
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Letizia Angiolella | -- | 1545 | 2024-03-12 17:33:54 | | | |
| 2 | Peter Tang | Meta information modification | 1545 | 2024-03-13 02:17:05 | | |
Video Upload Options
Plant essential oils (EOs) are produced predominantly using steam distillation, but can also be generated using fermentation, crushing, extraction, hydrolysis, and airing. EOs are used extensively in cosmetics in many different aspects as perfumes, in antiseptic applications, and in domestic cleaning products. The essential oils of Mentha (the Lamiaceae family) have been extensively studied for their biological actions.
1. Introduction
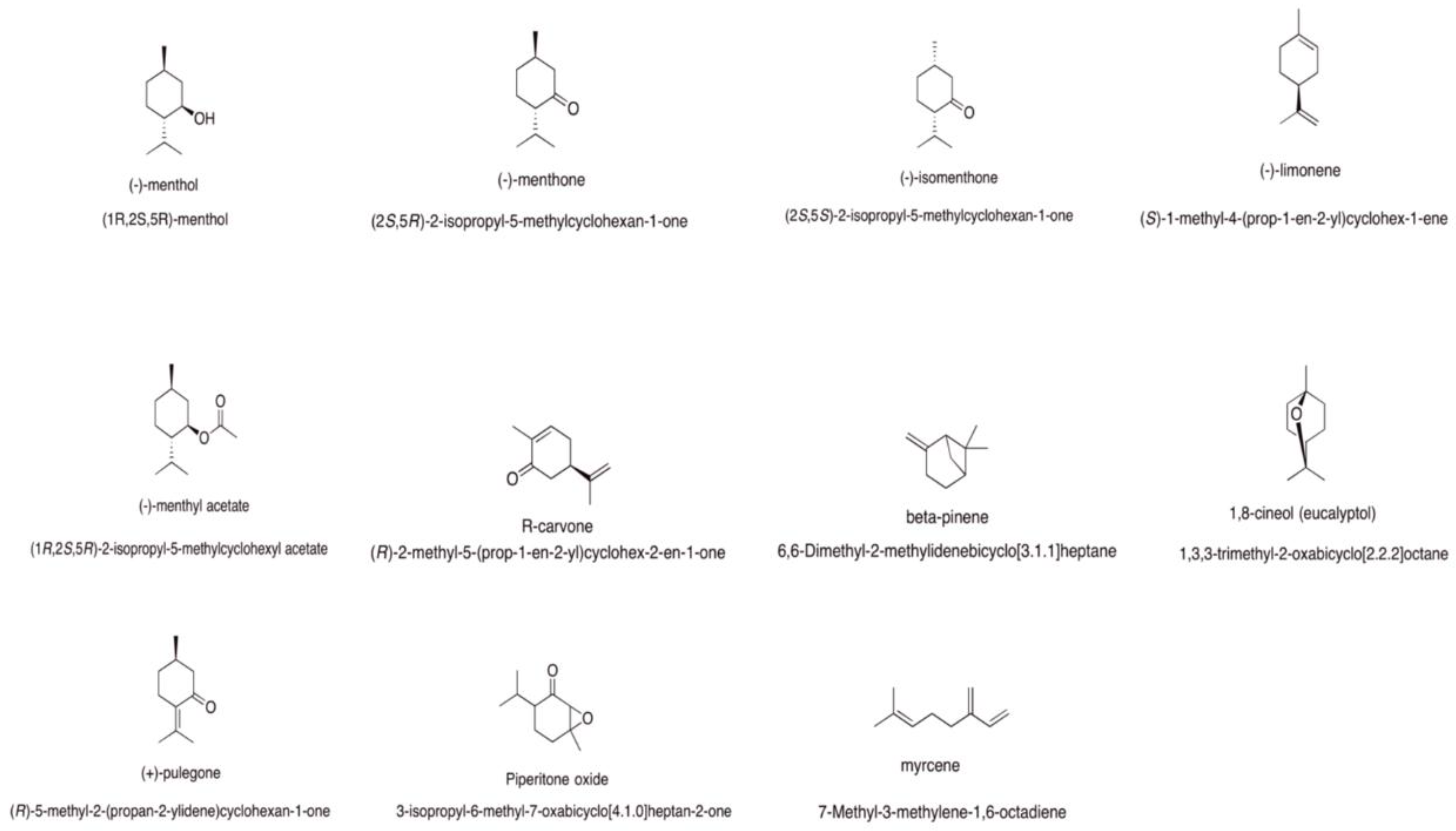
Mentha spp. Essential Oils
2. Antioxidant Properties
|
EOs |
DPPH Activity |
ABTS Activity |
References |
|---|---|---|---|
|
M. piperita |
860 μg/mL |
- |
[28] |
|
57.9 ± 1.34% |
80.6 ± 1.45% |
[29] |
|
|
600 μg/mL |
- |
[30] |
|
|
540 μg/mL |
- |
[31] |
|
|
11.289 ± 0.514 μg/g |
0.154 ± 0.006 mmol/g |
[32] |
|
|
M. pulegium |
14736 ± 156 μg/mL |
- |
[36] |
|
30.38 ± 0.8% |
- |
[37] |
|
|
69.60 μg/mL |
- |
[38] |
|
|
321.41 ± 2.53 μg/mL |
- |
[39] |
|
|
M. spicata |
3 μg/mL |
- |
[40] |
|
3450 ± 172.5 μg/mL |
40.2 ± 0.2 μg/mL |
[41] |
|
|
M. longifolia |
57.4 μg/mL |
- |
[42] |
|
M. suaveolens |
31 μg/mL |
- |
[43] |
|
52.4 ± 2.5% |
- |
[44] |
References
- Prabuseenivasan, S.; Jayakumar, M.; Ignacimuthu, S. In vitro antibacterial activity of some plant essential oils. BMC Complement. Altern. Med. 2006, 6, 39–50.
- Aburjai, T.; Natsheh, F.M. Plants used in cosmetics. Phytother. Res. 2003, 17, 987–1000.
- Wallace, R.J. Antimicrobial properties of plant secondary metabolites. Proc. Nutr. Soc. 2004, 63, 621–629.
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475.
- Nakatsu, T.; Lupo, A.T.; Chinn, J.W.; Kang, R.K.L. Studies in Natural Products Chemistry; Elsevier: New York, NY, USA, 2000; Volume 21, pp. 571–631.
