Collagen (CLG) is a structural protein composed of amino acids that create collagen fibers, characterized by exceptional strength and high elasticity. This protein is composed of three left-handed α polypeptides that wind around themselves and their axis to form a right-handed superhelix. Its structure varies depending on its functions and place of occurrence. CLG is one of the most important proteins in the human body because it is responsible for maintaining the appropriate structure of tissues and organs and constitutes as much as one-third of the total body protein mass. It occurs, among the main organs in the body that provide appropriate elasticity and strength. It is also an essential building block of the skin; without CLG, it would not be able to perform its functions properly.
1. Structure and Biosynthesis
Collagens are family of fibrillar proteins that dominate the extracellular matrix of most connective tissues in mammals
[1][2]. They are the basic proteins responsible for the structure and biochemical properties of connective tissue, and in the case of skin, they constitute approximately 70% of its dry weight. These are natural polymers constituting 1/3 of the total mass of proteins in the human body, mainly performing building functions
[3]. They are characterized by a unique form that varies depending on their function and place of occurrence
[1][4]. CLG commonly found in organisms is type I. Its presence, e.g., in bone tissue, provides elasticity and strength. Collagens are a fibrous components of the skin, tendons, ligaments, cartilage, and blood vessel walls. They are also present in bones and teeth and internal organs such as the heart, lungs, and liver
[1][3].
In terms of structure, collagens are among the most complex natural polymers
[5][6][7][8][9][10]. More than 20 amino acids may be involved in the construction of these protein molecules. Regardless of the type of CLG, the most abundant amino acids are proline (Pro), hydroxyproline (Pro-OH), glycine (Gly), and hydroxylysine (Lys-OH). The unique structure of CLG proteins consists of three left-handed polypeptide α chains, the so-called procollagen. They are wrapped around each other and have a common axis and thus form a right-handed conformation of a superhelix called tropocollagen
[1][5][6].
The superhelix is held together by hydrogen bonds, electrostatic interactions, and van der Waals forces
[5]. In addition to three-helical domains, non-helical fragments, i.e., telopeptides, are also involved in the structure of collagens. They occur at the ends of CLG macromolecules or are built into superhelix structures. The presence of a triple helix and its content ranges from 96% in type I CLG to less than 10% in type XII CLG
[1][5]. The fibers of most types of collagen form delicate networks, except types I and II, whose fibers are thick and resistant to stretching. Natural collagen superhelices are resistant to proteases such as pepsin, trypsin, and chymotrypsin
[1][8][10][11].
The only enzymes that can degrade these macromolecules are called collagenases; they are extracellular matrix metalloproteinases
[12]. Each chain contains about 1000 amino acids. A characteristic feature of the amino acid composition of CLG polypeptides is the equimolar amount of acidic amino acids, i.e., glutamic acid (Glu) and aspartic acid (Asp), and basic amino acids, i.e., lysine (Lys) and arginine (Arg)
[12]. The most frequently repeated sequence in the collagen polypeptide chain is Gly-X-Y-, of which Gly constitutes the third amino acid residue, and X and Y are the amino acid residues: Pro (approx. 28%) and Pro-OH (approx. 38%)
[5][6][12]. The number of kinks occurring in the collagen structure, resulting from disruptions in the regularly repeating Gly-X-Y sequence, determines its elasticity. Better aggregation possibilities are provided by greater flexibility, e.g., in membrane structures. The unique structure of the triple helix and strong bonds between amino acids make collagen fibers maintain their flexibility and are resistant to stretching. Collagen proteins are encoded by many genes, which is why they are structurally and functionally heterogeneous
[12]. Thanks to this, organisms protect themselves against the loss of these important macromolecules. Also, changes occurring during post-translational modification are important in maintaining the heterogeneity of collagen proteins, which are constantly synthesized and degraded in the extracellular space
[6]. This means that the vast majority of CLG fibers are made of more than one type of CLG
[6][8]. Before CLG appears in the extracellular space, it undergoes some modifications like hydroxylation, glycosylation, and the process of creating triplet procollagen particles. The resulting procollagen is expelled outside the cell. Then, using peptidases, telopeptides are removed and tropocollagen molecules are formed
