Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Weidong Xie | -- | 3390 | 2024-03-12 10:12:57 | | | |
| 2 | Mona Zou | Meta information modification | 3390 | 2024-03-14 08:30:07 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Gao, X.; Zhang, N.; Xie, W. Cultivation and Pharmacological Activities of Taxus mairei. Encyclopedia. Available online: https://encyclopedia.pub/entry/56154 (accessed on 08 February 2026).
Gao X, Zhang N, Xie W. Cultivation and Pharmacological Activities of Taxus mairei. Encyclopedia. Available at: https://encyclopedia.pub/entry/56154. Accessed February 08, 2026.
Gao, Xinyu, Ni Zhang, Weidong Xie. "Cultivation and Pharmacological Activities of Taxus mairei" Encyclopedia, https://encyclopedia.pub/entry/56154 (accessed February 08, 2026).
Gao, X., Zhang, N., & Xie, W. (2024, March 12). Cultivation and Pharmacological Activities of Taxus mairei. In Encyclopedia. https://encyclopedia.pub/entry/56154
Gao, Xinyu, et al. "Cultivation and Pharmacological Activities of Taxus mairei." Encyclopedia. Web. 12 March, 2024.
Copy Citation
Taxus mairei (Lemée and H.Lév.) S.Y.Hu, indigenous to the southern regions of China, is an evergreen tree belonging to the genus Taxus of the Taxaceae family. Owing to its content of various bioactive compounds, it exhibits multiple pharmacological activities and has been widely applied in clinical medicine.
T. mairei
phytochemical constituents
pharmacological activity
1. Origin and Cultivation
Taxus, belonging to the Taxaceae family, is one of the world’s precious tree species. Due to its unique ecological and medical values in China, Taxus has been classified as a first-class protected plant [1][2][3]. Globally, there are eleven species of Taxus, with China being home to four species and one variety, namely Taxus chinensis (Pilger.) Rehd, Taxus mairei (Lemée and H.Lév.) S.Y.Hu, Taxus wallichiana Zucc., Taxus cuspidata Siebold and Zucc., and Taxus yunnanensis W.C.Cheng and L.K.Fu. In addition, there are also the hybrid species Taxus × media Rehder [4][5]. Figure 1 shows the geographical distribution of native and hybrid species of Taxus in China.
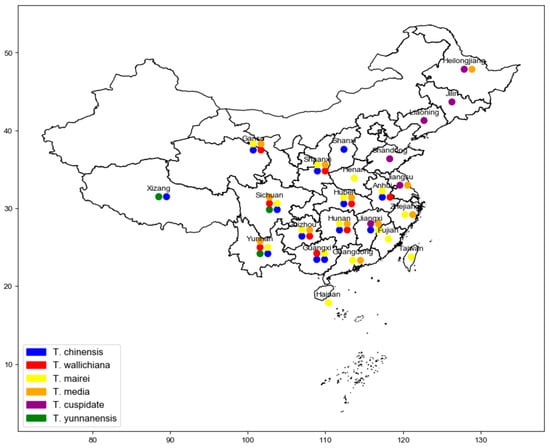
Figure 1. The geographical distribution of native and hybrid species of Taxus in China.
T. mairei has an extensive distribution in China. It is primarily concentrated in Shaanxi, Gansu, and the areas south of the Yangtze River. T. mairei is found from the northern part of Guangdong and Guangxi to the southeastern part of Shanxi at altitudes of 600 to 1200 m in subtropical mountainous areas [6]. This species typically grows in mountains or valleys at 800 to 1600 m. The uniqueness of its growing environment makes T. mairei the most widely distributed Taxus species in China [7]. Studies have shown that this species has a solid adaptability to soil types but prefers well-drained, fertile soil conditions [8].
As a relict plant from the Tertiary period, T. mairei is excellently protected and propagated in the Nanling region of China. Along the Nanling mountain range, from the Yuanbao and Mao’er mountains in the north of Guangxi, through Hunan’s Dupang and Mang mountains, eastward to the Dayuling at the junction of Jiangxi and Guangdong, and to the southern end of Wuyi Mountain in western Fujian, natural populations of T. mairei can be found in this continuous geographical area [9]. Nature reserves play a significant role in biodiversity conservation, and there are nature reserves for T. mairei in various provinces in China, such as the Guangdong Ruyuan T. mairei Nature Reserve located in the subtropical climate zone and the Sichuan Tangjiahe National Nature Reserve [10]. Hubei province has wild T. mairei resources, but the growth rate could be faster and meet clinical needs. Therefore, artificial cultivation bases have been established in Enshi, Zhongxiang, and Xianning of Hubei province [11]. These bases provide valuable plant resources for the medical industry and play an essential role in protecting this rare species.
2. Pharmacological Activity Studies
T. mairei has been listed as a provincial standard medicinal material in many provinces such as Zhejiang, Shanghai, Guangdong, Hunan, Jiangsu, etc. The “Zhejiang province Traditional Chinese Medicine Pieces Processing Specification” records the medicinal parts, taste, meridian tropism, and primary functions of T. mairei: the main medicinal parts are the branches with leaves, “slightly sweet, bitter, neutral, entering the kidney and heart meridians”, with the effects of reducing swelling, dispersing masses, promoting menstruation, diuresis, and clinically used for masses, edema, difficulty in urination, rheumatic pain, etc. [12]. Below, the anticancer, anti-inflammatory, antihypertensive, antidiabetic, and antimicrobial effects are described respectively:
2.1. Anticancer Activity
2.1.1. Anticancer Activity of Extracts
T. mairei contains many active chemical components; thus, its total extract shows specific anticancer activity. The aqueous extract of T. mairei (AETC) has shown significant effects in both in vitro and in vivo studies in anticancer research.