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253.
- Edris, A. Pharmaceutical and therapeutic potentials of essential oils and their individuals’ volatile constituents. A review. Phytother. Res. 2007, 21, 308–323.
- Garzoli, S.; Pirolli, A.; Vavala, E.; Di Sotto, A.; Sartorelli, G.; Bozovic, M.; Angiolella, L.; Mazzanti, G.; Pepi, F.; Ragno, R. Multidisciplinary approach to determine the optimal time and period to extract the essential oil from Mentha suaveolens ehrh. Molecules 2015, 20, 9640–9655.
- Nieto, G.; Ros, G.; Castillo, J. Antioxidant and Antimicrobial Properties of Rosemary (Rosmarinus officinalis, L.): A Review. Medicines 2018, 5, 98.
- Tucker, A.O.; Naczi, R.F.C. Mentha: An overview of its classification and relationships. In Mint: The Genus Mentha: Medicinal and Aromatic Plants-Industrial Profiles; Lawrence, B.M., Ed.; CRC Press: Boca Raton, FL, USA, 2006; p. 3.
- Sakkas, H.; Papadopoulou, C. Antimicrobial Activity of Basil, Oregano, and Thyme Essential Oils. J. Microbiol. Biotechnol. 2017, 27, 429–438.
- Peixoto, I.T.A.; Furlanetti, V.F.; Anibal, P.C.; Duarte, M.C.T.; Höfling, J.F. Potential pharmacological and toxicological basis of the essential oil from Mentha spp. Rev. Ciênc. Farm. Básica Apl. 2009, 30, 235–239.
- Chávez-González, M.L.; Rodríguez-Herrera, R.; Aguilar, C.N. Essential oils: A natural alternative to combat antibiotics resistance antibiotic resistance in mechanisms and new antimicrobial approaches. In Antibiotic Resistance; Kateryna, K., Mahendra, R., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 227–237.
- Sharma, V.; Hussain, S.; Gupta, M.; Saxena, A. In vitro anticancer activity of extracts of Mentha spp. against human cancer cells. Indian J. Biochem. Biophys. 2014, 51, 416–419.
- Amabeoku, G.J.; Erasmus, S.J.; Ojewole, J.A.; Mukinda, J.T. Antipyretic and antinociceptive properties of Mentha longifolia Huds. (Lamiaceae) leaf aqueous extract in rats and mice. Meth. Find. Exp. Clin. Pharmacol. 2009, 31, 645–649.