[6][8]. The next stage of CLG biosynthesis is the formation of crosslinks between tropocollagen molecules. Finally, spontaneous association occurs into microfibrils, and then into fibrils and mature collagen fibers. Type I CLG is the most common type, found in the human body
[13]. It constitutes 85–90% of the organic content of bone connective tissue, skin, or other organs. It is primarily responsible for the tensile strength of tissues and bone stiffness
[7][8]. It can be found in bones, which is a product of the same genes as skin type I CLG. Both of these collagens differ significantly from each other, which is the result of post-translational modifications
[5][6]. Many factors can stimulate or inhibit collagen biosynthesis. This occurs at various stages of gene expression. TGF-β (transforming growth factor beta), which is one of the most important stimulating factors, influences the production of type I CLG in fibroblasts as a result of inducing the activity of the promoter of the gene encoding this protein
[14]. In addition to TGF-β, the following also have a stimulating effect on the synthesis: FGF (fibroblast growth factor), EGF (epidermal growth factor), and various isoforms of PDGF (platelet-derived growth factor). The opposite effect, i.e., inhibition of CLG biosynthesis, is demonstrated by, among others, interferon α and TNF (tumor necrosis factor)
[14].
2. Types of Collagen
A characteristic feature of the structure of all collagen proteins is the formation of the so-called rope, i.e., a right-handed superhelix, which is formed by winding three left-handed, single polypeptide chains around its own axis
[5]. Each type of CLG has a different initiation stage in the biosynthesis process. Common steps include intracellular reactions and triple helix formation, post-translational modifications, enzymatic glycosylation and hydroxylation of lysine, proteolytic cleavage of procollagen, and natural crosslinking
[5][15]. So far, 30 different forms of collagen, which perform various functions and have different structures (
Table 1), have been detected in human tissues
[5]. Moreover, they differ in their location and content in the tissues (
Table 1).
Table 1. Types of fibrillar collagens and their basic functions and occurrence in the body
[1][4][16].
| Type |
Structure |
Localization |
Function |
| I |
Triple superhelix composed of two identical α1 and one α2 chain; a form consisting of three α1 chains (homotrimeric) is also found |
Bones, tendons, ligaments, skin, cornea |
Tensile strength of tissues and bone stiffness |
| II |
Three identical α1[III]3 chains—homotrimer; similar properties and size to type I, but higher content of hydroxylysine and glucose and galactose residues |
The dominant component of vitreous tissue (approx. 80%), corneal epithelium, and cartilage |
One of the main components of ECM (extracellular matrix), maintains chondrocyte functions, induces cell adhesion and proliferation |
| III |
Three identical α1[III]3 chains |
Element of the dermis, liver and lung tissues, spleen and blood vessels |
Gives elasticity to tissues |
| V |
Three different chains—heterotrimers |
Bones, skin, placenta, cornea |
Initiation of collagen fibrillogenesis |
| XI |
Three different chains—heterotrimers |
Cartilage, intervertebral discs |
Initiation of collagen fibrillogenesis |
| XXIV |
The fragment of the Gly-Xaa-Yaa repeated sequence is relatively short, constitutes approximal |
Bones, cornea |
A marker of osteoblast differentiation and bone formation in mice |
| XXVII |
Forms non-striated, thread-like structures, unlike other members of the subfamily |
Cartilage |
Deposited mainly in skeletal tissues at the junction of cartilage and bone |
The polymorphism of CLG may result from differences in the expression of genes encoding enzymes that are involved in the biosynthesis of these proteins [5][6][13][17]. Another hypothesis is that the diversity of these proteins is caused by changes occurring in post-translational modifications [12]. The description of the subunit composition facilitates the nomenclature based on numbering in Roman numerals (I–XXIX) [1][4][16]. Arabic numerals indicate the polypeptide chains they are composed of (α1–α6). The family of collagen proteins is divided into two main groups: fibrillar collagens and non-fibrillar collagens [6][8][10]. In mammals, they are encoded by 11 genes and were discovered as the first among the CLG protein family. A common feature of this group is a long central triple helix. The fibrillar molecule has a diameter of 1.5 nm and a length of 300 nm. Table 1 presents the group of fibrillar collagens, taking into account the functions, locations, and characteristics of individual types of CLG [6][8][10]. The characteristic transverse striations of CLG fibers visible under an electron microscope are caused by the aggregation of macromolecules. The vast majority of them are heterotypical. This means that they are made of more than one type of collagen (Table 2). This phenomenon is confirmed by the presence of CLG fibers in the bones and cornea, composed mainly of type I and V collagens [6][8][10].
