
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alberto Kousuke De la Herrán-Arita | -- | 2271 | 2024-03-12 05:23:40 | | | |
| 2 | Catherine Yang | Meta information modification | 2271 | 2024-03-12 06:13:19 | | |
Video Upload Options
The endocannabinoid system (ECS), comprising endogenous cannabinoids, receptors, and enzymes, has emerged as a critical modulator of sleep patterns, influencing both the initiation and maintenance of sleep. Concurrently and once considered tolerant support cells, glial cells are now recognized as active contributors to neuroinflammatory processes and synaptic regulation. The intricate relationships between the ECS and glia-mediated immune responses in the context of sleep regulation present a novel avenue for understanding the broader implications of disrupted sleep on neural health. The bidirectional communication within the ECS–Glia Axis intersects with sleep regulation, creating a dynamic relationship between neuroinflammation and sleep–wake patterns. Sleep disturbances often coincide with increased neuroinflammation, and chronic activation of the ECS–Glia Axis may contribute to disrupted sleep. Conversely, alterations in sleep architecture influence the activity of the ECS–Glia Axis, suggesting a reciprocal modulation.
1. Endocannabinoid Regulation of Glial Function
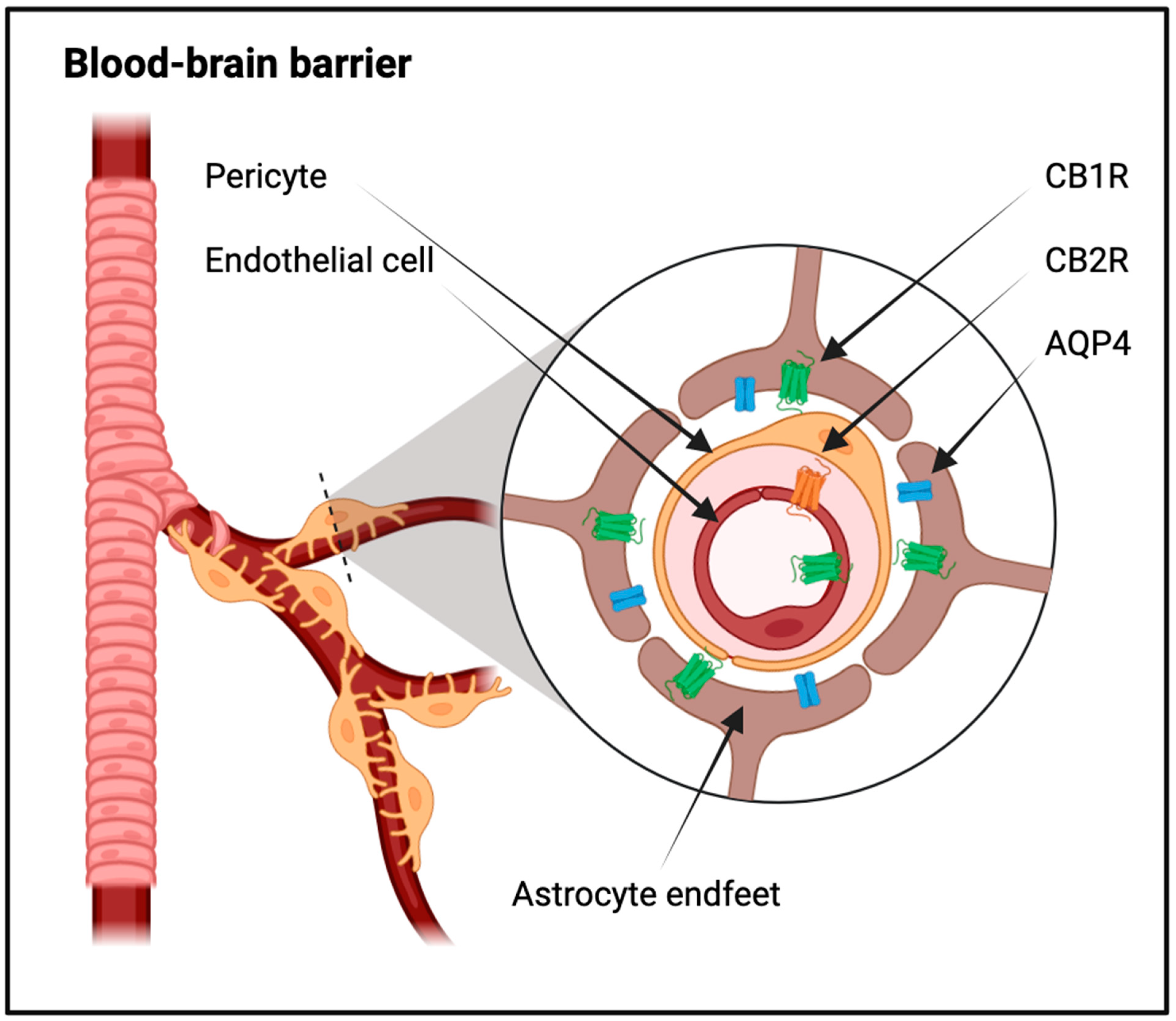
2. Endocannabinoid Regulation of the Central Nervous System Fluid Dynamics
3. Glial Regulation of Endocannabinoid Function
4. Sleep, Synaptic Plasticity, and the Endocannabinoid System in Memory Consolidation
References
- Navarrete, M.; Díez, A.; Araque, A. Astrocytes in endocannabinoid signalling. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130599.
