anti-inflammatory properties. Additionally, compounds such as quercetin-3-methyl ether, 5-deoxykaempferol, and β-sitosterol glucoside have been identified in A. frutescens, although their pharmacological properties have not been thoroughly investigated yet [1][14].
Nine compounds were obtained and identified from an ethereal extract of A. spinosa L. var. sinaica. These compounds were characterized as N-trans-p-coumaroyl-3′,4′-dihydroxyphenylethylamine, N-trans-feruloyl-3′,4′-dihydroxyphenylethylamine, (−)-fisetinidol, (−)-catechin, butin, quercetin, quercetin-3-methyl ether, 5-deoxykaempferol, and β-sitosterol glucoside. The compounds N-trans-p-coumaroyl-3′,4′-dihydroxyphenylethylamine and N-trans-feruloyl-3′,4′-dihydroxyphenylethylamine, which were isolated from natural sources for the first time, exhibited cytotoxic effects on leukemic P388 cells [13][15][17].
The comprehensive exploration of A. pyrifolia and A. laetevirens concerning their biologically active compounds remains incomplete. Nevertheless, limited research has acknowledged the presence of certain valuable compounds within these species. For instance, chrysophanol, physcion, nepodin, and emodin represent natural compounds that exist in diverse plant species, including A. laetevirens. Classified as anthraquinones, these compounds fall into the category of organic molecules and have displayed various pharmacological properties in scientific investigations. Specifically, chrysophanol and nepodin have gained recognition for their effectiveness in combating insects and bacteria [16].
Studies of A. pyrifolia have identified flavonoid glycosides, including compounds such as 7-methylgossypetin 8-β-D-glucopyranoside 3-O-α- L-rhamnopyranoside, and 7-methylgossypetin 8-β-D-glucopyranoside. However, despite their detection, extensive research into their potential pharmacological applications is yet to be conducted.
Phenols, a class of organic compounds characterized by a hydroxyl (OH) group attached to an aromatic ring, exhibit diverse pharmacological activities that have significant implications for human health. These compounds are renowned for their potent antioxidant properties, effectively scavenging free radicals and reducing oxidative stress, as indicated in numerous studies [18]. Their anti-inflammatory effects make them valuable in alleviating symptoms associated with conditions such as arthritis and inflammatory bowel disease, with research supporting their role in modulating inflammatory pathways [19].
Phenolic compounds also demonstrate antimicrobial and antibacterial activity, inhibiting the growth of bacteria, fungi, and viruses. Their potential in promoting heart health through blood pressure reduction and cholesterol management is well-documented [20]. Furthermore, some phenolic compounds exhibit anti-cancer properties by inhibiting tumor cell growth and promoting apoptosis [21]. Additionally, these compounds can have neuroprotective effects and may reduce the risk of neurodegenerative diseases, such as Alzheimer’s and Parkinson’s disease [22].
Moreover, phenolic compounds play a pivotal role in supporting liver health by protecting the liver from damage and aiding in tissue regeneration. They also have anti-diabetic potential by regulating blood sugar levels and improving insulin sensitivity. The immunomodulatory effects of phenols enhance immune responses against infections and diseases, and their use in wound healing and skin health applications is based on their ability to promote tissue repair and protect the skin from oxidative damage. The versatile pharmacological activities of phenols underscore their importance in preventive and therapeutic approaches to various health conditions [23][24][25].