In in vivo studies, AETC exhibited significant inhibition of NCI-N87 human gastric cancer cell xenograft tumors in nude mice and induced apoptosis. Administered via gavage at doses of 2.080–0.520 g/kg, AETC effectively inhibited the growth of NCI-N87 gastric cancer xenograft tumors in nude mice expressing high levels of HER2 and enhanced its inhibitory effect when combined with Herceptin treatment [13]. The immunoglobulin protein CD47 is overexpressed in malignant tumor cells, allowing them to evade host immunity by inhibiting macrophage-mediated phagocytosis. AETC reduced CD47 levels in non-small cell lung cancer (NSCLC) cells and Lewis tumor xenograft mice, enhancing immunity to NSCLC by triggering ubiquitination and degradation of CD47 [14]. In studies exploring the effect of AETC on the growth of A549 lung cancer xenografts in nude mice and its mechanisms, oral administration of AETC for seven weeks, compared to the control group, showed significantly reduced levels of EGFR and Survivin mRNA in the xenograft tissues, indicating a potential mechanism by which AETC inhibits tumor growth by affecting these molecular targets [15]. Research on the effect of the aqueous extract of T. mairei combined with Erlotinib on the growth of A549 tumor xenografts in nude mice found that, compared to the control group, the tumor weight in the experimental group significantly decreased, with marked reductions in EGFR mRNA expression, COX-2 mRNA expression, Bcl-2 mRNA expression, and COX-2 protein expression [16].
In in vitro studies, AETC and paclitaxel exhibited significant inhibitory effects on gastric cancer cells SGC-7901 and breast cancer cells MCF-7, showing a dose–effect relationship. Studies indicated that the IC50 of AETC for these two cell types were (2.23 ± 0.13) mg/mL and (2.29 ± 0.15) mg/mL, respectively, demonstrating its significant inhibitory effect on tumor cell proliferation [17]. Further research showed that AETC inhibited NSCLC cell proliferation, especially significant in lung cancer cells NCI-1975, and could induce apoptosis. The anti-tumor effect of AETC is associated with the upregulation and downregulation of ATF3 expression, involving inhibiting the Hippo pathway and reducing YAP degradation. AETC also reduced tumor volume and weight in nude mice, upregulated ATF3, p-MOB1, and p-YAP (Ser397), actively regulated cleaved PARP, and caspase-9/8/3, showing its role in inducing apoptosis in NSCLC cells in vitro and in vivo through the ATF3-Hippo-YAP pathway [18]. The MTT assay detected the inhibitory effect of T. mairei Aqueous Extract (TAE) and paclitaxel on the proliferation of gastric cancer cells SGC-7901 and breast cancer MCF-7 cells. The effect of TAE on the morphology of SGC-7901 and MCF-7 cells was observed under a microscope. The results indicated that the extract of T. mairei inhibits tumor cell proliferation, which is related to inducing tumor cell apoptosis [17]. Additionally, the CCK-8 assay showed that TAE has a selective inhibitory effect on the growth of non-small cell lung cancer A549 and HCC827 cells and is dose-dependent, with minimal impact on everyday human lung cells. Its mechanism may be related to inhibiting tumor cell proliferation and metastasis by inactivating the JAK/STAT3 axis [19][20]. Research also indicated that taxane compounds extracted from T. mairei exhibit a specific inhibitory effect on the proliferation of A549 non-small cell lung cancer cells (IC50 between 26–167 μg/mL). Additionally, these compounds showed potent inhibitory activity against B16 mouse melanoma cells (IC50 between 20–768 μg/mL) and a strong inhibitory effect on the proliferation of BEL7402 human hepatoma cells (IC50 between 30–273 μg/mL) [21].
2.1.2. Anticancer Activity of Monomers and Major Effective Components
T. mairei is rich in taxane compounds with anticancer effects [22]. Paclitaxel, as the most critical anticancer monomer in the taxane series of T. mairei, has been widely used in the treatment of various cancers since its approval by the FDA in December 1992, becoming a recognized effective broad-spectrum anticancer drug [23][24][25]. The IC50 of paclitaxel against tumor cells in vitro ranges from 2.5 to 7.5 nM [26]. Paclitaxel inhibits cancer cell division and proliferation by stabilizing microtubule structure and preventing the normal function of microtubules during mitosis. Additionally, paclitaxel can block the cell cycle in the G2/M phase, further inhibiting cancer cell growth [27][28].
In clinical applications, postoperative treatment of breast cancer patients with paclitaxel can effectively reduce recurrence and mortality rates, further confirming the significant effect of paclitaxel in breast cancer treatment [29]. In ovarian cancer treatment, paclitaxel is used as a second-line drug in dose-dense regimens for salvage therapy after relapse [30]. Approximately 80–85% of lung cancers are pathologically classified as non-small cell lung cancer (NSCLC). Paclitaxel, by interfering with microdynamics, is a first-line chemotherapeutic drug for treating advanced NSCLC [31]. These study results fully demonstrate the multifaceted role and clinical value of paclitaxel in treating different cancers.
