Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Karina Džermeikaitė | -- | 4924 | 2024-03-12 12:20:06 | | | |
| 2 | Rita Xu | Meta information modification | 4924 | 2024-03-13 02:30:49 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Džermeikaitė, K.; Krištolaitytė, J.; Antanaitis, R. Dairy Cow Health and Methane Emissions. Encyclopedia. Available online: https://encyclopedia.pub/entry/56163 (accessed on 01 March 2026).
Džermeikaitė K, Krištolaitytė J, Antanaitis R. Dairy Cow Health and Methane Emissions. Encyclopedia. Available at: https://encyclopedia.pub/entry/56163. Accessed March 01, 2026.
Džermeikaitė, Karina, Justina Krištolaitytė, Ramūnas Antanaitis. "Dairy Cow Health and Methane Emissions" Encyclopedia, https://encyclopedia.pub/entry/56163 (accessed March 01, 2026).
Džermeikaitė, K., Krištolaitytė, J., & Antanaitis, R. (2024, March 12). Dairy Cow Health and Methane Emissions. In Encyclopedia. https://encyclopedia.pub/entry/56163
Džermeikaitė, Karina, et al. "Dairy Cow Health and Methane Emissions." Encyclopedia. Web. 12 March, 2024.
Copy Citation
The dairy industry is facing criticism for its role in exacerbating global GHG emissions, as climate change becomes an increasingly pressing issue. These emissions mostly originate from methane (CH4), nitrous oxide (N2O), and carbon dioxide (CO2). An optimal strategy involves the creation of an economical monitoring device to evaluate methane emissions from dairy animals.
global warming
greenhouse gas
methane emissions
1. Introduction
Climate change is the gradual alteration of temperature and weather patterns caused by the accumulation of heat-trapping gases in the atmosphere over an extended period. The Bulletin of the American Meteorological Society’s global climate research reveals that the seven hottest years since the mid to late 1800s took place between 2015 and 2021 [1][2]. In the last ten years, there has been a growing level of consciousness regarding climate change resulting from the rise in GHG emissions [3][4]. The dairy industry is facing growing criticism for its contribution to global GHG emissions [5]. As a result, there has been an extraordinary sustainable intensification in advanced dairy farming worldwide in order to produce milk more efficiently [6]. Globally, ambitious goals have been established to decrease GHG emissions. At the recent UNFCCC 26th Conference in Glasgow, over 120 countries made a solemn commitment to achieve net zero emissions by 2050–2070 [7]. The primary sources of GHG emissions from ruminants are methane (CH4), nitrous oxide (N2O), and carbon dioxide (CO2) [8]. Enteric fermentation, feed production, manure production, and management operations are the main sources of these emissions [9]. Dairy production contributes to global warming because of the emission of methane, a highly potent GHG [10]. Methane is created in the rumen during the regular fermentation process by methanogenic archaea using either CO2 and hydrogen (H2), methylamines or methanol, or acetate and H2 to produce CH4 [11]. CH4 is a gas with a greenhouse potential of 25 times that of CO2 [12].
The scientific community regards global warming as a substantial issue [13]. It is widely accepted that human activities, particularly the burning of fossil fuels, are the main cause of the increase in greenhouse gases in the atmosphere. This has resulted in a rise in global temperatures, leading to numerous environmental consequences such as melting ice caps, rising sea levels, and extreme weather events. Numerous studies and data support the scientific consensus that there is an urgent need to address global warming, so it is crucial for governments, organisations, and individuals to take immediate action to mitigate its effects [14].
Ruminant animals, such as beef and dairy cattle, are significant methane producers due to enteric fermentation that takes place in their rumen during the digesting process [11][15]. Research reveals that cattle, which comprise both meat and milk production, produce around 3.8 gigatons of carbon dioxide equivalent each year, accounting for 62 percent of the overall emissions from livestock. Pigs are responsible for 14 percent of the emissions, chickens for 9 percent, buffaloes for 8 percent, and small ruminants for 7 percent (Figure 1) [16].
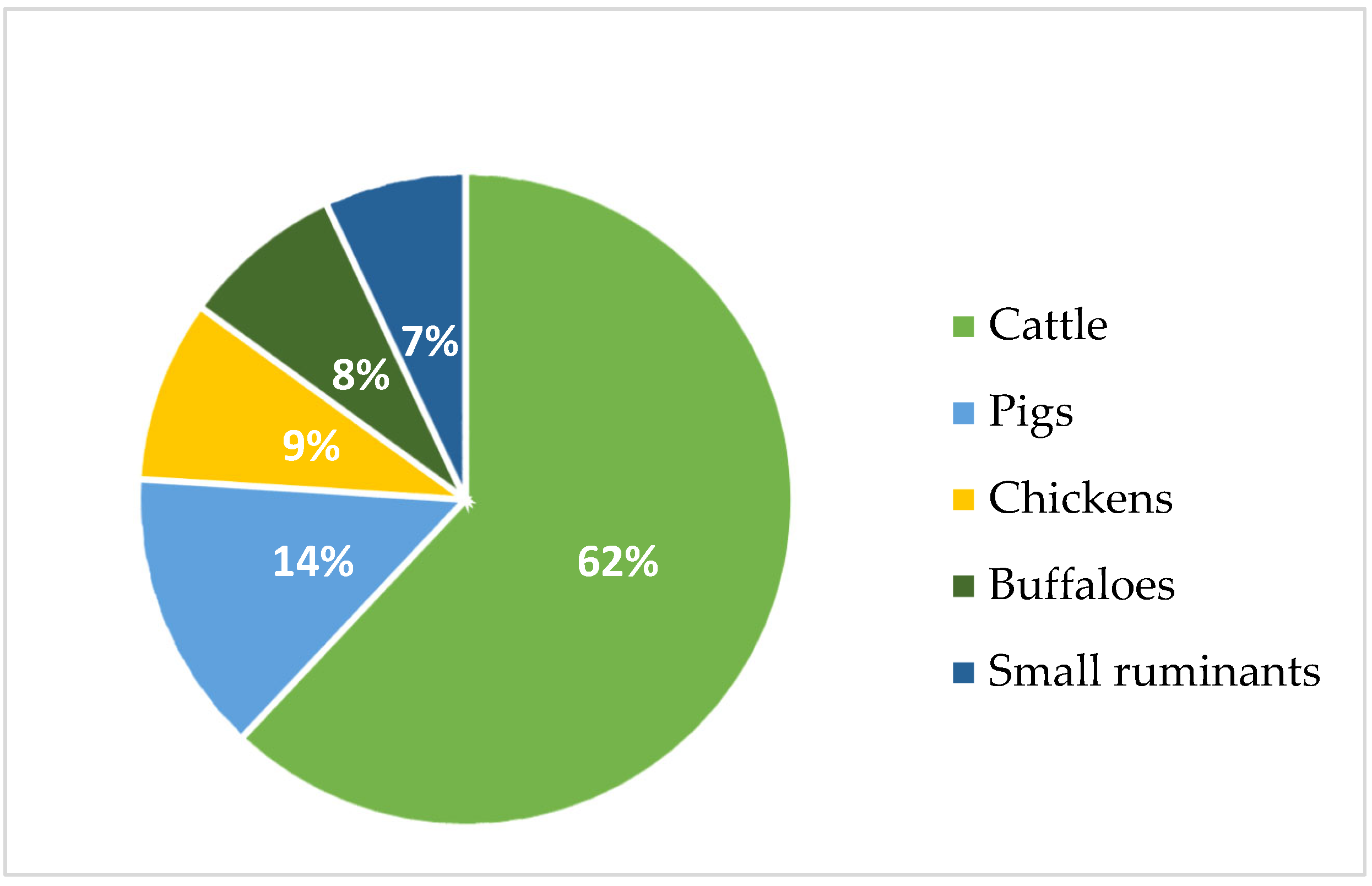
Figure 1. Animal category proportions on enteric methane emissions (FAO 2023).