Flavonoids, a diverse group of polyphenolic compounds found abundantly in plants, have captivated the attention of the medical and scientific communities for their remarkable potential in medicine. These natural compounds, responsible for the vibrant colors in fruits, vegetables, and flowers, go beyond aesthetics; they hold significant therapeutic promise. Flavonoids, including quercetin, kaempferol, and catechin, are potent antioxidants. They neutralize harmful free radicals and unstable molecules that can damage cells and the DNA. This antioxidant capacity is pivotal in preventing and managing a range of diseases, including cancer, cardiovascular ailments, and neurodegenerative disorders. Inflammation is a double-edged sword, being necessary for defense but destructive in excess. Certain flavonoids like quercetin, rutin, and luteolin exhibit anti-inflammatory properties. They mitigate excessive inflammation and alleviate symptoms in conditions such as arthritis and inflammatory bowel diseases [26]. Flavonoids contribute to heart health. By reducing oxidative stress, enhancing endothelial function, and improving blood vessel elasticity, they lower the risk of hypertension, atherosclerosis, and heart disease. Prominent flavonoids such as resveratrol, found in red wine, are associated with cardiovascular benefits. Moreover, the brain benefits from flavonoids too. Compounds such as epicatechin from dark chocolate may enhance cognitive function, and quercetin could protect against neurodegenerative disorders by reducing oxidative stress and inflammation in the brain. Additionally, flavonoids have shown antiviral properties, including inhibiting the replication of viruses, such as influenza and HIV [27]. Additionally, they possess antimicrobial capabilities, which can aid in combating infections and antibiotic-resistant bacteria. Another benefit is that flavonoids have gained recognition for their potential in cancer prevention and treatment. They can induce apoptosis (programmed cell death) in cancer cells, inhibit angiogenesis (formation of blood vessels that feed tumors), and modulate signaling pathways that drive cancer growth. Certain flavonoids, such as quercetin and kaempferol, promote wound healing and skin health by aiding tissue repair, reducing inflammation, and protecting against UV-induced damage. Some flavonoids can help manage diabetes by improving insulin sensitivity and glucose metabolism, potentially reducing the risk of diabetes-related complications [28].
Flavonoids and their derivatives offer a rich palette of therapeutic possibilities, and ongoing research continues to unveil their potential applications. However, it is important to consider that the efficacy and safety of flavonoid-based treatments may vary depending on the specific compound, dosage, and individual factors. Integrating flavonoid-rich foods and supplements into a balanced diet may offer a holistic approach to harnessing their health benefits. As science explores this colorful world of natural compounds, the future of medicine may hold exciting breakthroughs thanks to flavonoids [29].
Flavonoids, specifically flavonol glycosides, such as myricitrin, have been identified in A. frutescens [14] and A. spinosa L. [15]. Myricitrin exhibits noteworthy antioxidant properties and showcases anti-inflammatory as well as anti-nociceptive effects [30]. This flavonoid compound has garnered attention for its potential role in combating oxidative stress, reducing inflammation, and mitigating pain responses within the biological system.
Another flavonoid compound, emodin 8-O-β-D-glucopyranoside, discovered in A. frutescens [14], displays significant pharmacological activities. Emodin 8-O-β-D-glucopyranoside has shown promise as an anticancer agent [31], demonstrating potential in the fight against cancerous cell proliferation. Additionally, this compound exhibits neuroprotective properties [32], indicating its potential role in preserving neural health and potentially mitigating neurodegenerative conditions.
These flavonoid glycosides, found within Atraphaxis species, particularly myricitrin and emodin 8-O-β-D-glucopyranoside in A. frutescens, showcase a range of pharmacological effects. Their antioxidant, anti-inflammatory, anti-nociceptive, anticancer, and neuroprotective attributes demonstrate their potential therapeutic significance in various medical applications and merit further exploration in the domain of pharmacology and medical research. However, other flavonoid glycosides, specifically 3,4,5-trimethoxyphenyl 1-O-β-D-glucopyranoside, 8-O-β-D-glucopyranosyl-7-O-methyl-3-O-α-L-rhamnopyranosylgossypetin, 8-O-acetyl-7-O-methyl-3-O-α-L-rhamnopyranosylgossypetin, 7-O-methyl-3-O-α-rhamnopyranosylgossypetin, quercitrin, quercetin 3-O-β-D-glucuronide-6-O-methyl ester, quercetin-3-O-β-D-glucuronide, afzelin, 7-methylgossypetin 8-β-D-glucopyranoside 3-O-α-L-rhamnopyranoside, and 7-methylgossypetin 8-β-D-glucopyranoside, have not been thoroughly studied for their pharmacological properties, and their effects remain largely unclear.
5. Pharmacological Activities of Found Compounds from Four Atraphaxis Species
A vast reservoir of selected species remains untapped in terms of phytochemical constituents, as well as pharmacology, and this is the research gap for future studies. Further investigations of Atraphaxis species, along with their phytochemical constituents, are necessary to completely understand the molecular mechanisms of their action in vivo and in vitro and to ensure the plant extracts are safe for human use. According to findings related to bioactive compounds, it is possible to use Atraphaxis species for different purposes, as shown in Figure 2.