Other T. mairei components, such as 10-DAB, Cephalomannine, etc., also possess good anticancer activity. 10-DAB is a crucial precursor compound in T. mairei, providing an essential intermediate for synthesizing paclitaxel-like drugs. Studies indicate that while 10-DAB has weak anticancer activity, it is indispensable in developing potent anticancer drugs such as paclitaxel and docetaxel as a precursor [32]. Cephalomannine, an alkaloid with anticancer activity, has an IC50 of 1.458–1.499 µg/mL [33][34] and is a derivative of the taxane diterpene class, mainly reported in Taxus species [34]. Research has explored the effect of Cephalomannine on lung cancer cells under hypoxic conditions, finding it inhibits lung cancer cell growth, reactive oxygen species (ROS) production, intracellular pH, and migration, as well as angiogenesis of HUVECs under hypoxic conditions by inhibiting the APEX1/HIF-1α interaction [33]. Additionally, Taxinine from T. mairei also has anticancer activity [35], with IC50 values against tumor cells A549, B16, and BEL7402 being 46.17, 350.64, and 113.97 µg/mL, respectively [21][36]. Studies have shown that Baccatin III has anti-tumor immunomodulatory activity at very low doses (0.05–0.5 mg/kg). Oral Baccatin III significantly reduced tumor growth induced by 4 T1 breast cancer or CT26 colon cancer cell transplantation in BALB/c mice by reducing tumor progression by inhibiting the accumulation and inhibition of MDSCs [37]. However, the monomer activity of the above components is weaker than paclitaxel, and they have not been developed into marketed anticancer drugs alone, mainly serving as intermediates for the synthesis of paclitaxel.
With more profound research into T. mairei, it has been found that taxanes are not the only components with anti-tumor activity; some polysaccharides, and among various compounds, flavonoids were specifically noted for their ability to inhibit cancer cell proliferation. These flavonoids show dose-dependent antiproliferative activities, effectively inducing apoptosis in cancer cells. Studies demonstrated that at concentrations ranging from 55.51 to 82.75 µg/mL, these flavonoids significantly inhibit the growth of human breast cancer MDA-MB-231 cells, underscoring the critical importance of dosage in their anti-tumor efficacy [38][39]. Taxus polysaccharides can inhibit S180 sarcoma, HepA liver cancer, and Lewis lung cancer, significantly improve mice’s hypoxia tolerance, enhance endurance, and increase survival rate [40]. Cultured human cervical cancer HeLa cells in the logarithmic growth phase were treated with different concentrations of Taxus polysaccharides (30,60,90,120 μg/L), resulting in a significant increase in cell proliferation inhibition rate and apoptosis rate compared to the control group, possibly related to downregulating Survivin, Bcl–2, and Caspase–3 expression and upregulating P53 expression [41]. Zhao’s study indicated that polysaccharides extracted from the fruits of T. mairei showed a 76.33% inhibition rate against S180 cells, with no toxicity to organs such as the liver, kidney, and heart [42]. The polysaccharide component PSY-1 can inhibit tumor growth in mouse models of S180 sarcoma, Lewis lung cancer, and HepA liver cancer, potentially related to inhibiting the expression of matrix metalloproteinases MMP-2 and MMP-9 and the phosphorylation of Iκβ [43]. Total flavonoids in T. mairei can enhance the inhibitory effect of paclitaxel on mouse breast cancer 4T1 and lung cancer A549 cells. Total polysaccharides can enhance the inhibitory effect of paclitaxel on breast cancer MCF-7 cells and mitigate the myelosuppressive effect of paclitaxel, with the most significant inhibitory effect on S180 sarcoma activity at a dose of 0.4 mL 66.6 mg/mL total polysaccharides, 0.4 mL 20 mg/mL total flavonoids, and 0.1 mL 1.25 mg/mL paclitaxel, with an inhibition rate of 38.86% [44]. Related research studies suggest that while T. mairei polysaccharides exert potent anti-tumor effects, their impact on non-cancerous, benign cells appears minimal, indicating a selective toxicity profile [45][46]. This selectivity is paramount for therapeutic agents to ensure efficacy against cancer cells while preserving the integrity and function of healthy tissues.
The T. mairei extracts, mainly when used in conjunction with other drugs, also demonstrate excellent anticancer effects. This is particularly the case with paclitaxel. The combination of paclitaxel and cisplatin shows significant effects against various cancers, and the combined chemotherapy of paclitaxel and carboplatin is a first-line cancer chemotherapy regimen for ovarian cancer. Liu and others treated 40 cases of ovarian cancer with a combination of paclitaxel and cisplatin, supplemented by comprehensive nursing interventions, achieving a total effective rate of 95% [47]. Another study involving 110 ovarian cancer patients treated with a combination of paclitaxel and cisplatin chemotherapy found significant therapeutic effects, with a total of 76 compelling cases, accounting for 98.70% [48]. In studies involving 40 lung cancer patients each, intravenous drip of 135 mg/m2 paclitaxel and 70 mg/m2 cisplatin on days 7 and 14 showed that the combination treatment had a significant effect on lung cancer, with higher rates of gastrointestinal reactions, leukopenia, thrombocytopenia, and bone marrow suppression in the treatment group compared to the control group [49]. The combination of paclitaxel and emodin has a synergistic inhibitory effect on the proliferation of A549 cells in vitro. Increasing the expression of Bax and active caspase three and reducing Bcl-2, p-Akt, and p-ERK levels significantly promotes ptx-induced apoptosis in A549 cells [50]. Research by Li and others found that the aqueous extract of T. mairei combined with paclitaxel can also inhibit the growth of human lung cancer A549 cells, downregulate the expression of Bcl-2 and Survivin genes, and upregulate Bax expression [51]. The aqueous extract of T. mairei used in combination with erlotinib enhances the effect of erlotinib, possibly through the downregulation of COX-2 and MMP-2 protein expression [52].
