The endocannabinoid system (ECS), comprising endogenous cannabinoids, receptors, and enzymes, has emerged as a critical modulator of sleep patterns, influencing both the initiation and maintenance of sleep. Concurrently and once considered tolerant support cells, glial cells are now recognized as active contributors to neuroinflammatory processes and synaptic regulation. The intricate relationships between the ECS and glia-mediated immune responses in the context of sleep regulation present a novel avenue for understanding the broader implications of disrupted sleep on neural health. The bidirectional communication within the ECS–Glia Axis intersects with sleep regulation, creating a dynamic relationship between neuroinflammation and sleep–wake patterns. Sleep disturbances often coincide with increased neuroinflammation, and chronic activation of the ECS–Glia Axis may contribute to disrupted sleep. Conversely, alterations in sleep architecture influence the activity of the ECS–Glia Axis, suggesting a reciprocal modulation.
1. Endocannabinoid Regulation of Glial Function
The nervous system, once viewed solely through the lens of neurons, now reveals a complex symphony where glial cells and the ECS emerge as essential players. Endocannabinoid signaling is deeply intertwined with glial cell activity.
Astrocytes, with their fine processes ensheathing synapses, express both CB1R and CB2R. Activation of CB1R on astrocytes modulates neurotransmitter release and synaptic plasticity, contributing to the fine-tuning of neural networks. Upon activation of CB1R on astrocytes, a cascade of intracellular events is initiated. The primary consequence is the modulation of neurotransmitter release from neighboring neurons. Astrocytes, in response to endocannabinoid signaling, regulate the release of neurotransmitters such as glutamate and GABA. This modulation occurs through the control of calcium signaling and exocytosis machinery within astrocytic processes. The activation of CB1R on astrocytes also exerts profound effects on synaptic plasticity, a fundamental mechanism underlying learning and memory. Astrocytes, through the release of gliotransmitters, influence synaptic strength and contribute to the dynamic regulation of neural circuits. The release of factors such as ATP, D-serine, and prostaglandins by astrocytes modulates the efficacy of synaptic transmission and the induction of LTP or LTD. The orchestrated effects of CB1R activation on astrocytes collectively contribute to the fine-tuning of neural networks. By modulating both neurotransmitter release and synaptic plasticity, astrocytes actively shape the dynamics of neural circuits. This fine-tuning is essential for maintaining homeostasis in the CNS, optimizing signal transmission, and ensuring the appropriate processing of information within neural networks. While CB1R dominate the discussion of astrocytic modulation, the expression of CB2R on astrocytes introduces an additional layer of complexity. CB2R, traditionally associated with immune modulation, may play a role in astrocytic responses to inflammatory stimuli or pathological conditions. Further research is needed to elucidate the specific contributions of CB2R in astrocytic function and their potential impact on neural network dynamics
[1][2][3][4][5][130,131,132,133,134] (
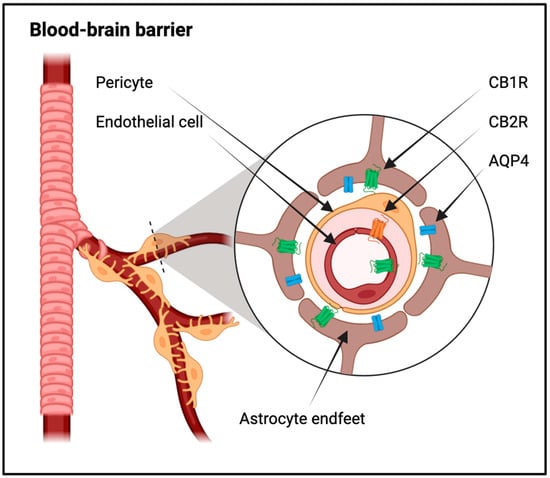
Figure 12).
Figure 12. Schematic representation of the blood–brain barrier. The Blood–Brain Barrier (BBB) is a pivotal interface regulating molecular exchange between the bloodstream and the brain parenchyma. The blood vessel, portrayed as a capillary, is defined by endothelial cells forming its walls. The endothelial cells are accentuated to underscore the presence of tight junctions, crucial components restricting the free movement of macromolecules. These tight junctions between adjacent endothelial cells constitute the primary foundation of the BBB, ensuring a highly selective barrier. BBB endothelial cells are further highlighted, with an emphasis on the luminal and abluminal sides. On the luminal side, CB1 receptors (CB1R) are depicted, illustrating their strategic location facing the bloodstream. Conversely, CB2 receptors (CB2R) are situated on the abluminal side, engaging with the brain parenchyma. This spatial distribution of cannabinoid receptors signifies their distinct roles in modulating molecular transport and signaling processes at the BBB. The perivascular space, a microenvironment situated between endothelial cells, is portrayed as a conduit for controlled diffusion. This space allows fluid and solutes to move from the bloodstream into the brain parenchyma, contributing to the dynamic exchange of substances. Astrocytic endfeet extend from astrocytes toward the blood vessel, forming a close association with endothelial cells. These endfeet express Aquaporin-4 (AQP4) channels, facilitating water transport, and CB1 receptors (CB1R), indicating their active involvement in regulatory processes. This sophisticated interaction between endothelial cells and astrocytic endfeet showcases the collaborative efforts in maintaining BBB integrity.