Table 2. Types of non-fibrillar collagens and their location in the body
[6][8][10].
| Collagen |
Type |
Localization |
| Basement membrane |
IV |
Basement membranes, capillaries |
| Forming microfibers |
XVI |
Bones, vessels, skin, cornea, cartilage |
| XXVIII |
Cells of the nervous system |
| XXIX |
Skin |
| Anchoring |
VII |
Mucosa, skin, bladder, umbilical cord, amnion |
| Forming hexagonal lattice systems |
VIII |
Mucosa, skin, bladder, umbilical cord, amnion |
| X |
Cartilage |
| FACITs type |
IX |
Cornea, vitreous body, cartilage |
| XII |
Cartilage, tendons, skin |
| XIV |
Vessels, eye, nerves, tendons, bones, skin, cartilage |
| XVI |
Heart, smooth muscles, skin, kidney |
| XIX |
Band of basement membranes in skeletal muscles, skin, kidneys, liver, placenta, spleen, prostate |
| XX |
Corneal epithelium |
| XXI |
Stomach, kidneys, vessels, heart, placenta, skeletal muscles |
| XXII |
Tissue connections |
| XXVI |
Testicles, ovaries |
| MACITs type |
XII |
Skeletal muscles, heart, eye, skin, endothelial cells |
| XVII |
Skin |
| XXIII |
Metastatic carcinogenic cells |
| XXV |
Eye, brain, heart, testicles |
| MULTIPLEXINs type |
X |
Capillaries, ovaries, heart, testicles, skin, placenta, kidneys |
| XVIII |
Kidney, lungs, liver |
However, the structure of the skin is characterized by the coexistence of types I and III, and in the cartilage, there are combinations of types II, III, IX, and XI. The composition of the fibrillar collagen group constitutes approximately 90% of all CLG proteins found in animal organisms. Fibrillar CLG types are I, II, III, V, XI, XXIV, and XXVI
[5][6][7][8]. Non-fibrillar CLGs do not form typical fibrils. Among this group of collagen proteins, we can distinguish basement membrane, forming microfibers, anchoring, forming hexagonal network systems, the FACITs type, containing transmembrane domains—MACITs and collagens of the MULTIPLEXINs type. The affiliation of individual types of CLG with the above groups and their location in the body
[8] are presented in
Table 2.
3. Physicochemical Characteristic of Collagen
The molecular weight of collagen is approximately 300,000 Da, the diameter is approximately 14–15 Å (Angstrom), and the length is 2800 Å
[5]. Collagen has high water-binding capacity, which makes it a good ingredient for texturizing, thickening, and creating gels
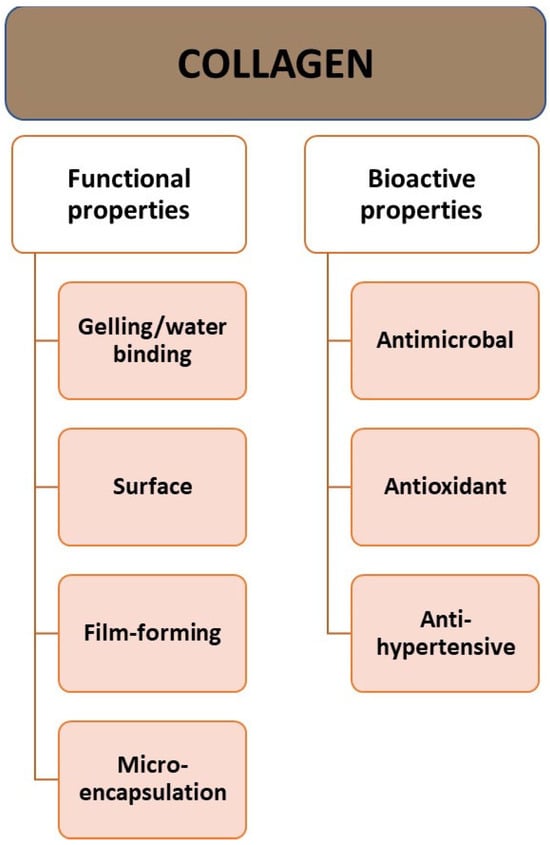
[5][8]. It has properties related to its behavior on the surface: emulsion formation, foam formation, stabilization, adhesion, cohesion, film-forming properties, and a protective function for the colloid (
Figure 1). Additionally, CLG is a good surfactant. It can penetrate lipid-free structures. It has good biocompatibility and low immunogenicity
[18]. The properties of CLG depend on the age of the body. With age, solubility tends to decrease due to the greater crosslinking of collagen in older animals
[19].