2.2. Antidiabetic and Antihypertensive Effects
Recent studies have revealed the significant potential of extracts from the leaves and twigs of T. mairei in regulating blood sugar, protecting organs, and their antioxidant properties. Research indicates that alcoholic extract and crude polysaccharides from T. mairei leaves and twigs can effectively inhibit weight loss, significantly reduce fasting blood glucose levels, regulate dyslipidemia, and protect the liver, kidney, and pancreas in diabetic rats. These components also improve glucose tolerance, demonstrating their potential for diabetes management. Different extract fractions have varied effects on blood sugar reduction in normal and insulin-resistant HepG2 cells, highlighting the importance of concentration in their efficacy. Further studies found that different extract fractions from the leaves and twigs of T. mairei have varying effects on reducing blood sugar. In normal HepG2 cells, the alcoholic extract and crude polysaccharides showed the best antidiabetic effects at 0.05 mg/mL. At the same time, the ethyl acetate and n-butanol fractions were most effective at a concentration of 0.01 mg/mL. For the HepG2 cell insulin resistance model, the n-butanol fraction and crude polysaccharides were most effective at reducing blood sugar at a concentration of 0.05 mg/mL, with ethyl acetate and alcoholic extracts being most effective at a concentration of 0.01 mg/mL [53].
Studies on the fruits of T. mairei also showed good antioxidant and anti-hyperglycemic activities and the potential for safety and bioactive components. The antioxidant activity of T. mairei fruit is good, with a DPPH radical scavenging rate of 82.1%, slightly lower than that of Vitamin C (96.04%) but still showing significant effects; its hydroxyl radical scavenging ability is lower than Vitamin C, with an EC50 of 1.306 mg/mL. Acute oral toxicity tests in mice indicated that the methanol extract of yew is safe [54].
Yang W X’s team extracted volatile components from fresh T. mairei leaves and tested them on a rat model of hypertension. By administering the extract orally at a dose of 5 mg/kg once daily for six weeks, it was found that the treatment significantly inhibited the increase in systolic blood pressure and plasma angiotensin II in rats. Although it did not significantly reduce blood triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C), it reduced total cholesterol (TC) and dose-dependently increased plasma NO levels [55].
2.3. Anti-Inflammatory Effects
T. mairei exhibits significant anti-inflammatory activity, notably due to its terpenoid content, which is recognized for its potent anti-inflammatory properties. These terpenoids function by inhibiting various upstream kinase signaling pathways (such as TLR, RAGE, TNFR, and IL-6R receptors), MAPK/p38/ERK/JAK signaling, HMGB-1 release, NF-κB activation, translocation, or reducing NF-κB DNA binding ability, thereby suppressing inflammation [56]. The NF-κB signaling pathway is crucial in regulating the expression of a wide array of cytokines involved in airway inflammation, remodeling, asthma, and other respiratory diseases [57].
In studies targeting specific components, the ethyl acetate fraction of the Southern Yew fruit showed a definite anti-inflammatory effect. Administering 200 mg/kg/day of this extract to twenty female SD rats for four weeks resulted in a significant decrease in the serum levels of IL-1β, IL-6, and IL-18 compared to the control group [58]. One of the polysaccharides from the Southern Yew (PTM) has been found to inhibit oxidative stress and apoptosis. Treating C57BL/6J mice modeled with Alzheimer’s disease (AD) with PTM (0.4 g/kg/day) for 14 days resulted in reduced levels of MDA and ROS, increased expression of NRF2, and improved cognitive functions, thus suggesting that PTM could reduce neurotoxicity and cognitive dysfunction [59]. The ethyl acetate extract of the Southern Yew fruit significantly lowered the serum levels of IL-1β, IL-6, and IL-18 after administration to SD rats at 200 mg/kg/day, demonstrating its anti-inflammatory effect [59]. The stems and leaves of the Southern Yew have certain analgesic and anti-inflammatory effects, with a clinical dose of 8 g/(case·day), equivalent to 0.13 g/kg/day (assuming a human body weight of 60 kg). Oral administration to Kunming mice at doses ranging from 0.133–0.667 g/kg/day resulted in inhibition of pain induced by thermal stimuli, with analgesic rates of 23.77–47.83%, and a reduction in the number of writhes induced by acetic acid in mice [60]. The carrageenan-induced rat paw edema model, a standard model for screening anti-inflammatory drugs, was used with an ethanol-percolated extract from the Southern Yew stems and leaves rich in terpenoids. This was prepared into a topical application for SD rats and ICR mice models. The study found that the Southern Yew ethanol extract inhibited paw swelling in rats at all tested doses, with the high dose group showing significant suppression of paw swelling, superior to the positive control drug, suggesting its potential development into a topical formulation for treating local swelling or arthritis [61].