Presently, the annual global methane emissions amount to around 500–600 tera-gram. Methanogenesis from diverse ecosystems accounts for over 70% of these emissions [17]. This is the last stage of the process of breaking down organic matter without the presence of oxygen, after all the inorganic substances that accept electrons, such as nitrate, ferric iron, or sulphate, have been used up [18]. Acetolactic methanogens decompose acetate into methane and carbon dioxide. They inhabit environments where hydrogenotrophic methanogens decrease hydrogen gas (H2) levels to a point where optimal circumstances for abundant acetate production are created. Acetolactic methanogens are primary methane producers in anaerobic digesters, rice fields, and wetlands, responsible for two-thirds of biologically produced methane emissions. They decompose acetate into methane and carbon dioxide, thriving in environments where hydrogenotrophic methanogens decrease H2 levels. Hydrogenotrophic methanogens utilise H2, formate, or a small number of simple alcohols as energy sources. They then convert CO2 into CH4 through reduction and are the main producers of methane in deep marine sediments, termite hindguts, and the gastrointestinal tracts of humans and animals [17][18]. Methanogens are the primary producers of methane in deep sea sediments, termite hindguts, and the gastrointestinal systems of humans and animals. Collectively, these sources account for one-third of the methane emissions produced by living organisms (Figure 2) [17].
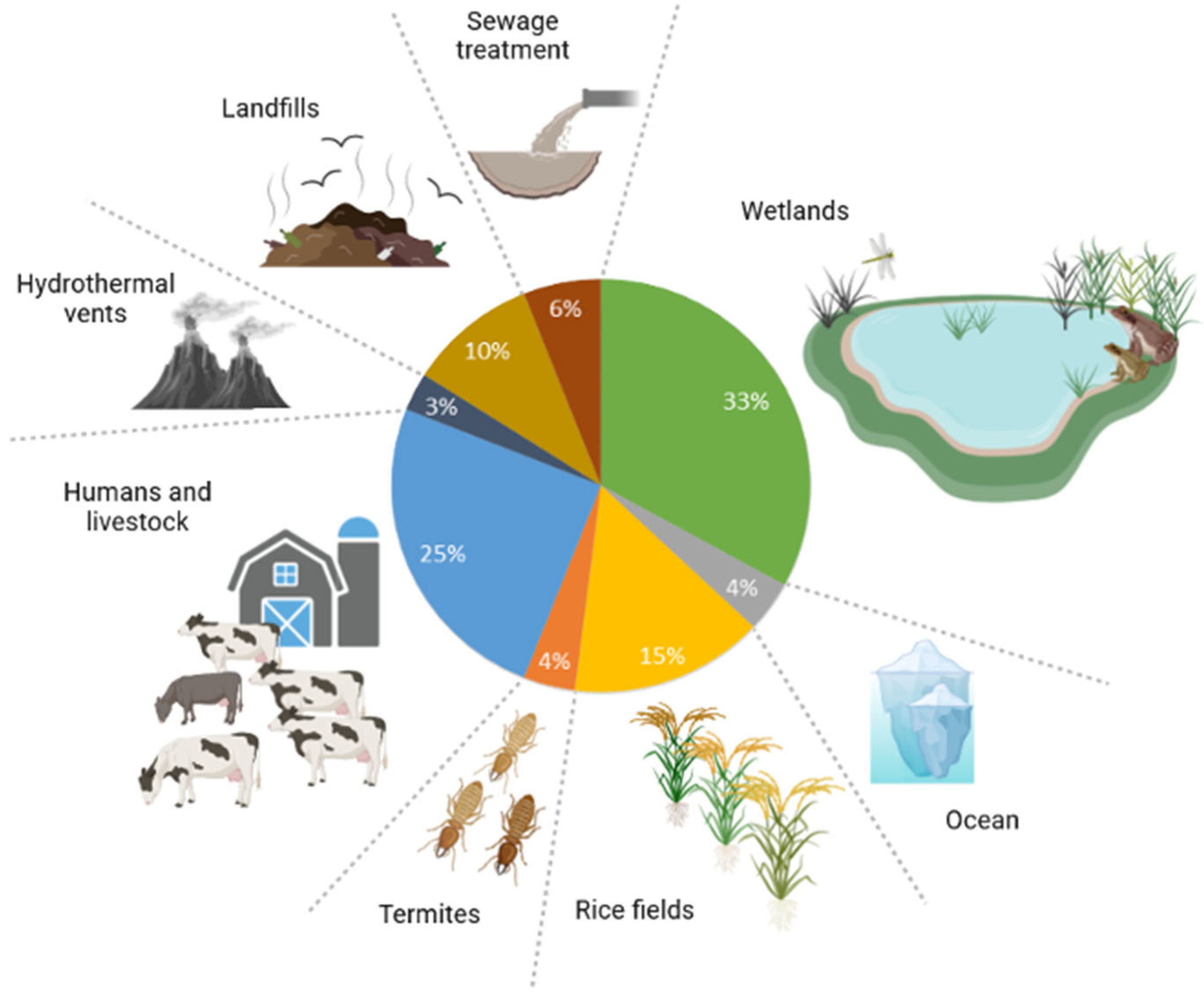
Figure 2. Biological entities that release methane into the atmosphere.
Approximately 90% of the CH4 that dairy cows produced comes from their breath and ejected rumen gases; CH4 is also a loss in energy and the result of fermentation by methanogens like archaea [12]. Therefore, a desirable approach would be to develop a straightforward, resilient, and cost-effective monitoring technology that can be widely implemented to assess CH4 emissions from dairy animals [19].
Livestock production systems face challenges posed by increasing food demand and environmental issues. When animal productivity is improved through nutrition, feeding management, reproduction, or genetics, CH4 production per unit of meat or milk is reduced. A 20% reduction in total CH4 production could allow growing cattle to gain an additional 75 g/d of body weight and 1 L/d more milk yield (MY) from dairy cows [20]. The rise in animal productivity led to a decrease in enteric CH4 emissions per unit of animal production (milk and average daily gain) and an enhancement in feed efficiency [21]. According to research, there is a heritability of enteric CH4 production and a genetic correlation with the intake of milk lactose, protein, fat, and DM [22]. Milk production and lifetime performance play a significant role in the breeding of high-production dairy cattle like Holstein cows. Enteric methane production is strongly associated with the genetics, health, and productivity of dairy cows, as well as with feeding and nutrition management [23]. On a global scale, it is estimated that livestock diseases result in a 25% decrease in productivity [24]. Infectious disorders can worsen these contributions by increasing methane emissions linked to animal production (Figure 3). The rise in the prevalence of numerous contagious illnesses has led to the development of a dangerous cycle involving climate, livestock, and disease, which poses a significant and imminent danger [7]. Improving the health and reproductive state of a herd could help by reducing the number of animals that have to be culled against their will and increasing fertility traits like calving intervals. This would cut down on unnecessary costs and CH4 production [25]. Therefore, addressing livestock health issues may boost output while also lowering the intensity of GHG emissions and enhancing animal welfare [24].
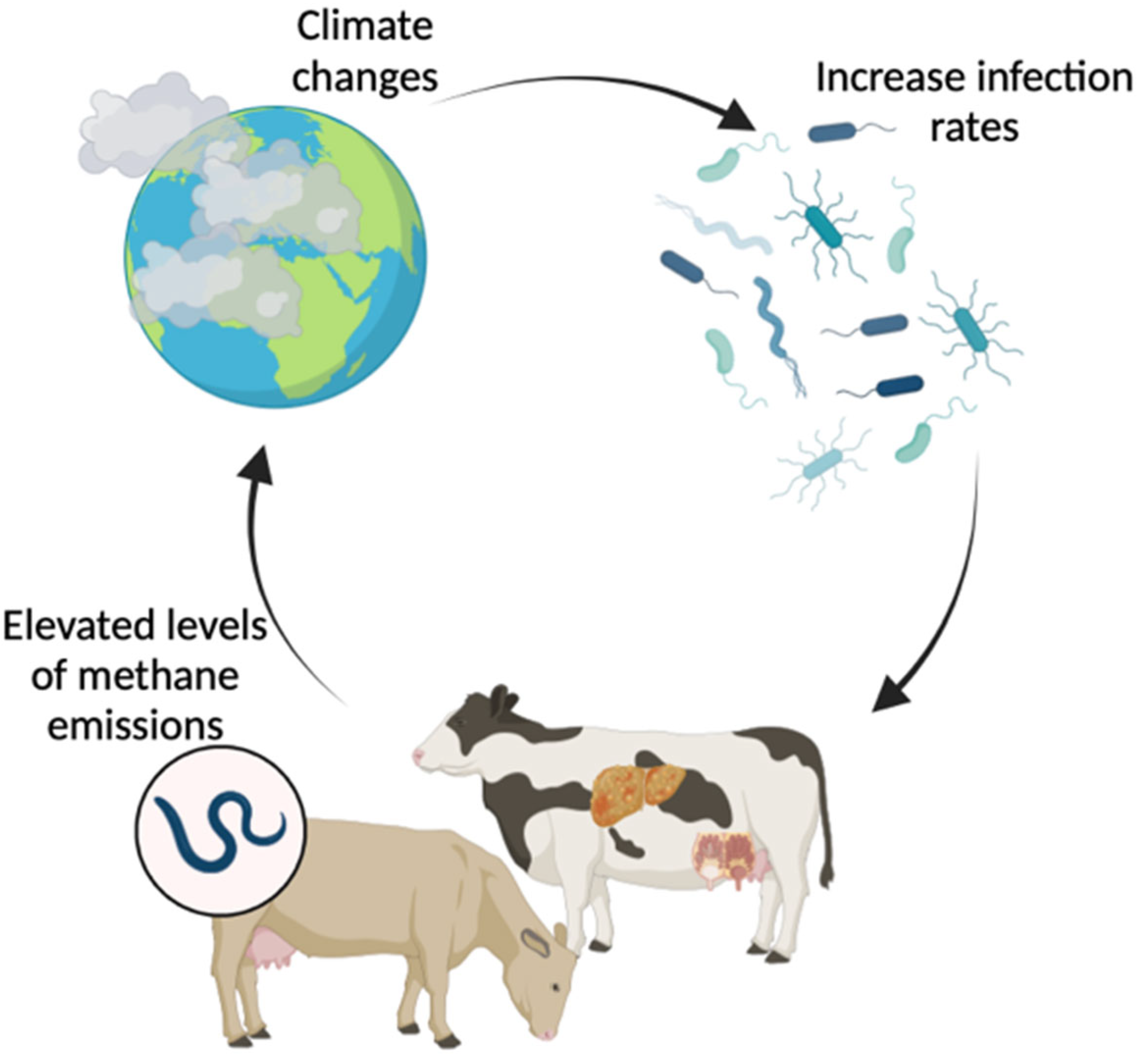
Figure 3. The relationship between climate change and cattle infections.