Figure 1. Properties of collagen.
Aging processes lead to crosslinking of the protein structure and affect its mechanical properties
[15][19][20]. Mature CLG has a highly crosslinked structure and is usually insoluble in water. Water-soluble and acid-soluble CLG can only be obtained from young tissues. Age-related differences in CLG solubility have been examined based on susceptibility to pepsin digestion. Research has established that the solubility of collagen in acetic acid decreases rapidly as it matures
[15]. Like other proteins, it is denatured under the influence of certain factors. If collagen is denatured by heat, the weak bonds (hydrogen, dipole–dipole, ionic bonds, or van der Waals forces) are broken, while the covalent bonds remain intact. The temperature at which thermal denaturation of CLG occurs (the so-called melting point) depends on the water content, pH of the environment, and the degree of crosslinking. The triple helix unwinds, and the chains separate. The denatured mass of tangled chains cools and absorbs all the water from the surroundings. This denatured collagen is called gelatin. Gelatin itself is a mixture of water-soluble proteins, mainly derived from collagen. It usually binds more water than CLG because it is partially degraded and more active groups are exposed to interactions with water through hydrogen bonds. Due to their structural role and compatibility with the body, collagen and gelatin are commonly used biomaterials in medicine, the pharmaceutical and cosmetics industries, with gelatin being a much cheaper material
[21][22]. Collagen is a highly crosslinked material, usually insoluble in water and oils, so in the case of cosmetic preparations, it is usually hydrolyzed into smaller peptides
[15][19].
4. The Role of Collagen in the Body
Collagen proteins perform various functions in the body (
Figure 2). The most important of them are maintaining structural integrity, and being responsible for the processes of cell adhesion, differentiation, growth, survival, and regeneration
[6][14][23]. CLG is found in various tissues of the body (
Table 1 and
Table 2), and its structure varies depending on its location and function.
Figure 2. Main role of collagen in the body.
The basic task of collagen is to connect cells, which is why it is a building block of most organs, especially skin, bones, teeth, cartilage, blood vessels, and the cornea of the eye
[11]. At the same time, it protects internal organs such as the kidneys, stomach, and liver, creating a flexible scaffolding around them. It also takes part in regenerative processes and ensures proper hydration of the skin thanks to its ability to bind water
[1][4][24]. In the immune system, it prevents the entry of pathogenic microorganisms and toxic substances
[14]. It ensures the continuity of cell renewal processes in the skin and maintains the appropriate level of hydration, which affects its elasticity, appearance, and condition
[20][25][26]. It accelerates wound healing, creates scars, and promotes the reconstruction of connective tissue
[27][28]. Collagen increases the absorption of minerals and increases bone density. It stimulates the activity of cartilage cells and supports protective processes within cartilage tissue, providing cartilage with the appropriate shape and resistance to stretching
[25]. It is responsible for the production of synovial fluid and the condition of cartilage. Moreover, it reduces the activity of enzymes responsible for causing inflammation and rheumatic pain
[14][29]. CLG fibers can also be carriers of some drugs, including interferon. It provides essential amino acids that nourish hair bulbs and ensure their proper growth