Regarding the effects of individual components, one of the polysaccharides from the Southern Yew (PTM) used in treating C57BL/6J mice modeled with Alzheimer’s disease (AD) was found to lower MDA and ROS levels, increase NRF2 expression, and improve cognitive functions, showing its effectiveness in inhibiting oxidative stress and apoptosis [55]. Incubation with different extracts from the Southern Yew, including taxane and volatile oil components, could regulate the NF-κB signaling pathway, thus playing a role in inhibiting airway inflammation [62]. Taxifolin is a bioflavonoid which has been used to treat Inflammatory Bowel Disease. Taxifolin prevented the increase in serum aminotransferase activity during inflammation [63]. Treatment of mice with Taxifolin and fecal transplantation showed a lower diarrhea score, reduced colonic inflammation, and less mucosal damage, possibly related to increased levels of butyrate in fecal metabolites [64]. Amentoflavone, a biflavonoid naturally occurring compound, exhibits significant anti-inflammatory properties. It has demonstrated efficacy in mitigating pilocarpine-induced epileptic seizures in mouse kindling models by inhibiting nuclear factor-κB (NF-κB) activation and expression. This inhibition curtails the excessive firing of hippocampal neurons, thereby reducing the frequency and duration of epileptic episodes. Additionally, amentoflavone contributes to decreasing neuronal loss and apoptosis in the hippocampus, further underscoring its potential therapeutic benefits in epilepsy management [65]. Baccatin III, an essential precursor to paclitaxel, exhibits notable anti-inflammatory effects with reduced toxicity. The findings revealed that Baccatin III administration, in a dose-dependent manner, lessened inflammatory infiltration and the release of the pro-fibrotic mediator TGF-β1. It also decreased the accumulation of collagen and various extracellular matrix (ECM) components, such as alpha smooth muscle actin (α-SMA) and fibronectin [66].
2.4. Antimicrobial Effects
Extracts from T. mairei, specifically flavonoid-rich fractions, have demonstrated antimicrobial activities. Fifty-nine flavonoid compounds have been identified from T. mairei, demonstrating significant pharmacological activity [67]. A study exploring the antifungal effects of amentoflavone showed vigorous antifungal activity against several pathogenic fungi but low hemolytic activity against human red blood cells. As a stress response to the drug, amentoflavone induced trehalose accumulation inside Candida albicans cells and disrupted pseudohyphae formation during pathogenesis, showing potential as a lead compound for antifungal drug development [68]. Additionally, amentoflavone’s impact on inducing mitochondrial dysfunction and apoptotic cell death in Candida albicans has been investigated [69]. Ginkgetin, another flavonoid from these extracts, has been noted for its anticancer properties, including cell cycle arrest, apoptosis induction, autophagy stimulation, and interference with dysregulated pathways such as JAK/STAT and MAPKs [70]. Moreover, Quercetin 3-O-β-D-glucoside has been identified to inhibit HIV-RT activity with an IC50 value of 50 μmol/L, showcasing anti-HIV virus effects [71].
References
- Shao, F.; Wilson, I.W.; Qiu, D. The Research Progress of Taxol in Taxus. Curr. Pharm. Biotechnol. 2021, 22, 360–366.
- Hao, D.C.; Xiao, P.G.; Peng, Y.; Liu, M.; Huo, L. Research progress and trend analysis of biology and chemistry of Taxus medicinal resources. Yao Xue Xue Bao 2012, 47, 827–835.
- Nižnanský, Ľ.; Osinová, D.; Kuruc, R.; Hengerics Szabó, A.; Szórádová, A.; Masár, M.; Nižnanská, Ž. Natural Taxanes: From Plant Composition to Human Pharmacology and Toxicity. Int. J. Mol. Sci. 2022, 23, 15619.
- Truus, K.; Vaher, M.; Borissova, M.; Robal, M.; Levandi, T.; Tuvikene, R.; Toomike, P.; Kaljurand, M. Characterization of yew tree (Taxus) varieties by fingerprint and principal component analyses. Nat. Prod. Commun. 2012, 7, 1143–1146.
- Gallego-Jara, J.; Lozano-Terol, G.; Sola-Martínez, R.A.; Cánovas-Díaz, M.; de Diego Puente, T. A Compressive Review about Taxol(®): History and Future Challenges. Molecules 2020, 25, 5986.
- Tan, L.; Chen, Z. Taxus resources in China. J. Northwest For. Coll. 2006, 21, 5. (In Chinese)
- Fang, C. Content Determination method and Time variation of the Year of Taxanes, Flavonoids and Polysaccharide in the Needles of T. mairei; Xinjiang Agricultural University: Urumchi, China, 2014.
- Wang, Z.; Peng, H.; Li, D. Flora of China; Germplasm Bank of Wild Life in Southwest China: Kunming, China, 2004; Volume 24, p. 4.
- Wen, Y.; Xie, W.; Han, W.; Zhou, H.; Cao, J.; Chen, G. Resource status and distribution characteristics of Taxus chinensis in southern Nanling Mountains. J. Cent. South Univ. For. Technol. 2012, 32, 5. (In Chinese)
- Wu, N.; Guo, M.; Zhang, T.; Shu, F.; You, J.; Xu, Y.; Liu, J. Status Quo of Species Resources and Develop ment Countermeasures in Taxus wallichiana var. mairei Nature Reserve e of Ruyuan, Guangdong. Cent. South For. Inventory Plan. 2022, 41, 48–52.
- Fei, Y.; Zhou, Y.; Yong, L.; Shen, J.; Qi, R. A New Forma of the Genus Taxus L. from Hubei, China. Acta Bot. Boreali Occident. Sin. 2016, 36, 1707–1709. (In Chinese)
- Jing, Z. Construction of Administrative Talents in Zhejiang Food and Drug Administration. Chin. Pharm. Aff. 2016.