A worldwide goal is to lessen the damage that animal production does to the environment. Innovative ideas and tools can help the change to a more environmentally friendly livestock system [26]. Additionally, addressing livestock health issues can also have positive effects on human health. By reducing the prevalence of contagious illnesses in livestock, the risk in zoonotic diseases spreading to humans can be minimised. This would not only protect human populations from potential outbreaks but also reduce the burden on healthcare systems and resources. Overall, prioritising livestock health can have far-reaching benefits for both animal welfare and public health.
The advancements in animal health present the possibility of a future where the risk of animal diseases is significantly diminished. This is due to enhanced immunity, better prevention methods, earlier and more precise detection, and novel therapies. Maintaining the well-being of animals not only mitigates pollutants stemming from livestock production, but also diminishes the likelihood of spreading infections to people [27].
2. Dairy Cow Health and Methane Emissions
Enteric fermentation, the process of food digestion in ruminant animals like cattle and sheep, results in the production and release of methane [15]. CH4 is primarily expelled or eructated via the nasal and oral cavities as a by-product of anaerobic fermentation in the rumen, a process in which ruminal microorganisms convert feed into nutrients that are readily absorbable by the host animal, with 97% being expelled by the mouth and 3% through the rectum [28][29][30]. Methanogens are primarily responsible for producing CH4 during the anaerobic degradation of plant biomass in the rumen (Figure 4) [29]. The molecular process of methane production in the gastrointestinal tract is extensively understood. Elevated fibre intake leads to an increase in rumen pH and a reduction in the rate at which digesta moves through the gastrointestinal tract. Consequently, a change in ruminal fermentation towards acetate leads to an increase in the amount of dissolved hydrogen equivalents available to produce CH4 by rumen methanogens [31].
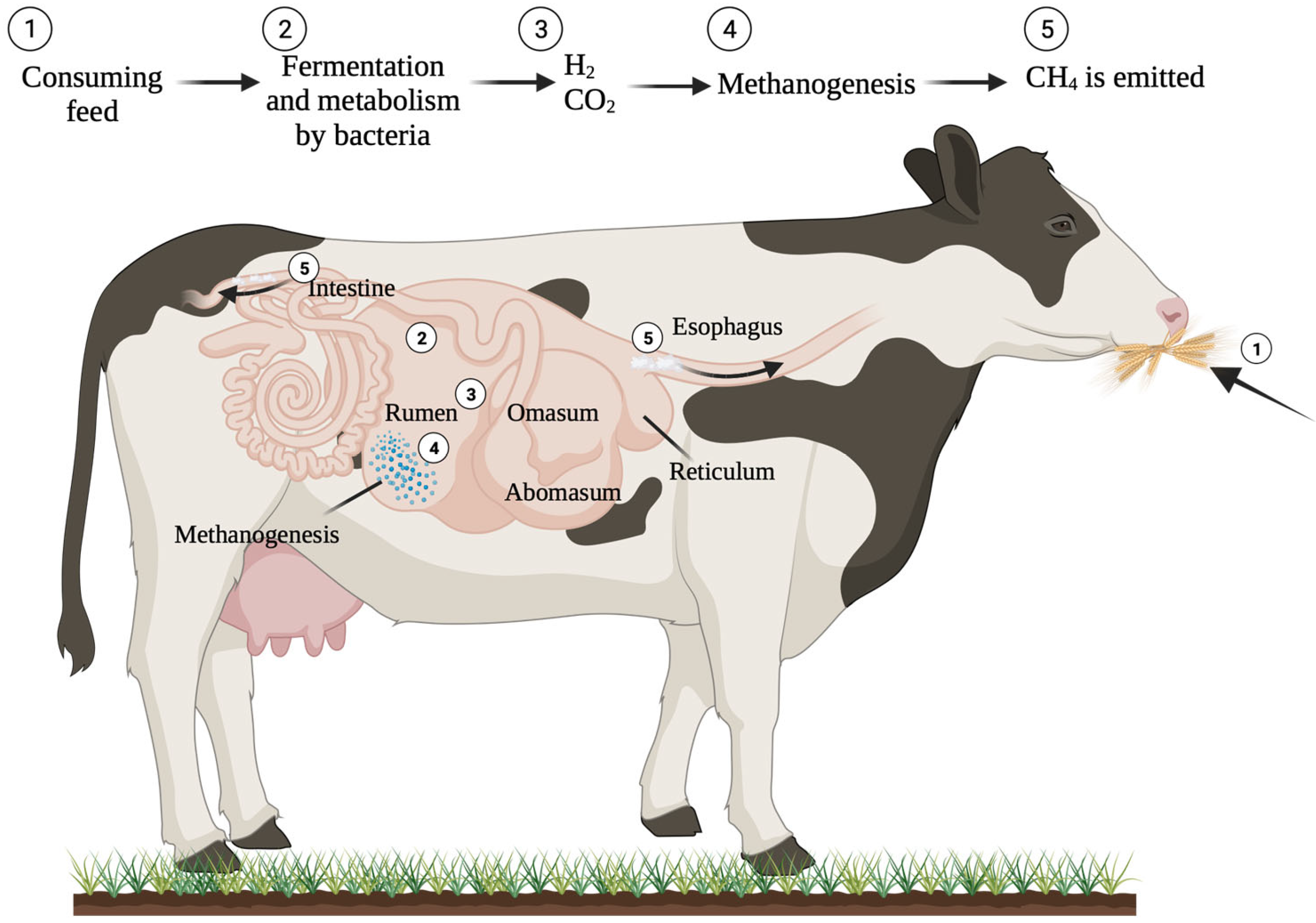
Figure 4. Mechanisms involved in the generation of methane in ruminant.
Microbial fermentation is a complex series of steps that begins with the breakdown of dietary polysaccharides into easily digestible sugars through the action of microbial enzymes. Additionally, after a series of multi-step processes, these sugars that can undergo hydrolysis go through fermentation to produce volatile fatty acids (VFAs), frequently acetate, propionate, and butyrate, without the presence of oxygen [32]. These VFAs serve as an energy source [33]. Throughout the process, elemental hydrogen (H), which acts as a reducing agent and is commonly referred to as metabolic hydrogen, is generated as a secondary product. Hydrogen-producing bacterial species convert the metabolic hydrogen into molecular H2, which methanogens then convert into CH4 [32].
Animal health is fundamental to the establishment of a sustainable animal agriculture industry [34]. The major global health challenge lies in establishing the correlations between climate change and infectious disease [35]. Enhancing animal well-being and minimising animal sickness and death to improve the effectiveness of the animal production system present possibilities for decreasing both CH4 and N2O emissions resulting from digestive fermentation and animal manure [24][36].