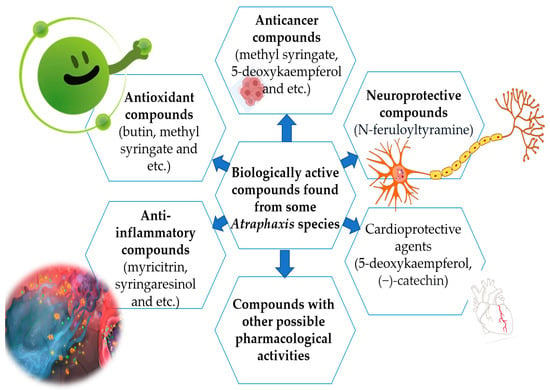
Figure 2. Biological active compounds with pharmacological activities.
Atraphaxis species are known to contain polyphenols, such as flavonoids and tannins, which are well-regarded for their potent antioxidant properties. These compounds have the ability to counteract oxidative stress, inflammation, and cellular damage, making them valuable assets in the realm of health and medicine. Their therapeutic potential extends to conditions associated with these processes, including cardiovascular diseases and cancer. Another class of compounds found in Atraphaxis species includes triterpenoids. These compounds exhibit anti-inflammatory and antioxidant attributes, which can be invaluable for addressing inflammatory disorders and promoting overall health and wellness. The anti-inflammatory properties make them particularly attractive for conditions involving excessive inflammation, such as arthritis [33][34]. In addition to polyphenols and triterpenoids, Atraphaxis species may also contain alkaloids with diverse pharmacological actions. Alkaloids are nitrogen-containing compounds with a wide range of potential effects, including analgesic and antimicrobial properties. These compounds have shown promise in managing pain and combating various pathogens. Moreover, Atraphaxis species may harbor lignans with potential hormonal activity. Lignans are phytochemicals with estrogenic effects and may hold promise in addressing hormonal imbalances and managing menopausal symptoms [1][13][14][15][17].
In summary, Atraphaxis species represent a rich source of bioactive molecules with diverse pharmacological activities. The compounds found in these plants have the potential to contribute to the development of novel medicines and health-promoting products [35][36]. However, it is essential to note that the specific pharmacological activities and applications may vary among different Atraphaxis species and may require further research and investigation. Continued scientific exploration of these natural compounds may unlock their full therapeutic potential, leading to valuable contributions to healthcare and wellness. The pharmacological activities of the compounds found in Atraphaxis species, namely A. laetevirens, A. frutescens, A. spinosa L., and A. pyrifolia, encompass a diverse range of potential effects, although comprehensive studies on these activities are still needed for a more definitive understanding. Some identified compounds have shown promising pharmacological properties in preliminary research. For instance, known to be present in A. frutescens, myricitrin exhibits antioxidant, anti-inflammatory, and anti-nociceptive effects [14]. Emodin 8-O-β-D-glucopyranoside was discovered in A. frutescens. This compound demonstrates potential as both an anticancer and a neuroprotective agent [14][31][32]. Compounds such as 3,4,5-Trimethoxyphenyl 1-O-β-D-glucopyranoside, 8-O-β-D-glucopyranosyl-7-O-methyl-3-O-α-L-rhamnopyranosylgossypetin, 8-O-acetyl-7-O-methyl-3-O-α-L-rhamnopyranosylgossypetin, 7-O-methyl-3-O-α-rhamnopyranosylgossypetin, quercitrin, quercetin 3-O-b-D-glucuronide-6″-methyl ester, quercetin-3-O-bD-glucuronide, afzelin, 7-methylgossypetin 8-β-D-glucopyranoside 3-O-α-L-rhamnopyranoside, and 7-methylgossypetin 8-β-D-glucopyranoside are found in Atraphaxis species, but their pharmacological activities have not been fully elucidated [16].
The identified compounds display potential pharmacological activities, ranging from antioxidant and anti-inflammatory effects to anticancer and neuroprotective properties. However, further in-depth investigations and studies are necessary to fully comprehend and establish the therapeutic potential and mechanisms of action of these compounds from Atraphaxis species.
References
- Sanchez, A.; Kron, K.A. Phylogenetics of Polygonaceae with an Emphasis on the Evolution of Eriogonoideae. Systematic Bot. 2008, 33, 87–96.