- Wang, K.; Pei, J.; Xie, C.; Dai, F.; Sun, X. Taxus chinensis var. mairei (AETC) Inhibiting HER2 Positive Human Gastric Cancer Cells NCI–N87 Transplantation Tumor and Inducing Apoptosis. Chin. Arch. Tradit. Chin. Med. 2016, 34, 5. (In Chinese)
- Dai, S.; Liu, Y.; Zhao, F.; Wang, H.; Shao, T.; Xu, Z.; Shou, L.; Chen, S.; Zhang, G.C.; Shu, Q. Aqueous extract of Taxus chinensis var. mairei targeting CD47 enhanced antitumor effects in non-small cell lung cancer. Biomed. Pharmacother. 2022, 154, 113628.
- Cui, Q.L.; Shao, M.; Shu, Q.J. Study on inhibitory effect of aqueous extract of Taxus chinensis var. mairei on growth of A549 lung cancer xenografts in nude mice and its mechanism. Zhongguo Zhong Yao Za Zhi 2013, 38, 3549–3553.
- Cui, Q.L.; Ye, P.; Shu, Q.J.; Shao, M. Study on Inhibitory Effect of Aqueous Extract of Taxus chinensis var. mairei Combined Erlotnib on A549 Xenograft in Nude Mice and Its Mechanism. Zhongguo Zhong Xi Yi Jie He Za Zhi 2015, 35, 572–577.
- Zhang, J.; Shu, Q.-J.; Gao, J.-L.; Zhang, L. Study on Inhibitory Effects of Taxus chinensis var. mairei Aqueous Extract on the Proliferation of Tumor Cells. Chin. J. Integr. Tradit. West. Med. 2013, 33, 0805–0809. (In Chinese)
- Zhang, G.; Dai, S.; Chen, Y.; Wang, H.; Chen, T.; Shu, Q.; Chen, S.; Shou, L.; Cai, X. Aqueous extract of Taxus chinensis var. mairei regulates the Hippo-YAP pathway and promotes apoptosis of non-small cell lung cancer via ATF3 in vivo and in vitro. Biomed. Pharmacother. 2021, 138, 111506.
- Sun, L.; Ding, S.; Luo, Q.; Wang, P.; Yang, X.; Wu, L.; Chen, Y.; Zheng, X.; Zhang, H.; Yuan, L.; et al. Taxus wallichiana var. chinensis (Pilg.) Florin Aqueous Extract Suppresses the Proliferation and Metastasis in Lung Carcinoma via JAK/STAT3 Signaling Pathway. Front. Pharmacol. 2021, 12, 736442.
- Shu, Q.J.; Li, P.; Wang, B.B. Experimental study on apoptosis induced by aqueous extract of Taxus chinensis in human pulmonary carcinoma cell A549 and its molecular mechanisms. Zhongguo Zhong Xi Yi Jie He Za Zhi 2011, 31, 1243–1247.
- Bingyi, W. Study on Effective Components of Taxus chinensis; Suzhou University: Suzhou, China, 2013.
- Zhang, S.; Lu, X.; Zheng, T.; Guo, X.; Tang, Z. Investigation of bioactivities of Taxus chinensis, Taxus cuspidata, and Taxus × media by gas chromatography-mass spectrometry. Open Life Sci. 2021, 16, 287–296.
- Yang, C.H.; Horwitz, S.B. Taxol(®): The First Microtubule Stabilizing Agent. Int. J. Mol. Sci. 2017, 18, 1733.
- Kingston, D.G. Taxol: The chemistry and structure-activity relationships of a novel anticancer agent. Trends Biotechnol. 1994, 12, 222–227.
- Guchelaar, H.J.; Ten Napel, C.H.; de Vries, E.G.; Mulder, N.H. Clinical, toxicological and pharmaceutical aspects of the antineoplastic drug taxol: A review. Clin. Oncol. R. Coll. Radiol. 1994, 6, 40–48.
- Liebmann, J.E.; Cook, J.A.; Lipschultz, C.; Teague, D.; Fisher, J.; Mitchell, J.B. Cytotoxic studies of paclitaxel (Taxol) in human tumour cell lines. Br. J. Cancer 1993, 68, 1104–1109.
- Weaver, B.A. How Taxol/paclitaxel kills cancer cells. Mol. Biol. Cell 2014, 25, 2677–2681.
- Zhu, L.; Chen, L. Progress in research on paclitaxel and tumor immunotherapy. Cell. Mol. Biol. Lett. 2019, 24, 40.
- Yan, C. Ertraction Technology Optimization and Content Detemination of Taxol in the Braches and Leaves of Taxus madia; Nanchang University: Nanchang, China, 2015.
- Joly, F.; Hilpert, F.; Okamoto, A.; Stuart, G.; Ochiai, K.; Friedlander, M. Fifth Ovarian Cancer Consensus Conference of the Gynecologic Cancer InterGroup: Recommendations on incorporating patient-reported outcomes in clinical trials in epithelial ovarian cancer. Eur. J. Cancer 2017, 78, 133–138.
- Xu, X.; Jin, S.; Ma, Y.; Fan, Z.; Yan, Z.; Li, W.; Song, Q.; You, W.; Lyu, Z.; Song, Y.; et al. miR-30a-5p enhances paclitaxel sensitivity in non-small cell lung cancer through targeting BCL-2 expression. J. Mol. Med. 2017, 95, 861–871.
- Sun, C. Study on Dynamic Changes of Biomass and 10-DAB Content of Taxus media var CL; Sichuan Agricultural University: Yaan, China, 2017.
- Ullah, A.; Leong, S.W.; Wang, J.; Wu, Q.; Ghauri, M.A.; Sarwar, A.; Su, Q.; Zhang, Y. Cephalomannine inhibits hypoxia-induced cellular function via the suppression of APEX1/HIF-1α interaction in lung cancer. Cell Death Dis. 2021, 12, 490.