The primary objective of veterinary medicine in livestock production systems that depend on small herds is the complete elimination of clinical infectious illnesses, with a particular emphasis on treating each animal individually. Nevertheless, when the number of animals in a herd and their output rise, the attention turns to proactive veterinary treatment and places more importance on addressing subclinical diseases and implementing comprehensive health management programmes that aim to enhance productivity. Irrespective of the stage of development of a livestock production system, a decrease in the number of deaths and illnesses results in a higher amount of products that can be sold, which in turn, reduces GHG emissions per unit of product [36]. Animals typically respond to diseases by initially decreasing their consumption of food, which subsequently leads to decreased productivity and increased GHG emissions per unit of output [37]. The primary factors influencing the fluctuation in methane emissions from ruminant animals, including cattle, buffalo, sheep, goats, and camels, are the intake and quality of their feed. Increased consumption of feed and/or decreased quality of feed results in higher methane emission. Typically, larger animals exhibit higher feed intake demands [38].
Furthermore, the process of calving induces significant stress in cows, with around 65% of all disease occurrences in the dairy herd taking place during this period [39]. Additional research indicates that around 75% of infections in dairy cows tend to arise within the initial month following calving. The periparturient phase, also known as the transition period, typically spans from 3 weeks before giving birth to 3 weeks after giving birth [40]. During this stage, cows experience a condition known as negative energy balance. This occurs when the demand for nutrients for milk production increases rapidly and surpasses the supply of nutrients obtained from food consumption [41].
The epidemiology of infectious animal diseases can be significantly influenced by climate change, which is directly connected to production environments and their subsequent consequences [42]. The increase in GHG emissions per ton of milk in ill cows compared to healthy cows might reach up to 25%, depending on the specific health disorder. The estimated increases in GHG emissions per unit of milk and per case are 7%, 8%, and 16% for mastitis, lameness, and infertility, respectively [8].
Only a limited number of studies have investigated the relationship between dairy cow health and its impact on GHG emissions [37]. Ensuring the future viability of the dairy business necessitates the careful management of dairy cow health in conjunction with environmental sustainability. This requires adopting a comprehensive approach that considers both the well-being of the animals and the ecological consequences. The economic viability of livestock is significantly affected by the repercussions of livestock disease. However, there is a lack of comprehensive and up-to-date literature on the economic and environmental consequences of cattle diseases, which is a matter of worry for producers [43].
2.1. Metabolic Diseases
During the transition from late gestation to early lactation, metabolic and hormonal profile changes occur. Homeorhetic modifications take place during this phase to supply nutrients to the neonate and facilitate lactogenesis. The alterations linked to the heightened nutritional requirements and reduced food consumption that transpire throughout this stage contribute to the formation of an adverse energy balance (NEB). This metabolic and nutritional imbalance has the potential to contribute to the development of an immunosuppressive state [44]. An energy deficit causes a decrease in blood glucose levels and prompts the body to use its stored reserves for more energy. This leads to higher levels of non-esterified fatty acids (NEFA) and β-hydroxybutyric acid (BHBA) in the blood [41]. These parameters are generated as metabolites during the lipid oxidation of fatty acids in the liver. The liver in ruminants maintains energy balance by converting propionic acid, which is taken from the rumen, into glucose. Additionally, it controls fat metabolism by both oxidising and synthesising fat. The liver plays a crucial role in regulating the body’s metabolism and maintaining energy equilibrium. Moreover, the liver’s metabolic and pathological states have a significant impact on animal productivity. Due to its strong association with feed efficiency and energy level, liver metabolism is thought to play a direct and indirect role in intestinal methane generation [45]. Understanding and optimising liver metabolism in ruminants is, therefore, important for reducing methane emissions and improving overall animal productivity.
Additionally, calcium and phosphorus are released to produce milk, resulting in a reduction in their levels in the bloodstream. These metabolic alterations can result in hypocalcaemia, ketosis, displaced abomasum, and hepatic lipidosis [41]. These illnesses are associated with reduced milk production, decreased conception rates, longer intervals between calving, lameness, and diminished well-being and productive lifetime in the herd [46].
Ketosis is a pronounced metabolic disorder that manifests in dairy cows during the initial stages of lactation. It is defined by increased concentrations of ketone bodies in the bloodstream, which frequently result in decreased efficiency, reproductive problems, and occasionally, mortality or culling [47]. For instance, a study demonstrated that by decreasing subclinical ketosis (SCK) and associated illnesses in dairy cows, it is possible to lower GHG emissions per unit of milk produced. The mean rise in GHG emissions per unit of SCK was 20.9 kg of carbon dioxide equivalent per metric ton of fat-and-protein-corrected milk (CO2e/t FPCM), representing a 2.3% increase [48]. Hence, the occurrence of ketosis in a dairy cow herd may contribute to the intensity of GHG emissions.
Sub-acute ruminal acidosis (SARA) is a widely acknowledged digestive condition that affects high-producing dairy cows. It has detrimental effects on both the health of the animals and the profitability of the herd, especially in well-managed dairy farms [49]. SARA in cattle can cause physiological disruptions resulting from decreased dry matter intake (DMI), which may lead to acute ruminal acidosis. Diagnosing SARA at an individual level is challenging due to the vague and frequently observable clinical signs mostly seen at the herd level. Nevertheless, it can be recognised by a decrease in ruminal pH [50][51]. SARA typically arises when the pH level in the rumen remains between 5.2 and 6 for an extended duration [49]. Using real-time measured reticulorumen parameters, Antanaitis et al. [52] discovered that dairy cows whose reticulorumen pH ranged from 6.22 to 6.42 had an average total methane emission increase of 46.18% [52]. The data demonstrate that ruminal pH has a significant impact on the physiology and fermentation of the rumen, which in turn, affects methanogenesis [53]. On the other hand, several studies have shown that a decrease in pH promotes the synthesis of propionate, providing alternative routes for the elimination of hydrogen (H) ions. This leads to a decrease in the amount of hydrogen accessible for butyrate, which affects fibrolytic bacteria and methanogens, thus resulting in a reduction in CH4 synthesis [54][55][56]. Moreover, it may be linked to a decrease in the diversity of methanogens and changes in composition such as Methanobrevibacter spp. and Methanosphera spp. that were observed in SARA [53]. The bacterial population underwent alterations in response to acidosis in the laboratory investigation of M. Eger et al. [57], and it was restored to its original state 5 days following the acidosis challenge [57]. Considering that fibrolytic and amylolytic bacteria, as well as lactobacilli, have different pH preferences, the drop in pH may reflect most of the changes that were pointed out. Unlike bacteria, the population of methane-producing archaea was unable to be restored after acidosis. It is crucial to emphasise that the decrease in rumen pH can only be used to identify the ideal pH ranges that are advantageous for developing methods to reduce methane emissions, such as using feeding management techniques and supplementing with prebiotics and probiotics while not jeopardising the animal’s well-being [58].
2.2. Mastitis
Ensuring optimal udder health is crucial for both dairy farmers and the entire dairy production chain to provide milk of superior quality. Nevertheless, the production industry faces ongoing challenges from infections, with mastitis being a disease that has significant economic implications. Mastitis is an inflammatory condition of the mammary gland that occurs in dairy cows around the time of calving. It is categorised as one of the periparturient diseases [59]. Clinical mastitis (CM) is an infection that occurs inside the mammary gland and leads to a decrease in milk production and fertility. It also increases the rate at which cows are removed from the herd and the rate of cow deaths. As a result, CM has a detrimental effect on the efficiency of milk production, which is measured by the ratio of output to input. This could potentially lead to an increase in GHG emissions per unit of product [60].
Monitoring mastitis in cows has become a regular practice since the 1980s, with monthly somatic cell count (SCC) examinations being conducted. Even in the present day, this strategy continues to be widely acknowledged and embraced [61]. Özkan Gülzari et al. [62] showed that by lowering the SCC in milk production from 800,000 cells/mL to 50,000 cells/mL, it is possible to decrease the overall emissions intensity of farms by 3.7% [62]. Subclinical mastitis, a variant of the disease characterised by the absence of obvious indications of infection in the udders, diminishes both milk production and feed consumption in cows [63]. The anticipated disparity in DMI between a cow with a higher SCC of 250,000 cells/mL and a cow with a lower SCC of 50,000 cells/mL would result in an additional 12 grammes of methane per day (equivalent to 4.4 kg of methane per year). This accounts for approximately 2.8% of the annual enteric methane emissions from a dairy cow in the United States in 2017. Additionally, there would be an extra 0.34 g of methane per kilogramme of milk, representing a 2.8% increase in methane emissions per kilogramme of milk produced [64][65]. According to a recent study, subclinical mastitis can cause a significant increase in both enteric and manure methane emissions [63]. Specifically, infected cows can produce up to 8% more methane per kilogramme of milk compared to healthy cows [62].