- Covelo, A.; Eraso-Pichot, A.; Fernández-Moncada, I.; Serrat, R.; Marsicano, G. CB1R-dependent regulation of astrocyte physiology and astrocyte-neuron interactions. Neuropharmacology 2021, 195, 108678.
- Krishnan, K.S.; Billups, B. ASC Transporters Mediate D-Serine Transport into Astrocytes Adjacent to Synapses in the Mouse Brain. Biomolecules 2023, 13, 819.
- Beltrán-Castillo, S.; Olivares, M.J.; Contreras, R.A.; Zúñiga, G.; Llona, I.; von Bernhardi, R.; Eugenín, J.L. D-serine released by astrocytes in brainstem regulates breathing response to CO2 levels. Nat. Commun. 2017, 8, 838.
- Neame, S.; Safory, H.; Radzishevsky, I.; Touitou, A.; Marchesani, F.; Marchetti, M.; Kellner, S.; Berlin, S.; Foltyn, V.N.; Engelender, S.; et al. The NMDA receptor activation by d-serine and glycine is controlled by an astrocytic Phgdh-dependent serine shuttle. Proc. Natl. Acad. Sci. USA 2019, 116, 20736–20742.
- Romero-Sandoval, E.A.; Horvath, R.; Landry, R.P.; DeLeo, J.A. Cannabinoid Receptor Type 2 Activation Induces a Microglial Anti-Inflammatory Phenotype and Reduces Migration via MKP Induction and ERK Dephosphorylation. Mol. Pain 2009, 5, 25.
- Garland, E.F.; Hartnell, I.J.; Boche, D. Microglia and Astrocyte Function and Communication: What Do We Know in Humans? Front. Neurosci. 2022, 16, 824888.
- Komorowska-Müller, J.A.; Schmöle, A.-C. CB2 Receptor in Microglia: The Guardian of Self-Control. Int. J. Mol. Sci. 2020, 22, 19.
- Young, A.P.; Denovan-Wright, E.M. The Dynamic Role of Microglia and the Endocannabinoid System in Neuroinflammation. Front. Pharmacol. 2022, 12, 806417.
- Olabiyi, B.F.; Schmoele, A.-C.; Beins, E.C.; Zimmer, A. Pharmacological blockade of cannabinoid receptor 2 signaling does not affect LPS/IFN-γ-induced microglial activation. Sci. Rep. 2023, 13, 11105.
- Sánchez-de la Torre, A.; Aguado, T.; Huerga-Gómez, A.; Santamaría, S.; Gentile, A.; Chara, J.C.; Matute, C.; Monory, K.; Mato, S.; Guzmán, M.; et al. Cannabinoid CB1 receptor gene inactivation in oligodendrocyte precursors disrupts oligodendrogenesis and myelination in mice. Cell Death Dis. 2022, 13, 585.
- Tomas-Roig, J.; Wirths, O.; Salinas-Riester, G.; Havemann-Reinecke, U. The Cannabinoid CB1/CB2 Agonist WIN55212.2 Promotes Oligodendrocyte Differentiation In Vitro and Neuroprotection During the Cuprizone-Induced Central Nervous System Demyelination. CNS Neurosci. Ther. 2016, 22, 387–395.
- Valeri, A.; Mazzon, E. Cannabinoids and Neurogenesis: The Promised Solution for Neurodegeneration? Molecules 2021, 26, 6313.
- Galea, I. The blood–brain barrier in systemic infection and inflammation. Cell. Mol. Immunol. 2021, 18, 2489–2501.
- Shao, F.; Wang, X.; Wu, H.; Wu, Q.; Zhang, J. Microglia and Neuroinflammation: Crucial Pathological Mechanisms in Traumatic Brain Injury-Induced Neurodegeneration. Front. Aging Neurosci. 2022, 14, 825086.
- Muzio, L.; Viotti, A.; Martino, G. Microglia in Neuroinflammation and Neurodegeneration: From Understanding to Therapy. Front. Neurosci. 2021, 15, 742065.
- Hagan, K.; Varelas, P.; Zheng, H. Endocannabinoid System of the Blood–Brain Barrier: Current Understandings and Therapeutic Potentials. Cannabis Cannabinoid Res. 2021, 7, 561–568.
- Adermark, L.; Stomberg, R.; Söderpalm, B.; Ericson, M. Astrocytic Regulation of Endocannabinoid-Dependent Synaptic Plasticity in the Dorsolateral Striatum. Int. J. Mol. Sci. 2024, 25, 581.