- Helson, L. Cephalomannine and 10-deacetyltaxol cytotoxicity in human glial and neuroblastoma cell-lines. Int. J. Oncol. 1993, 2, 297–299.
- Hosoyama, H.; Shigemori, H.; Tomida, A.; Tsuruo, T.; Kobayashi, J. Modulation of multidrug resistance in tumor cells by taxinine derivatives. Bioorg. Med. Chem. Lett. 1999, 9, 389–394.
- Zhang, S.; Wang, J.; Hirose, K.; Ando, M. An efficient conversion of taxinine to taxinine NN-1, an anticancer agent and a modulator of multidrug-resistant tumor cells. J. Nat. Prod. 2002, 65, 1786–1792.
- Lee, Y.H.; Lee, Y.R.; Park, C.S.; Im, S.A.; Song, S.; Hong, J.T.; Whang, B.Y.; Kim, K.; Lee, C.K. Baccatin III, a precursor for the semisynthesis of paclitaxel, inhibits the accumulation and suppressive activity of myeloid-derived suppressor cells in tumor-bearing mice. Int. Immunopharmacol. 2014, 21, 487–493.
- Leong, C.N.; Tako, M.; Hanashiro, I.; Tamaki, H. Antioxidant flavonoid glycosides from the leaves of Ficus pumila L. Food Chem. 2008, 109, 415–420.
- Zhang, H.W.; Hu, J.J.; Fu, R.Q.; Liu, X.; Zhang, Y.H.; Li, J.; Liu, L.; Li, Y.N.; Deng, Q.; Luo, Q.S.; et al. Flavonoids inhibit cell proliferation and induce apoptosis and autophagy through downregulation of PI3Kγ mediated PI3K/AKT/mTOR/p70S6K/ULK signaling pathway in human breast cancer cells. Sci. Rep. 2018, 8, 11255.
- Xie, Z.; Du, L.; Li, X.; Xiong, Y. New progress in the study of Taxus australis. Chin. Pharm. Ind. 2009, 18, 3–5. (In Chinese)
- Yan, S.; Jie, Y. Effects of Taxus Chinensis Polysaccharide on Proliferation and Apoptosis of Cervical Cancer Cells. Shenzhen J. Integr. Tradit. Chin. West. Med. 2021, 31, 3. (In Chinese)
- Zhao, C.; Li, Z.; Li, C.; Yang, L.; Yao, L.; Fu, Y.; He, X.; Shi, K.; Lu, Z. Optimized extraction of polysaccharides from Taxus chinensis var. mairei fruits and its antitumor activity. Int. J. Biol. Macromol. 2015, 75, 192–198.
- Cai, W.; Yu, H.; Luo, Y.; Liu, S. Study on the mechanism of Taxus chinensis polysaccharides enhancing paclitaxel on tumor inhibition in S180 tumor-bearing mice. Mod. Chin. Med. Res. Pract. 2021, 035, 38–42. (In Chinese)
- Cai, W.; Xia, M.; Xiong, Y. Study on Enhancing Efficacy and Reducing Toxicity of Total Flavonoids, Total Polysaccharides in Taxus mairei Compatibility with Taxol. Mod. Tradit. Chin. Med. Mater. Med. World Sci. Technol. 2015, 17, 556–562.
- Ranjitkar, S.; Zhang, D.; Sun, F.; Salman, S.; He, W.; Venkitanarayanan, K.; Tulman, E.R.; Tian, X. Cytotoxic effects on cancerous and non-cancerous cells of trans-cinnamaldehyde, carvacrol, and eugenol. Sci. Rep. 2021, 11, 16281.
- Yu, J.; Sun, R.; Zhao, Z.; Wang, Y. Auricularia polytricha polysaccharides induce cell cycle arrest and apoptosis in human lung cancer A549 cells. Int. J. Biol. Macromol. Struct. Funct. Interact. 2014, 68, 67–71.
- Liu, L.; Zhang, W.; Wang, Y.; Zhang, C. Clinical nursing of ovarian cancer treated by paclitaxel combined with cisplatin. Chin. Foreign Womens Health Res. 2020, 1. 53+55 (In Chinese)
- Ming, N. Clinical nursing observation of paclitaxel combined with cisplatin in treatment of ovarian cancer. Chin. J. Mod. Drug Appl. 2014, 8, 2. (In Chinese)
- Lida, F.; Shang, W.; Lizhi, J. Clinical effect of paclitaxel combined with cisplatin concurrent radiotherapy and chemotherapy in the treatment of advanced non-small cell lung cancer. Clin. Med. Res. Pract. 2020, 5, 2. (In Chinese)
- Chen, S.; Zhang, Z.; Zhang, J. Emodin enhances antitumor effect of paclitaxel on human non-small-cell lung cancer cells in vitro and in vivo. Drug Des. Devel. Ther. 2019, 13, 1145–1153.
- Liu, C.; Cao, J. Research progress of water extracts of Taxus australis in tumor. Zhejiang Clin. Med. 2019, 21, 1729–1731. (In Chinese)
- Shang, S.; Shu, Q. Effects of Taxus chinensis aqueous extract and erlotinib on expression of COX-2 and MMP-2 in human lung cancer A549 cells. J. Xinjiang Med. Univ. 2013, 36, 789–792. (In Chinese)
- Bin, T. Study on Material Basis of Antidiabetic Effect of Branches and Leaves of Southern Taxus chinensis; Nanjing University of Traditional Chinese Medicine: Nanjing, China, 2014.