Therefore, research indicates that clinical mastitis has a detrimental effect on output, namely reducing feed efficiency (measured as kg of milk per kg of feed intake) in cows. Consequently, this leads to an increase in GHG emissions per unit of product produced. The scientific findings indicate that cows with clinical mastitis have, on average, a 57.5 (6.2%) kg CO2e/t FPCM higher emission compared to cows without clinical mastitis [60]. It is necessary to emphasise that a rise in temperature of 10 °C in different regions of the United States resulted in an increase in antibiotic resistance of 4.2%, 2.2%, and 2.7% for the common pathogens Escherichia coli, Klebsiella pneumoniae, and Staphylococcus aureus, respectively. This demonstrates the intricate and mutually influential relationship linking animals, disease, and climate [66]. Implementing measures to prevent clinical mastitis can serve as a viable approach for farmers to mitigate GHG emissions and promote the sustainable growth of the dairy industry. This practice can also enhance farmers’ revenue and improve the well-being of cows [60].
2.3. Lameness
Lameness is a principal issue in terms of health and well-being in dairy farming [67]. Numerous factors contribute to this painful condition, which has detrimental economic effects such as decreased production, decreased fertility, and an increased likelihood of culling [68]. When productivity goes down, environmental effects get worse per unit of production because each animal’s maintenance costs rise in relation to production, so more animals are needed to keep milk production steady [69]. As herd sizes expand, lameness is thought to become more widespread and severe [70]. Approximately 90 percent of instances of lameness are linked to foot lesions [71]. Implementing management measures aimed at decreasing foot lesions might potentially lead to a decrease in GHG emissions per kilogram of milk produced [72]. The different increase in global warming potential (GWP) with a time horizon of 100 years (kg CO2-eq) due to lameness indicated that higher prevalence of foot diseases has a more negative impact. The result suggests that implementing sensors as well as information and communication technology for lameness detection could enhance management on dairy farms. This, in turn, could reduce the negative environmental effects linked to lameness by addressing the increased cow–handler ratio caused by larger herd sizes [70]. A further study discovered that foot lesions led to a mean rise of 13.6 (1.5%) kg CO2e/t of fat-and-protein-corrected milk (FPCM) in GHG emissions. Moreover, it also varied based on parity, with a rise from 17 kg CO2e/t FPCM in parity 1 to 7 kg CO2e/t FPCM in parity 5. This was mostly attributed to the heightened influence of eliminating cows with a low parity, since the rearing of a young female bovine results in the generation of GHG without yielding any milk production. Additionally, the emissions varied according on the kind of foot injury. The impact of non-infected white line disease on GHG emissions was the most significant, whereas infectious digital dermatitis had the least impact. Nevertheless, despite its negligible effect, viral digital dermatitis has the highest occurrence rate, and hence made the greatest contribution to the total impact of foot lesions. The findings of this research demonstrate that the assessment of GHG emissions by type of foot lesion provides more valuable information and has the potential to successfully reduce GHG emissions from the dairy sector [72]. This research not only highlights the importance of understanding the diverse types of foot lesions in dairy cows, but also emphasises the need for targeted management strategies to reduce GHG emissions.
2.4. Parasites
In livestock production, helminth infections are pervasive and have detrimental effects on feed ingestion, growth, productivity, reproductive performance, welfare, and health status. According to reports, nematode infection ranks second in terms of healthcare expenditures among dairy producers, following mastitis. Additionally, they contribute to the escalation of GHG emissions linked to ruminant agriculture [73]. Managing gastrointestinal parasites has the potential to decrease GHG emissions in grazing livestock. Nevertheless, the impact of these factors on methane emissions remains uncertain due to a dearth of studies [74]. Sheep that were infected with larvae of Haemonchus contortus and Trichostrongylus colubriformis had greater levels of CH4 per unit of consumed DM compared to the uninfected sheep (10.72 vs. 6.75 CH4/DMI (g/kg DM), p < 0.05) throughout the assessed time [75]. Repeatedly infecting ewes with Teladorsagia circumcincta infective larvae resulted in a 16% increase in total methane yield and a 4% increase in total nitrous oxide yield per unit of dry matter intake. Similarly, per unit of digestible organic matter intake, there was a 46% increase in total methane yield and a 31% increase in total nitrous oxide yield [76]. Just recently, Fox et al. [74] discovered that lambs infected with abomasal parasites exhibited a 33% increase in total CH4 output compared to uninfected animals. As per the findings of N. J. Fox et al. [74] and J. G. Houdijk et al. [76], using deworming measures for female sheep may enhance both production efficiency and environmental sustainability in sheep farming. This approach may also be relevant for cattle [74][76].
One of the limited number of studies identified examined the effects of Fasciola hepatica in beef cattle. In beef cattle, the observed 1.5% rise in GHG emissions intensity in 2022 attributed to Fasciola hepatica seems to be moderate. Nevertheless, the presence of liver fluke also leads to alterations in feed conversion ratio, milk production levels and the quality of output. Therefore, eliminating the fluke challenge would have a significantly larger effect on emissions intensity in real-world scenarios than what is observed in current studies [77]. However, further research should be conducted on dairy cows of this nature.
2.5. Viral Infections
Given the significance of viral illnesses in worldwide cow production, it is crucial to make efforts to eliminate, or at least decrease, the occurrence of these diseases. Environmental change, trade globalisation, and livestock expansion have all played a role in the dissemination of established pathogens and the introduction of disease into formerly disease-free regions and animal populations [78]. Contagious viral infections in dairy cattle have significant consequences for milk supply, quality, and general animal well-being [79]. The GHG emissions intensity (kg CO2eq/kg product) of livestock-derived food production can be reduced by decreasing the incidence or eradicating diseases that negatively affect milk and meat production. However, the extent of specific disease effects differs based on factors such as output losses, disease prevalence, and baseline population characteristics [80].
Research conducted in the United Kingdom (UK) revealed that bovine viral diarrhea (BVD) might raise greenhouse gas emissions per unit of beef carcass by up to 113% and increase emissions by 14% compared to the healthy baseline for dairy beef production. However, it is important to note that this effect was not adjusted for the prevalence of the illness [81]. In addition, an experiment investigating the impact of dairy bovine illness on GHG emission revealed that eliminating BVD would result in a 4% reduction in GHG emission for average UK herds, while the most problematic 10% of herds would see an 11% decrease [81]. At the herd level, several studies have shown that infected bovine rhinotracheitis results in an 8% increase in GHG emissions per kilogramme of energy-corrected milk and a 20% increase per kilogramme of beef [43]. Another study has shown that by reducing the incidence of foot and mouth disease in beef cattle from 45% to 5%, there would be an 11.3% decrease in GHG emission intensities (CO2eq/kg CW) [80]. These findings highlight the significant contribution of diseases in livestock to GHG emission, and the potential for disease control measures to mitigate emissions in the agriculture sector.
Paratuberculosis has long been recognised as a latent issue in dairy cows on a global scale. In the research study conducted by McAloon et al. [82], it was demonstrated that dairy cows that tested positive for a certain condition saw a reduction in MY by 5.9%. There is a lack of data about the emission intensity, nevertheless, it can be inferred that if the nutrients are not well utilised, it would have an impact on the emission intensity [37]. Additionally, further investigation should be undertaken with dairy cows of this sort.
2.6. Methane Emissions and Cow Blood Parameters
Monitoring and optimising specific blood parameters in cattle can potentially enhance their overall health, increase efficiency by reducing methane emissions, and improve the growth performance of their offspring, so increasing profitability [83]. Macro- and microminerals are essential for maintaining good cattle production performance by fulfilling the basic physiological needs. Their presence in the bloodstream is crucial for various physiological activities, including health maintenance, growth, reproduction, and the proper functioning of the immunological and endocrine systems [84]. In a recent research investigation, Reintke et al. [83] discovered a correlation between a low blood serum BHB level and a reduction in CH4 emissions in ewe. Furthermore, in the Merinoland breed, elevated zinc levels during lactation were linked to decreased methane emissions. The features of interest were not significantly influenced by the serum levels of Na, K, P, glutamate dehydrogenase (GLDH), and Fe [83].