[21]. Its proteins also play an important role in the functioning of the circulatory system because they are a component of blood vessel walls. The functioning of the circulatory system directly depends on the composition and structure of the vessel walls
[3][18]. Collagen, as a fibrillar support protein, interacts with the structures of the extracellular matrix of vessel walls, which gives them appropriate elasticity and mechanical strength. CLG reorganization can impair the effective function of veins and arteries in transporting blood. It also significantly influences the development of circulatory system diseases
[1][4]. Collagens are the largest group of proteins that make up the blood vessels of the human body; types I and III constitute approximately 90% of all CLG in the vessels. They are the main supporting proteins of vessels—both healthy and damaged ones. However, among the non-fibrillar collagens that support the functioning of vessels, type VI CLG, which forms microfibers can be distinguished, and collagens that combine with fibrillar CLG—types XII and XIV
[7][9]. In the basement membrane of the inner layer of the vessel, there is also a network of CLG molecules of type IV, anchoring of type VII, and CLG of types XV, XVIII, and XIX, which act as connectors between cells and the basement membrane. Due to the high stiffness of their fibers, their main mechanical role is to limit the expansion of blood vessels under the influence of the pressure of blood flowing through them
[4][9][10]. The number of bonds between collagen molecules affects the quality of the mechanical functions of the vessel walls, as well as the organization of crosslinked collagen fibers within the tissue and their interaction with other matrix components
[8][9].
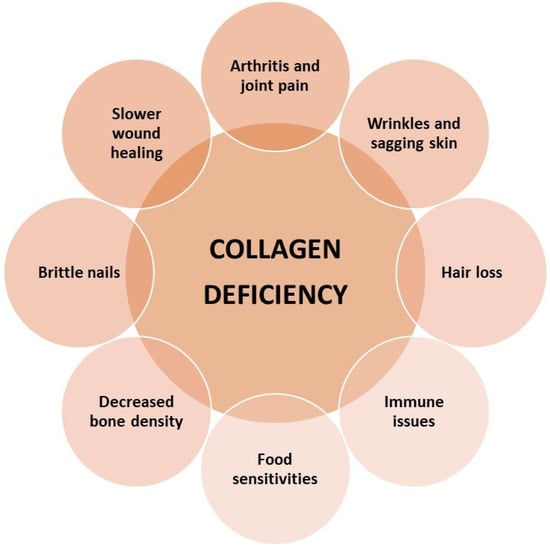
5. Effects of Collagen Deficiency in the Body
In a healthy, young body, collagen is regularly replaced—about 3 kg per year—and systematically rebuilt, and over time, the ability to regenerate CLG fibers disappears. The level in the body begins to decrease from the age of 25, its sharp decline occurs after the age of 50, and after the age of 60, this protein ceases to be synthesized by the body. However, the reduction in endogenous collagen biosynthesis is not limited to adulthood
[5][8][10]. A decrease (
Figure 3) in its concentration in the body can also be observed in young people, which is genetically and hormonally determined. This process is also favored by stress and other external factors, and the CLG biosynthesis process is also disrupted during menopause.
Figure 3. The main effects of collagen deficiency in the body.
Then, the level of estrogens decreases, which affects the synthesis of this protein and changes its properties. CLG fibers become less elastic and thinner
[1][3][10][23][30][31]. Changes in the structure can occur under the influence of various external factors, such as mechanical loads, hormonal changes, or diseases
[5][17][32][33]. Increased physical activity and practicing extreme sports contribute to the increased destruction of collagen fibers and disturbances in their synthesis. Accelerated fiber breakdown is also caused by excessive exposure to the sun, too high and low temperatures, and some compounds contained in cosmetics
[34][35]. These changes may result (
Figure 3) in problems with movement, spine and joint pain. With age, the concentration of vitamins A, C, and E, and minerals (copper), which are responsible for the natural renewal of collagen, decreases. Disturbances in the synthesis of CLG protein and its transformation in bone tissue may cause bone decalcification, brittleness, and susceptibility to fractures. The most noticeable symptom is the loss of skin firmness and general deterioration of its condition. Hair becomes brittle and falls out excessively, which may lead to premature baldness
[24][36][37][38].