- Vicente-Acosta, A.; Ceprian, M.; Sobrino, P.; Pazos, M.R.; Loría, F. Cannabinoids as Glial Cell Modulators in Ischemic Stroke: Implications for Neuroprotection. Front. Pharmacol. 2022, 13, 888222.
- Gaffuri, A.-L.; Ladarre, D.; Lenkei, Z. Type-1 Cannabinoid Receptor Signaling in Neuronal Development. Pharmacology 2012, 90, 19–39.
- Rom, S.; Zuluaga-Ramirez, V.; Dykstra, H.; Reichenbach, N.L.; Pacher, P.; Persidsky, Y. Selective Activation of Cannabinoid Receptor 2 in Leukocytes Suppresses Their Engagement of the Brain Endothelium and Protects the Blood-Brain Barrier. Am. J. Pathol. 2013, 183, 1548–1558.
- Vendel, E.; de Lange, E.C.M. Functions of the CB1 and CB2 receptors in neuroprotection at the level of the blood-brain barrier. Neuromolecular Med. 2014, 16, 620–642.
- Jing, N.; Fang, B.; Li, Z.; Tian, A. Exogenous activation of cannabinoid-2 receptor modulates TLR4/MMP9 expression in a spinal cord ischemia reperfusion rat model. J. Neuroinflamm. 2020, 17, 101.
- D’Souza, D.C.; Pittman, B.; Perry, E.; Simen, A. Preliminary evidence of cannabinoid effects on brain-derived neurotrophic factor (BDNF) levels in humans. Psychopharmacology 2008, 202, 569–578.
- Maglio, L.E.; Noriega-Prieto, J.A.; Maraver, M.J.; Fernández, D. Endocannabinoid-Dependent Long-Term Potentiation of Synaptic Transmission at Rat Barrel Cortex. Cereb. Cortex 2017, 28, 1568–1581.
- Moreno-García, Á.; Bernal-Chico, A.; Colomer, T.; Rodríguez-Antigüedad, A.; Matute, C.; Mato, S. Gene Expression Analysis of Astrocyte and Microglia Endocannabinoid Signaling during Autoimmune Demyelination. Biomolecules 2020, 10, 1228.
- Bedse, G.; Bluett, R.J.; Patrick, T.A.; Romness, N.K.; Gaulden, A.D.; Kingsley, P.J.; Plath, N.; Marnett, L.J.; Patel, S. Therapeutic endocannabinoid augmentation for mood and anxiety disorders: Comparative profiling of FAAH, MAGL and dual inhibitors. Transl. Psychiatry 2018, 8, 92.
- Eraso-Pichot, A.; Pouvreau, S.; Olivera-Pinto, A.; Gomez-Sotres, P.; Skupio, U.; Marsicano, G. Endocannabinoid signaling in astrocytes. Glia 2022, 71, 44–59.
- Ferré, S.; Lluís, C.; Justinova, Z.; Quiroz, C.; Orru, M.; Navarro, G.; Canela, E.I.; Franco, R.; Goldberg, S.R. Adenosine-cannabinoid receptor interactions. Implications for striatal function. Br. J. Pharmacol. 2010, 160, 443–453.
- Chang, C.-P.; Wu, K.-C.; Lin, C.-Y.; Chern, Y. Emerging roles of dysregulated adenosine homeostasis in brain disorders with a specific focus on neurodegenerative diseases. J. Biomed. Sci. 2021, 28, 70.
- Reichert, C.F.; Deboer, T.; Landolt, H. Adenosine, caffeine, and sleep–wake regulation: State of the science and perspectives. J. Sleep Res. 2022, 31, e13597.
- Huang, Z.-L.; Zhang, Z.; Qu, W.-M. Roles of Adenosine and Its Receptors in Sleep–Wake Regulation. Int. Rev. Neurobiol. 2014, 119, 349–371.
- Ditmer, M.; Gabryelska, A.; Turkiewicz, S.; Sochal, M. Investigating the Role of BDNF in Insomnia: Current Insights. Nat. Sci. Sleep 2023, 15, 1045–1060.
- Diógenes, M.J.; Costenla, A.R.; Lopes, L.V.; Jerónimo-Santos, A.; Sousa, V.C.; Fontinha, B.M.; Ribeiro, J.A.; Sebastião, A.M. Enhancement of LTP in Aged Rats is Dependent on Endogenous BDNF. Neuropsychopharmacology 2011, 36, 1823–1836.
- Lu, Y.; Christian, K.; Lu, B. BDNF: A key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiol. Learn. Mem. 2008, 89, 312–323.
- Miao, H.-H.; Miao, Z.; Pan, J.-G.; Li, X.-H.; Zhuo, M. Brain-derived neurotrophic factor produced long-term synaptic enhancement in the anterior cingulate cortex of adult mice. Mol. Brain 2021, 14, 119.