Ľubomíra Grešáková et al. [23] conducted a study that sought to clarify the relationship between the mineral status of dairy cows and enteric methane production at various stages of lactation. Nevertheless, this investigation did not discover any association between exhaled methane emissions and blood plasma mineral status at various stages of breastfeeding [23].
Due to its strong association with feed efficiency and energy level, liver metabolism is assumed to have a direct and indirect role in intestinal methane generation. Scientists conducting experiments on Japanese Black cattle discovered that cattle with high methane emissions (HME) had elevated levels of blood β-hydroxybutyric acid concentration and total ketone bodies compared to cattle with low methane emissions (LME). The rumen-generated butyrate is then transformed into BHBA and delivered via circulation to be used as energy in different tissues. Therefore, the elevated BHBA concentrations detected in the serum of the HME cattle in this investigation may be attributed, at least in part, to the increased rate of butyrate generation in the rumen [45]. The blood metabolites and CH4 production is affected by both DMI and diet. L. T. C. Ornelas et al. [85] observed that despite feeding the animals the same diet and them exhibiting identical DMI values during respiration and digestibility assays, the group with low emissions’ CH4 production (LPr) exhibited a reduced insulin concentration in comparison to the group with high emissions’ CH4 production (HPr). The elevated glucose-to-insulin ratios and reduced insulin levels can be rationalised by variations in DMI during the pre-experimental phase. These distinctions suggest that insulin and the glucose-to-insulin ratio may serve as indirect indicators for animals whose methane production is below one gramme per day [85]. Consistent findings were shown in research conducted by M. Kim et al. [45]. The insulin levels in HME cattle were significantly increased compared to the LME group, indicating that the HME group effectively used amino acids as energy sources in muscle and peripheral tissues to offset the energy depletion caused by methane generation. Insulin facilitated the transportation of amino acids, hence sustaining elevated amounts [45].
References
- Woolery, S.; Osei, E.; Yu, M.; Guney, S.; Lovell, A.; Jafri, H. The Carbon Footprint of a 5000-Milking-Head Dairy Operation in Central Texas. Agriculture 2023, 13, 2109.
- Blunden, J.; Boyer, T. State of the Climate in 2021. Bull. Am. Meteorol. Soc. 2022, 103, S1–S465.
- Starsmore, K.; Lopez-Villalobos, N.; Shalloo, L.; Egan, M.; Burke, J.; Lahart, B. Animal Factors That Affect Enteric Methane Production Measured Using the GreenFeed Monitoring System in Grazing Dairy Cows. J. Dairy Sci. 2023, S0022-0302(23)00805-6.
- Murphy, P.; Crosson, P.; O’Brien, D.; Schulte, R.P.O. The Carbon Navigator: A Decision Support Tool to Reduce Greenhouse Gas Emissions from Livestock Production Systems. Animal 2013, 7, 427–436.
- Reyes, D.C.; Meredith, J.; Puro, L.; Berry, K.; Kersbergen, R.; Soder, K.J.; Quigley, C.; Donihue, M.; Cox, D.; Price, N.N.; et al. Maine Organic Dairy Producers’ Receptiveness to Seaweed Supplementation and Effect of Chondrus Crispus on Enteric Methane Emissions in Lactating Cows. Front. Vet. Sci. 2023, 10, 1153097.
- Britt, J.H.; Cushman, R.A.; Dechow, C.D.; Dobson, H.; Humblot, P.; Hutjens, M.F.; Jones, G.A.; Mitloehner, F.M.; Ruegg, P.L.; Sheldon, I.M.; et al. Review: Perspective on High-Performing Dairy Cows and Herds. Animal 2021, 15, 100298.
- Soliman, T.; Barnes, A.; Helgesen, I.S. The Hidden Carbon Impact of Animal Disease. PLoS ONE 2023, 18, e0292659.
- Herzog, A.; Winckler, C.; Zollitsch, W. In Pursuit of Sustainability in Dairy Farming: A Review of Interdependent Effects of Animal Welfare Improvement and Environmental Impact Mitigation. Agric. Ecosyst. Environ. 2018, 267, 174–187.
- Singaravadivelan, A.; Sachin, P.B.; Harikumar, S.; Vijayakumar, P.; Vindhya, M.V.; Farhana, F.M.B.; Rameesa, K.K.; Mathew, J. Life Cycle Assessment of Greenhouse Gas Emission from the Dairy Production System—Review. Trop. Anim. Health Prod. 2023, 55, 320.
- Wang, Q.; Bovenhuis, H. Validation Strategy Can Result in an Overoptimistic View of the Ability of Milk Infrared Spectra to Predict Methane Emission of Dairy Cattle. J. Dairy Sci. 2019, 102, 6288–6295.
- Aryee, G.; Luecke, S.M.; Dahlen, C.R.; Swanson, K.C.; Amat, S. Holistic View and Novel Perspective on Ruminal and Extra-Gastrointestinal Methanogens in Cattle. Microorganisms 2023, 11, 2746.
- Lassen, J.; Løvendahl, P. Heritability Estimates for Enteric Methane Emissions from Holstein Cattle Measured Using Noninvasive Methods. J. Dairy Sci. 2016, 99, 1959–1967.
- McGuffey, R.K. A 100-Year Review: Metabolic Modifiers in Dairy Cattle Nutrition. J. Dairy Sci. 2017, 100, 10113–10142.
- Bačėninaitė, D.; Džermeikaitė, K.; Antanaitis, R. Global Warming and Dairy Cattle: How to Control and Reduce Methane Emission. Animals 2022, 12, 2687.
- Ghassemi Nejad, J.; Ju, M.-S.; Jo, J.-H.; Oh, K.-H.; Lee, Y.-S.; Lee, S.-D.; Kim, E.-J.; Roh, S.; Lee, H.-G. Advances in Methane Emission Estimation in Livestock: A Review of Data Collection Methods, Model Development and the Role of AI Technologies. Animals 2024, 14, 435.
- New FAO Report Maps Pathways towards Lower Livestock Emissions. Available online: https://www.fao.org/newsroom/detail/new-fao-report-maps-pathways-towards-lower-livestock-emissions/en (accessed on 2 February 2024).
- Lyu, Z.; Shao, N.; Akinyemi, T.; Whitman, W.B. Methanogenesis. Curr. Biol. 2018, 28, R727–R732.
- Conrad, R. Importance of Hydrogenotrophic, Aceticlastic and Methylotrophic Methanogenesis for Methane Production in Terrestrial, Aquatic and Other Anoxic Environments: A Mini Review. Pedosphere 2020, 30, 25–39.
- van Gastelen, S.; Dijkstra, J. Prediction of Methane Emission from Lactating Dairy Cows Using Milk Fatty Acids and Mid-Infrared Spectroscopy. J. Sci. Food Agric. 2016, 96, 3963–3968.
- Beauchemin, K.A.; McGinn, S.M. Reducing Methane in Dairy and Beef Cattle Operations: What Is Feasible? Prairie Soil Crop 2008, 1, 17–21.
- Min, B.-R.; Lee, S.; Jung, H.; Miller, D.N.; Chen, R. Enteric Methane Emissions and Animal Performance in Dairy and Beef Cattle Production: Strategies, Opportunities, and Impact of Reducing Emissions. Animals 2022, 12, 948.
- de Haas, Y.; Veerkamp, R.F.; de Jong, G.; Aldridge, M.N. Selective Breeding as a Mitigation Tool for Methane Emissions from Dairy Cattle. Animal 2021, 15, 100294.
- Grešáková, Ľ.; Holodová, M.; Szumacher-Strabel, M.; Huang, H.; Ślósarz, P.; Wojtczak, J.; Sowińska, N.; Cieślak, A. Mineral Status and Enteric Methane Production in Dairy Cows during Different Stages of Lactation. BMC Vet. Res. 2021, 17, 287.
- Özkan, Ş.; Vitali, A.; Lacetera, N.; Amon, B.; Bannink, A.; Bartley, D.J.; Blanco-Penedo, I.; De Haas, Y.; Dufrasne, I.; Elliott, J.; et al. Challenges and Priorities for Modelling Livestock Health and Pathogens in the Context of Climate Change. Environ. Res. 2016, 151, 130–144.
- Zetouni, L.; Kargo, M.; Norberg, E.; Lassen, J. Genetic Correlations between Methane Production and Fertility, Health, and Body Type Traits in Danish Holstein Cows. J. Dairy Sci. 2018, 101, 2273–2280.
- Caprarulo, V.; Ventura, V.; Amatucci, A.; Ferronato, G.; Gilioli, G. Innovations for Reducing Methane Emissions in Livestock toward a Sustainable System: Analysis of Feed Additive Patents in Ruminants. Animals 2022, 12, 2760.