- Xu, Z.; Zhang, M.L. Phylogeography of the arid shrub Atraphaxis frutescens (Polygonaceae) in northwestern China: Evidence from cpDNA sequences. J. Hered. 2015, 106, 184–195.
- Khojimatov, O.K.; Gafforov, Y.; Bussmann, R. Ethnobiology of Uzbekistan: Ethnomedicinal Knowledge of Mountain Communities, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2023; pp. 107–159.
- Yurtseva, O.V.; Severova, E.E.; Bovina, I.Y. Pollen morphology and taxonomy of Atraphaxis (Polygoneae, Polygonaceae). Plant Syst. Evol. 2014, 300, 749–766.
- Brandbyge, J. The Families and Genera of Vascular Plants, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 1993; pp. 531–544.
- Bao, B.; Grabovskaya, B. Flora of China, 5th ed.; Science Press: Beijing, China; Missouri Botanical Press: St. Louis, MI, USA, 2003; pp. 328–333.
- Hong, S.P. Pollen morphology of Parapteropyrum and some putatively related genera (Polygonaceae-Atraphaxideae). Grana 1995, 34, 153–159.
- Cullen, J. Flora of Turkey and the East Aegean Islands, 2nd ed.; Edinburgh University Press: Edinburgh, UK, 1967; pp. 266–267.
- Akhani, H. Studies on the flora and vegetation of the Golestan National Park, NE Iran. III. Three new species, one new subspecies and fifteen new records for Iran. Edinb. J. Bot. 1999, 56, 1–31.
- Tavakkoli, S.; Kazempour Osaloo, S.; Mozaffarian, V.; Maassoumi, A.A. Molecular phylogeny of Atraphaxis and the woody Polygonum species (Polygonaceae): Taxonomic implications based on molecular and morphological evidence. Plant Syst. Evol. 2015, 301, 1157–1170.
- Rechinger, K.H. Flora Iranica; Achademische Druck–und Verlagsanstalt: Graz, Iran, 1968; pp. 30–35.
- Qaiser, M. Flora of Pakistan; Missouri Botanical Garden Press: St. Louis, MI, USA, 2001; pp. 128–136.
- Nurlybekova, A.; Kudaibergen, A.; Kazymbetova, A.; Amangeldi, M.; Baiseitova, A.; Ospanov, M.; Aisa, H.A.; Ye, Y.; Ibrahim, M.A.; Jenis, J. Traditional Use, Phytocemical Profiles and Pharmacological Properties of Artemisia Genus from Central Asia. Molecules 2022, 27, 5128.
- Odonbayar, B.; Murata, T.; Batkhuu, J.; Yasunaga, K.; Goto, R.; Sasaki, K. Antioxidant Flavonols and Phenolic Compounds from Atraphaxis frutescens and Their Inhibitory Activities against Insect Phenoloxidase and Mushroom Tyrosinase. J. Nat. Prod. 2016, 79, 3065–3071.
- Wang, X.; Khutsishvili, M.; Fayvush, G.; Tamanyan, K.; Atha, D.; Borris, R.P. Phytochemical investigations of Atraphaxis spinosa L (Polygonaceae). Bioch. Syst. Eco. 2018, 77, 44–47.
- Nakano, H.; Schrader, K.K.; Mamonov, L.K.; Kustova, T.S.; Mursaliyeva, V.K.; Cantrell, C.L. Isolation and identification of Flavobacterium columnare and Streptococcus iniae antibacterial compounds from the terrestrial plant Atraphaxis laetevirens. J. Agric. Food Chem. 2012, 24, 10415–10419.
- El-Gamal, A.A.; Takeya, K.O.I.C.H.I.; Itokawa, H.I.D.E.J.I.; Halim, A.F.; Amer, M.M.; Saad, H.E.A.; Awad, S.A. Studies on the chemical constituents of Atraphaxis spinosa L. var. sinaica Boiss. J. Nat. Med. 1994, 48, 304–306.
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and beyond. Am. J. Clin. Nutr. 2005, 81, 215S–217S.
- Pandey, K.B.; Syed, I.R. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278.
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural polyphenols: Chemical classification, definition of classes, subcategories, and structures. J AOAC Int. 2019, 102, 1397–1400.
- Dini, I.; Grumetto, L. Recent advances in natural polyphenol research. Molecules 2022, 27, 8777.