- Yadegarinia, D.; Gachkar, L.; Rezaei, M.B.; Taghizadeh, M.; Astaneh, S.A.; Rasooli, I. Biochemical activities of Iranian Mentha piperita L. and Myrtus communis L. essential oils. Phytochemistry 2006, 67, 1249–1255.
- Gonzales-Burgos, E.; Gomez-Serranillos, M.P. Terpene compounds in nature. A review of their potential antioxidant activity. Curr. Med. Chem. 2012, 19, 5319–5341.
- Kaufmann, H.; Dorhoi, A. Molecular determinants in phagocyte-bacteria interactions. Immunity 2016, 44, 476–491.
- Nathan, C.; Cunningham-Bussel, A. Beyond oxidative stress: An immunologist’s guide to reactive oxygen species. Nat. Rev. Immunol. 2013, 13, 349–361.
- Dawidowicz, A.L.; Olszowy, M. Does antioxidant properties of the main component of essential oil reflect its antioxidant properties? The comparison of antioxidant properties of essential oils and their main components. Nat. Prod. Res. 2014, 28, 1952–1963.
- Pisoschi, A.M.; Negulescu, G.P. Methods for total antioxidant activity determination: A review. Biochem. Anal. Biochem. 2011, 1.
- Olszowy, M.; Dawidowicz, A.L. Essential oils as antioxidants: Their evaluation by DPPH, ABTS, FRAP, CUPRAC, and β-carotene bleaching methods. Monatsh. Chem. 2016, 147, 2083–2091.
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675.
- Marc, F.; Davin, A.; Deglène-Benbrahim, L.; Ferrand, C.; Baccaunaud, M.; Fritsch, P. Studies of several analytical methods for antioxidant potential evaluation in food. Med. Sci. 2004, 20, 458–463.
- Pellegrini, N.; Serafini, M.; Colombi, B.; Del Rio, D.; Salvatore, S.; Bianchi, M.; Brighenti, F. Total antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays. J. Nutr. 2003, 133, 2812–2819.
- Su, L.; Yin, J.J.; Charles, D.; Zhou, K.; Moore, J.; Yu, L.L. Total phenolic contents, chelating capacities, and radical-scavenging properties of black peppercorn, nutmeg, rosehip, cinnamon and oregano leaf. Food Chem. 2007, 100, 990–997.
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant activity of essential oils. J. Agric. Food Chem. 2013, 61, 10835–10847.
- Schmidt, E.; Bail, S.; Buchbauer, G.; Stoilova, I.; Atanasova, T.; Stoyanova, A.; Krastanov, A.; Jirovetz, L. Chemical composition, olfactory evaluation and antioxidant effects of essential oil from Mentha x piperita. Nat. Prod. Commun. 2009, 4, 1107–1112.
- Yang, S.A.; Jeon, S.K.; Lee, E.J.; Shim, C.H.; Lee, I.S. Comparative study of the chemical composition and antioxidant activity of six essential oils and their components. Nat. Prod. Res. 2010, 24, 140–151.
- Sun, Z.; Wang, H.; Wang, J.; Zhou, L.; Yang, P. Chemical composition and anti-inflammatory, cytotoxic and antioxidant activities of essential oil from leaves of Mentha piperita grown in China. PLoS ONE 2014, 9, e114767.
- da Silva Ramos, R.; Lobato Rodrigues, A.B.; Ferreira Farias, A.L.; Simões, R.C.; Pinheiro, M.T.; dos Anios Ferreira, R.M.; Costa Barbosa, L.M.; Picanço Souto, R.N.; Fernandes, J.B.; da Silvas Santos, L.; et al. Chemical composition and in vitro antioxidant, cytotoxic, antimicrobial, and larvicidal activities of the essential oil of Mentha piperita L. (Lamiaceae). Sci. World J. 2017, 2017.
- Pellegrini, M.; Ricci, A.; Serio, A.; Chaves-López, C.; Mazzarrino, G.; D’Amato, S.; Lo Sterzo, C.; Paparella, A. Characterization of Essential Oils Obtained from Abruzzo Autochthonous Plants: Antioxidant and Antimicrobial Activities Assessment for Food Application. Foods 2018, 7, 19.