- Arnouts, S.; Brown, S.; de Arriba, M.L.; Donabedian, M.; Charlier, J. Technology Readiness Levels for Vaccine and Drug Development in Animal Health: From Discovery to Life Cycle Management. Front. Vet. Sci. 2022, 9, 1016959.
- Dittmann, M.T.; Hammond, K.J.; Kirton, P.; Humphries, D.J.; Crompton, L.A.; Ortmann, S.; Misselbrook, T.H.; Südekum, K.-H.; Schwarm, A.; Kreuzer, M.; et al. Influence of ruminal methane on digesta retention and digestive physiology in non-lactating dairy cattle. Br. J. Nutr. 2016, 116, 763–773.
- Zhao, Y.; Nan, X.; Yang, L.; Zheng, S.; Jiang, L.; Xiong, B. A Review of Enteric Methane Emission Measurement Techniques in Ruminants. Animals 2020, 10, 1004.
- Cameron, L.; Chagunda, M.G.G.; Roberts, D.J.; Lee, M.A. A Comparison of Milk Yields and Methane Production from Three Contrasting High-Yielding Dairy Cattle Feeding Regimes: Cut-and-Carry, Partial Grazing and Total Mixed Ration. Grass Forage Sci. 2018, 73, 789–797.
- Watt, L.J.; Clark, C.E.F.; Krebs, G.L.; Petzel, C.E.; Nielsen, S.; Utsumi, S.A. Differential Rumination, Intake, and Enteric Methane Production of Dairy Cows in a Pasture-Based Automatic Milking System. J. Dairy Sci. 2015, 98, 7248–7263.
- Kumari, S.; Fagodiya, R.K.; Hiloidhari, M.; Dahiya, R.P.; Kumar, A. Methane Production and Estimation from Livestock Husbandry: A Mechanistic Understanding and Emerging Mitigation Options. Sci. Total Environ. 2020, 709, 136135.
- Fu, Y.; Yao, S.; Wang, T.; Lu, Y.; Han, H.; Liu, X.; Lv, D.; Ma, X.; Guan, S.; Yao, Y.; et al. Effects of Melatonin on Rumen Microorganisms and Methane Production in Dairy Cow: Results from in Vitro and in Vivo Studies. Microbiome 2023, 11, 196.
- Nguyen, B.T.; Briggs, K.R.; Eicker, S.; Overton, M.; Nydam, D.V. Herd Turnover Rate Reexamined: A Tool for Improving Profitability, Welfare, and Sustainability. Am. J. Vet. Res. 2023, 84, 1.
- Ezenwa, V.O.; Civitello, D.J.; Classen, A.T.; Barton, B.T.; Becker, D.J.; Brenn-White, M.; Deem, S.L.; Kutz, S.; Malishev, M.; Penczykowski, R.M.; et al. Response to Charlier et al.: Climate–Disease Feedbacks Mediated by Livestock Methane Emissions Are Plausible. Trends Ecol. Evol. 2021, 36, 578–579.
- Hristov, A.N.; Ott, T.; Tricarico, J.; Rotz, A.; Waghorn, G.; Adesogan, A.; Dijkstra, J.; Montes, F.; Oh, J.; Kebreab, E.; et al. SPECIAL TOPICS—Mitigation of Methane and Nitrous Oxide Emissions from Animal Operations: III. A Review of Animal Management Mitigation Options1. J. Anim. Sci. 2013, 91, 5095–5113.
- von Soosten, D.; Meyer, U.; Flachowsky, G.; Dänicke, S. Dairy Cow Health and Greenhouse Gas Emission Intensity. Dairy 2020, 1, 20–29.
- Franzluebbers, A.J. Chapter 2—Cattle Grazing Effects on the Environment: Greenhouse Gas Emissions and Carbon Footprint. In Management Strategies for Sustainable Cattle Production in Southern Pastures; Rouquette, M., Aiken, G.E., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 11–34. ISBN 978-0-12-814474-9.
- Lehmann, J.O.; Mogensen, L.; Kristensen, T. Extended Lactations May Improve Cow Health, Productivity and Reduce Greenhouse Gas Emissions from Organic Dairy Production. Org. Agric. 2014, 4, 295–299.
- Kabir, M.; Hasan, M.M.; Tanni, N.S.; Parvin, M.S.; Asaduzzaman, M.; Ehsan, M.A.; Islam, M.T. Metabolic Profiling in Periparturient Dairy Cows and Its Relation with Metabolic Diseases. BMC Res. Notes 2022, 15, 231.
- Lacasse, P.; Vanacker, N.; Ollier, S.; Ster, C. Innovative Dairy Cow Management to Improve Resistance to Metabolic and Infectious Diseases during the Transition Period. Res. Vet. Sci. 2018, 116, 40–46.
- Kappes, A.; Tozooneyi, T.; Shakil, G.; Railey, A.F.; McIntyre, K.M.; Mayberry, D.E.; Rushton, J.; Pendell, D.L.; Marsh, T.L. Livestock Health and Disease Economics: A Scoping Review of Selected Literature. Front. Vet. Sci. 2023, 10, 1168649.
- Capper, J.L.; Williams, P. Investing in Health to Improve the Sustainability of Cattle Production in the United Kingdom: A Narrative Review. Vet. J. 2023, 296–297, 105988.
- Paiano, R.B.; Birgel, D.B.; Bonilla, J.; Birgel Junior, E.H. Evaluation of Biochemical Profile of Dairy Cows with Metabolic Diseases in Tropical Conditions. Reprod. Domest. Anim. 2020, 55, 1219–1228.
- Kim, M.; Masaki, T.; Ikuta, K.; Iwamoto, E.; Nishihara, K.; Hirai, M.; Uemoto, Y.; Terada, F.; Roh, S. Physiological Responses and Adaptations to High Methane Production in Japanese Black Cattle. Sci. Rep. 2022, 12, 11154.
- McFadden, J.W. Review: Lipid Biology in the Periparturient Dairy Cow: Contemporary Perspectives. Animal 2020, 14, s165–s175.
- Lei, M.A.C.; Simões, J. Invited Review: Ketosis Diagnosis and Monitoring in High-Producing Dairy Cows. Dairy 2021, 2, 303–325.
- Mostert, P.F.; van Middelaar, C.E.; Bokkers, E.A.M.; de Boer, I.J.M. The Impact of Subclinical Ketosis in Dairy Cows on Greenhouse Gas Emissions of Milk Production. J. Clean. Prod. 2018, 171, 773–782.
- Abdela, N. Sub-Acute Ruminal Acidosis (SARA) and Its Consequence in Dairy Cattle: A Review of Past and Recent Research at Global Prospective. Achiev. Life Sci. 2016, 10, 187–196.
- Simanungkalit, G.; Bhuiyan, M.; Bell, R.; Sweeting, A.; Morton, C.L.; Cowley, F.; Hegarty, R. The Effects of Antibiotic-Free Supplementation on the Ruminal pH Variability and Methane Emissions of Beef Cattle under the Challenge of Subacute Ruminal Acidosis (SARA). Res. Vet. Sci. 2023, 160, 30–38.
- Kaur, U.; Malacco, V.M.R.; Bai, H.; Price, T.P.; Datta, A.; Xin, L.; Sen, S.; Nawrocki, R.A.; Chiu, G.; Sundaram, S.; et al. Invited Review: Integration of Technologies and Systems for Precision Animal Agriculture—A Case Study on Precision Dairy Farming. J. Anim. Sci. 2023, 101, skad206.
- Antanaitis, R.; Anskienė, L.; Rapaliutė, E.; Bilskis, R.; Džermeikaitė, K.; Bačėninaitė, D.; Juškienė, V.; Juška, R.; Meškinytė, E. Relationship between Reticulorumen Parameters Measured in Real Time and Methane Emission and Heat Stress Risk in Dairy Cows. Animals 2022, 12, 3257.
- Mickdam, E.; Khiaosa-ard, R.; Metzler-Zebeli, B.U.; Klevenhusen, F.; Chizzola, R.; Zebeli, Q. Rumen Microbial Abundance and Fermentation Profile during Severe Subacute Ruminal Acidosis and Its Modulation by Plant Derived Alkaloids In Vitro. Anaerobe 2016, 39, 4–13.
- Wang, K.; Xiong, B.; Zhao, X. Could Propionate Formation Be Used to Reduce Enteric Methane Emission in Ruminants? Sci. Total Environ. 2023, 855, 158867.