- Szabo, B.; Dörner, L.; Pfreundtner, C.; Nörenberg, W.; Starke, K. Inhibition of GABAergic inhibitory postsynaptic currents by cannabinoids in rat corpus striatum. Neuroscience 1998, 85, 395–403.
- Shegebayev, Z.; Turgumbayeva, A.; Datkhayev, U.; Zhakipbekov, K.; Kalykova, A.; Kartbayeva, E.; Beyatli, A.; Tastambek, K.; Altynbayeva, G.; Dilbarkhanov, B.; et al. Pharmacological Properties of Four Plant Species of the Genus Anabasis, Amaranthaceae. Molecules 2023, 28, 4454.
- Zhakipbekov, K.; Turgumbayeva, A.; Issayeva, R.; Kipchakbayeva, A.; Kadyrbayeva, G.; Tleubayeva, M.; Akhayeva, T.; Tastambek, K.; Sainova, G.; Serikbayeva, E.; et al. Antimicrobial and Other Biomedical Properties of Extracts from Plantago major, Plantaginaceae. Pharmaceuticals 2023, 16, 1092.
- Chumbalov, T.K.; Omurkamzinova, V.B. Flavonoids of Atraphaxis pyrifolia. IV. Chem. Nat. Compd. 1976, 12, 593–594.
- Khalatbary, A.R.; Khademi, E. The green tea polyphenolic catechin epigallocatechin gallate and neuroprotection. Nutr. Neurosci. 2020, 23, 281–294.
- Bernatova, I. Biological activities of (-)-epicatechin and (-)-epicatechin-containing foods: Focus on cardiovascular and neuropsychological health. Biotechnol. Adv. 2018, 36, 666–681.
- Ling, J.; Wu, Y.; Zou, X.; Chang, Y.; Li, G.; Fang, M. (-)-Epicatechin Reduces Neuroinflammation, Protects Mitochondria Function, and Prevents Cognitive Impairment in Sepsis-Associated Encephalopathy. Oxid. Med. Cell Longev. 2022, 2022, 2657713.
- Arts, I.C.W.; Hollman, P.C.H. Polyphenols and disease risk in epidemiologic studies. Am. J. Clin. Nutr. 2005, 81 (Suppl. 1), 317S–325S.
- Zhang, X.; Zhang, K.; Wang, Y.; Ma, R. Effects of Myricitrin and Relevant Molecular Mechanisms. Curr. Stem. Cell Res. Ther. 2020, 15, 11–17.
- Li, Y.; Li, K.; Zhao, Y.; Li, Y.; Li, D.; Shen, L.; Wang, Q.; Yang, H.S.; Sun, Z. Emodin-8-O-β-D-glucopyranoside, a natural hydroxyanthraquinone glycoside from plant, suppresses cancer cell proliferation via p21-CDKs-Rb axis. Toxicol. Appl. Pharmacol. 2022, 438, 115909.
- Eom, M.R.; Weon, J.B.; Jung, Y.S.; Ryu, G.H.; Yang, W.S.; Ma, C.J. Neuroprotective compounds from Reynoutria sachalinensis. Arch. Pharm. Res. 2017, 40, 704–712.
- Serafini, M.; Peluso, I.; Raguzzini, A. Flavonoids as anti-inflammatory agents. Proc. Nutr. Soc. 2010, 69, 273–278.
- Chumbalov, T.K.; Mukhamed’yarova, M.M.; Omurkamzinova, V.B. 7-O-methylherbacetin 3-rhamnoside from Atraphaxis pyrifolia. Chem. Nat. Compd. 1974, 10, 817. Available online: https://link.springer.com/article/10.1007/BF00564016 (accessed on 12 October 2023).
- Umbetova, A.K.; Burasheva, G.S.; Ikhsanov, Y.S.; Abidkulova, K.T.; Beyatli, A.; Sagatova, S.N.; Askanova, D.K. Chemical research and biological activity of plants of the genus atraphaxis (a. spinosa). Известия НАН РК. Chem. Technol. Ser. 2020, 6, 127–133.
- Olas, B. New Perspectives on the Effect of Dandelion, Its Food Products and Other Preparations on the Cardiovascular System and Its Diseases. Nutrients 2022, 14, 1350.
More
Information
Subjects:
Pharmacology & Pharmacy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
682
Revisions:
2 times
(View History)
Update Date:
26 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