- Li, N.; Pan, Z.; Zhang, D.; Wang, H.X.; Yu, B.; Zhao, S.P.; Guo, J.J.; Wang, J.W.; Yao, L.; Cao, W.G. Chemical Components, Biological Activities, and Toxicological Evaluation of the Fruit (Aril) of Two Precious Plant Species from Genus Taxus. Chem. Biodivers. 2017, 14, e1700305.
- Yang, W.X.; Zhao, Z.G.; Wang, L.H.; Yu, S.J.; Liang, Z.S. Control of hypertension in rats using volatile components of leaves of Taxus chinensis var. mairei. J. Ethnopharmacol. 2012, 141, 309–313.
- Kumari, N.; Anand, S.; Shah, K.; Chauhan, N.S.; Sethiya, N.K.; Singhal, M. Emerging Role of Plant-Based Bioactive Compounds as Therapeutics in Parkinson’s Disease. Molecules 2023, 28, 7588.
- Prasher, P.; Sharma, M.; Chellappan, D.K.; Gupta, G.; Jha, N.K.; Singh, S.K.; MacLoughlin, R.; Terezinha, J.A.P.; Löbenberg, R.; Dua, K. Advanced drug delivery systems targeting NF-κB in respiratory diseases. Future Med. Chem. 2021, 13, 1087–1090.
- Huang, W.; Zhang, F.; Zhou, C.; Chen, M.; Yang, C. Effects of ethyl acetate extract of Taxus chinensis fruit on phenotype and inflammatory cytokines in rats with depression. World Chin. Med. 2023, 1–5. (In Chinese)
- Zhang, S.; Li, L.; Hu, J.; Ma, P.; Zhu, H. Polysaccharide of Taxus chinensis var. mairei Cheng et L.K.Fu attenuates neurotoxicity and cognitive dysfunction in mice with Alzheimer’s disease. Pharm. Biol. 2020, 58, 959–968.
- Tong, L.; Jiang, Y.; Guo, W.; Zhu, J. Experimental study on analgesic effect of stems and leaves of Taxus chinensis. Zhejiang Clin. Med. J. 2008, 10, 439–440. (In Chinese)
- Lou, P.; Xia, A.; Ying, Y. Experimental study on analgesic and anti-inflammatory effects of stem and leaf extracts of Taxus australis. Zhejiang J. Tradit. Chin. Med. 2015, 2, 556–567. (In Chinese)
- Wang, N.; Cai, T.; Tong, Y.; Liu, X.; Zhu, W.; Jiang, S.; Zhao, G. Study on the selection of anti-airway inflammation active ingredients in Taxus chinensis by HTRF method based on NF-κB signaling pathway. Zhejiang J. Tradit. Chin. Med. 2023, 58, 553–556. (In Chinese)
- Gupta, M.B.; Bhalla, T.N.; Gupta, G.P.; Mitra, C.R.; Bhargava, K.P. Anti-inflammatory activity of taxifolin. Jpn. J. Pharmacol. 1971, 21, 377–382.
- Li, W.; Zhang, L.; Xu, Q.; Yang, W.; Zhao, J.; Ren, Y.; Yu, Z.; Ma, L. Taxifolin Alleviates DSS-Induced Ulcerative Colitis by Acting on Gut Microbiome to Produce Butyric Acid. Nutrients 2022, 14, 1069.
- Zhang, Z.; Sun, T.; Niu, J.G.; He, Z.Q.; Liu, Y.; Wang, F. Amentoflavone protects hippocampal neurons: Anti-inflammatory, antioxidative, and antiapoptotic effects. Neural Regen. Res. 2015, 10, 1125–1133.
- Nie, Y.; Zhang, D.; Qian, F.; Wu, Y. Baccatin III ameliorates bleomycin-induced pulmonary fibrosis via suppression of TGF-β1 production and TGF-β1-induced fibroblast differentiation. Int. Immunopharmacol. 2019, 74, 105696.
- Wei, Q.; Li, Q.Z.; Wang, R.L. Flavonoid Components, Distribution, and Biological Activities in Taxus: A review. Molecules 2023, 28, 1713.
- Jung, H.J.; Sung, W.S.; Yeo, S.H.; Kim, H.S.; Lee, I.S.; Woo, E.R.; Lee, D.G. Antifungal effect of amentoflavone derived from Selaginella tamariscina. Arch. Pharm. Res. 2006, 29, 746–751.
- Hwang, I.S.; Lee, J.; Jin, H.G.; Woo, E.R.; Lee, D.G. Amentoflavone stimulates mitochondrial dysfunction and induces apoptotic cell death in Candida albicans. Mycopathologia 2012, 173, 207–218.
- Lou, J.S.; Zhao, L.P.; Huang, Z.H.; Chen, X.Y.; Xu, J.T.; Tai, W.C.; Tsim, K.W.K.; Chen, Y.T.; Xie, T. Ginkgetin derived from Ginkgo biloba leaves enhances the therapeutic effect of cisplatin via ferroptosis-mediated disruption of the Nrf2/HO-1 axis in EGFR wild-type non-small-cell lung cancer. Phytomedicine 2021, 80, 153370.
- Matsuse, I.T.; Lim, Y.A.; Hattori, M.; Correa, M.; Gupta, M.P. A search for anti-viral properties in Panamanian medicinal plants: The effects on HIV and its essential enzymes. J. Ethnopharmacol. 1998, 64, 15–22.
More
Information
Subjects:
Pharmacology & Pharmacy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
535
Revisions:
2 times
(View History)
Update Date:
14 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