- Beauchemin, K.A.; Ungerfeld, E.M.; Abdalla, A.L.; Alvarez, C.; Arndt, C.; Becquet, P.; Benchaar, C.; Berndt, A.; Mauricio, R.M.; McAllister, T.A.; et al. Invited Review: Current Enteric Methane Mitigation Options. J. Dairy Sci. 2022, 105, 9297–9326.
- Børsting, C.F.; Brask, M.; Hellwing, A.L.F.; Weisbjerg, M.R.; Lund, P. Enteric Methane Emission and Digestion in Dairy Cows Fed Wheat or Molasses. J. Dairy Sci. 2020, 103, 1448–1462.
- Eger, M.; Riede, S.; Breves, G. Induction of a Transient Acidosis in the Rumen Simulation Technique. J. Anim. Physiol. Anim. Nutr. 2018, 102, 94–102.
- Elmhadi, M.E.; Ali, D.K.; Khogali, M.K.; Wang, H. Subacute Ruminal Acidosis in Dairy Herds: Microbiological and Nutritional Causes, Consequences, and Prevention Strategies. Anim. Nutr. 2022, 10, 148–155.
- Kaseke, T.B.; Chikwambi, Z.; Gomo, C.; Mashingaidze, A.B.; Murungweni, C. Antibacterial Activity of Medicinal Plants on the Management of Mastitis in Dairy Cows: A Systematic Review. Vet. Med. Sci. 2023, 9, 2800–2819.
- Mostert, P.F.; Bokkers, E.A.; De Boer, I.J.; Van Middelaar, C.E. Estimating the impact of clinical mastitis in dairy cows on greenhouse gas emissions using a dynamic stochastic simulation model: A case study. Animal 2019, 13, 2913–2921.
- Luo, W.; Dong, Q.; Feng, Y. Risk Prediction Model of Clinical Mastitis in Lactating Dairy Cows Based on Machine Learning Algorithms. Prev. Vet. Med. 2023, 221, 106059.
- Özkan Gülzari, Ş.; Vosough Ahmadi, B.; Stott, A.W. Impact of Subclinical Mastitis on Greenhouse Gas Emissions Intensity and Profitability of Dairy Cows in Norway. Prev. Vet. Med. 2018, 150, 19–29.
- Ezenwa, V.O.; Civitello, D.J.; Barton, B.T.; Becker, D.J.; Brenn-White, M.; Classen, A.T.; Deem, S.L.; Johnson, Z.E.; Kutz, S.; Malishev, M.; et al. Infectious Diseases, Livestock, and Climate: A Vicious Cycle? Trends Ecol. Evol. 2020, 35, 959–962.
- Potter, T.L.; Arndt, C.; Hristov, A.N. Short Communication: Increased Somatic Cell Count Is Associated with Milk Loss and Reduced Feed Efficiency in Lactating Dairy Cows. J. Dairy Sci. 2018, 101, 9510–9515.
- Hockstad, L.; Hanel, L. Inventory of U.S. Greenhouse Gas Emissions and Sinks; Environmental System Science Data Infrastructure for a Virtual Ecosystem (ESS-DIVE): Washington, DC, USA, 2018.
- MacFadden, D.R.; McGough, S.F.; Fisman, D.; Santillana, M.; Brownstein, J.S. Antibiotic Resistance Increases with Local Temperature. Nat. Clim. Chang. 2018, 8, 510–514.
- Dolecheck, K.; Bewley, J. Animal board invited review: Dairy cow lameness expenditures, losses and total cost. Animal 2018, 12, 1462–1474.
- Weigele, H.C.; Gygax, L.; Steiner, A.; Wechsler, B.; Burla, J.-B. Moderate Lameness Leads to Marked Behavioral Changes in Dairy Cows. J. Dairy Sci. 2018, 101, 2370–2382.
- Herzog, A.; Hörtenhuber, S.; Winckler, C.; Kral, I.; Zollitsch, W. Welfare Intervention and Environmental Impacts of Milk Production—Cradle-to-Farm-Gate Effects of Implementing Rubber Mats in Austrian Dairy Farms. J. Clean. Prod. 2020, 277, 123953.
- Chen, W.; White, E.; Holden, N.M. The Effect of Lameness on the Environmental Performance of Milk Production by Rotational Grazing. J. Environ. Manag. 2016, 172, 143–150.
- Somers, J.; O’Grady, L. Foot Lesions in Lame Cows on 10 Dairy Farms in Ireland. Ir. Vet. J. 2015, 68, 10.
- Mostert, P.F.; van Middelaar, C.E.; de Boer, I.J.M.; Bokkers, E.A.M. The Impact of Foot Lesions in Dairy Cows on Greenhouse Gas Emissions of Milk Production. Agric. Syst. 2018, 167, 206–212.
- Podpečan, O.; Hajdinjak, M.; Posedi, J. Helminth Control as a Part of Animal Welfare Measure Protocol in Grazing Cattle in Slovenia. Agriculture 2023, 13, 1038.
- Fox, N.J.; Smith, L.A.; Houdijk, J.G.M.; Athanasiadou, S.; Hutchings, M.R. Ubiquitous Parasites Drive a 33% Increase in Methane Yield from Livestock. Int. J. Parasitol. 2018, 48, 1017–1021.
- Fernandes, M.A.; de Mello Tavares Lima, P.; do Amarante, A.F.T.; Abdalla, A.L.; Louvandini, H. Hematological, Biochemical Alterations and Methane Production in Sheep Submitted to Mixed Infection of Haemonchus Contortus and Trichostrongylus Colubriformis. Small Rumin. Res. 2022, 216, 106798.
- Houdijk, J.G.M.; Tolkamp, B.J.; Rooke, J.A.; Hutchings, M.R. Animal Health and Greenhouse Gas Intensity: The Paradox of Periparturient Parasitism. Int. J. Parasitol. 2017, 47, 633–641.
- Jonsson, N.N.; MacLeod, M.; Hayward, A.; McNeilly, T.; Ferguson, K.D.; Skuce, P.J. Liver Fluke in Beef Cattle—Impact on Production Efficiency and Associated Greenhouse Gas Emissions Estimated Using Causal Inference Methods. Prev. Vet. Med. 2022, 200, 105579.
- Wathes, D.C.; Oguejiofor, C.F.; Thomas, C.; Cheng, Z. Importance of Viral Disease in Dairy Cow Fertility. Engineering 2020, 6, 26–33.
- Brito, B.; Hick, P. Milk as a Diagnostic Fluid to Monitor Viral Diseases in Dairy Cattle. Aust. Vet. J. 2024, 102, 11–18.
- Capper, J.L. The Impact of Controlling Diseases of Significant Global Importance on Greenhouse Gas Emissions from Livestock Production. One Health Outlook 2023, 5, 17.
- Williams, A.; Chatterton, J.; Hateley, G.; Curwen, A.; Elliott, J. A Systems-Life Cycle Assessment Approach to Modelling the Impact of Improvements in Cattle Health on Greenhouse Gas Emissions. Adv. Anim. Biosci. 2015, 6, 29–31.
- McAloon, C.G.; Whyte, P.; More, S.J.; Green, M.J.; O’Grady, L.; Garcia, A.; Doherty, M.L. The Effect of Paratuberculosis on Milk Yield—A Systematic Review and Meta-Analysis. J. Dairy Sci. 2016, 99, 1449–1460.
- Reintke, J.; Brügemann, K.; Yin, T.; Wagner, H.; Wehrend, A.; Müller, A.; König, S. Associations between Minerals and Metabolic Indicators in Maternal Blood Pre- and Postpartum with Ewe Body Condition, Methane Emissions, and Lamb Body Weight Development. Anim. Int. J. Anim. Biosci. 2021, 15, 100034.
- Gul, F.; Amin, H.; Naz, S.; Khan, M.T.; Alhidary, I.A.; Khan, R.U.; Pugliese, G.; Tufarelli, V. Evaluation of Blood Minerals and Oxidative Stress Changing Pattern in Prepartum and Postpartum Achai and Holstein Friesian Dairy Cows. Reprod. Domest. Anim. 2024, 59, e14525.
- Ornelas, L.T.C.; Silva, D.C.; Tomich, T.R.; Campos, M.M.; Machado, F.S.; Ferreira, A.L.; Maurício, R.M.; Pereira, L.G.R. Differences in Methane Production, Yield and Intensity and Its Effects on Metabolism of Dairy Heifers. Sci. Total Environ. 2019, 689, 1133–1140.
More
Information
Subjects:
Veterinary Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
13 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