- Dorman, H.; Koşar, M.; Başer, K.; Hiltunen, R. Phenolic profile and antioxidant evaluation of Mentha x piperita L. (peppermint) extracts. Nat. Prod. Commun. 2009, 4, 535–542.
- Sroka, Z.; Fecka, I.; Cisowski, W. Antiradical and Anti-H2O2 properties of polyphenolic compounds from anaqueous peppermint extract. Z. Naturforsch. C 2005, 60, 826–832.
- Ferreira, P.; Cardoso, T.; Ferreira, F.; Fernandes-Ferreira, M.; Piper, P.; Sousa, M.J. Mentha piperita essential oil induces apoptosis in yeast associated with both cytosolic and mitochondrial ROS-mediated damage. FEMS Yeast Res. 2014, 14, 1006–1014.
- Kamkar, A.; Javan, A.J.; Asadi, F.; Kamalinejad, M. The antioxidative effect of Iranian Mentha pulegium extracts and essential oil in sunflower oil. Food Chem. Toxicol. 2010, 48, 1796–1800.
- Cherrat, L.; Espina, L.; Bakkali, M.; Pagan, R.; Laglaoui, A. Chemical composition, antioxidant and antimicrobial propertie of Mentha pulegium, Lavandula stoechas and Satureja calamintha Scheele essential oils and an evaluation of their bactericidal effect in combined processes. Innov. Food Sci. Emerg. Technol. 2014, 22, 221–229.
- Abdelli, M.; Moghrani, H.; Aboun, A.; Maachi, R. Algerian Mentha pulegium L. leaves essential oil: Chemical composition, antimicrobial, insecticidal and antioxidant activities. Ind. Crops Prod. 2016, 94, 197–205.
- Bouyahya, A.; Et-Touys, A.; Bakri, Y.; Talbaui, A.; Fellah, H.; Abrini, J.; Dakka, N. Chemical composition of Mentha pulegium and Rosmarinus officinalis essential oils and their antileishmanial, antibacterial and antioxidant activities. Microb. Pathog. 2017, 111, 41–49.
- Snoussi, M.; Noumi, E.; Trabelsi, N.; Flamini, G.; Papetti, A.; De Feo, V. Mentha spicata Essential Oil: Chemical Composition, Antioxidant and Antibacterial Activities against Planktonic and Biofilm Cultures of Vibrio spp. Strains. Molecules 2015, 20, 14402–14424.
- Bardaweel, S.K.; Bakchiche, B.; AL-Salamat, H.A.; Rezzoug, M.; Gherib, A.; Flamini, G. Chemical composition, antioxidant, antimicrobial and Antiproliferative activities of essential oil of Mentha spicata L. (Lamiaceae) from Algerian Saharan atlas. BMC Complement. Altern. Med. 2018, 18.
- Eissa, T.F.; González-Burgos, E.; Carretero, M.E.; Gómez-Serranillos, M.P. Compositional analysis and in vitro protective activity against oxidative stress of essential oils from egyptian plants used in traditional medicine. Nat. Prod. Commun. 2014, 9, 1377–1382.
- El-Askary, H.I.; El-Kashoury, E.A.; Kandil, Z.A.; Salem, M.A.; Ezzat, S.M. Biological activity and standardization of the ethanolic extract of the aerial parts of Mentha suaveolens Ehrh. World J. Pharm. Pharm. Sci. 2014, 3, 223–241.
- Ferreira, A.; Proenc, C.; Serralheiro, M.L.M.; Ara´ujo, M.E.M. The in vitro screening for acetylcholinesterase inhibition and antioxidant activity of medicinal plants from Portugal. J. Ethnopharmacol. 2006, 108, 31–37.
- Sitzmann, J.; Habegger, R.; Schnitzler, W.H.; Grassmann, J. Comparative analysis of antioxidant activities of fourteen Mentha essential oils and their components. Chem. Biodivers. 2014, 11, 1978–1989.
- Spagnoletti, A.; Guerrini, A.; Tacchini, M.; Vinciguerra, V.; Leone, C.; Maresca, I.; Simonetti, G.; Sacchetti, G.; Angiolella, L. Chemical Composition and Bio-efficacy of Essential Oils from Italian Aromatic Plants: M. suaveolens, C. capitatus, O. hirtum and R. officinalis. Nat. Prod. Commun. 2016, 11, 1517–1520.