Microglia, the immune cells of the CNS, express CB2R, suggesting a role for endocannabinoids in immune modulation. In response to neuronal injury or inflammation, microglia become activated, and the ECS acts as a regulator of this immune response, influencing the release of pro-inflammatory and anti-inflammatory mediators (
Figure 12). Upon activation in response to neuronal injury or inflammation, microglia undergo morphological and functional changes, transitioning to an activated state. The activation of CB2R on microglia serves as a crucial regulatory mechanism in shaping the ensuing immune response. The ECS, acting through CB2R, modulates the release of pro-inflammatory and anti-inflammatory mediators, influencing the overall immune milieu in the CNS. CB2R activation has been shown to dampen the release of pro-inflammatory cytokines, such as TNF-α and IL-1β, while concurrently promoting the release of anti-inflammatory mediators, including IL-10 and TGF-β. This delicate balance orchestrated by the ECS contributes to the resolution of inflammation and the restoration of neural homeostasis. In situations of neuronal injury or inflammation, microglial activation is a key component of the CNS’s defense and repair mechanisms. The ECS, through CB2R signaling, modulates the intensity and duration of this microglial response. By influencing the release of pro-inflammatory mediators, the ECS contributes to the containment of inflammation, preventing excessive damage to surrounding neurons. Simultaneously, the promotion of anti-inflammatory mediators aids in the resolution of the immune response, fostering an environment conducive to tissue repair
[6][7][8][9][10][135,136,137,138,139].
Oligodendrocytes, responsible for myelination, also express CB1R. The ECS is implicated in the regulation of myelination processes, influencing the conduction speed of nerve impulses and contributing to the structural integrity of neural circuits. The presence of CB1R on oligodendrocytes has opened a new avenue of investigation into the role of the ECS in myelination processes. CB1R activation on oligodendrocytes modulates intracellular signaling cascades that impact the synthesis and maintenance of myelin. The intricate interplay between endocannabinoid signaling and myelination highlights the adaptability of oligodendrocytes in response to environmental cues and the overall homeostasis of neural circuits. CB1R signaling on oligodendrocytes influences the thickness and compactness of myelin sheaths, directly impacting the conduction speed of nerve impulses. This modulation ensures the rapid and efficient transmission of electrical signals along axons, a critical aspect of neural communication. Beyond its influence on conduction speed, the ECS-mediated regulation of myelination contributes to the overall structural integrity of neural circuits. Oligodendrocyte-derived myelin provides essential support and insulation for axons, preventing signal leakage and maintaining the fidelity of neural communication
[11][12][13][140,141,142].
2. Endocannabinoid Regulation of the Central Nervous System Fluid Dynamics
Inflammation within the CNS serves as a pivotal mechanism contributing to the pathogenesis of various neurological conditions. This process unfolds through a complex cascade initiated by inflammatory signals stemming from diverse sources such as infection, injury, or neurodegenerative processes. The orchestrated migration of leukocytes, including neutrophils and monocytes, from the bloodstream across the BBB to the site of inflammation is a crucial aspect of this process. Upon reaching the BBB, leukocytes undergo a series of steps, including initial rolling and subsequent firm adhesion to endothelial cells, followed by transmigration into the CNS tissue
[14][143]. Within the CNS tissue, these migrating leukocytes interact with local immune cells, particularly microglia, triggering the activation of an immune response. This immune response involves the release of pro-inflammatory cytokines, chemokines, and other mediators, aimed at eliminating the source of inflammation. The interaction between circulating leukocytes and resident immune cells can amplify the immune response, intensifying the inflammatory process. Maintaining a balanced resolution of inflammation becomes crucial to prevent tissue damage and the onset of neurodegenerative disorders
[15][16][144,145].
Inflammatory conditions and neurodegenerative diseases often disrupt the BBB, resulting in increased permeability and compromised brain function. CB1R are primarily located on the luminal side of the BBB endothelium, and their expression has been observed in astrocytes, microglial cells, and pericytes (
Figure 12). Activation of CB1R in astrocytes has been linked to the regulation of metabolic activities, enhancing processes crucial for supplying energy to the brain, such as glucose oxidation and ketogenesis. Considering the role of perivascular astrocytes in delivering energy to neurons, the activation of astrocytic CB1R may have implications for regulating the energy supply to neurons
[17][18][19][20][146,147,148,149].
Studies demonstrate that cannabinoids, such as CBD, can modify detrimental effects associated with neuroinflammatory conditions by reducing leukocyte infiltration and down-regulating the expression of inflammatory molecules. CBD has shown the potential to prevent inflammation in endothelial cells, thereby mitigating observed BBB alterations. On the other hand, the activation of CB2R has been associated with decreased surface expression of adhesion molecules, increased tight junction protein levels, and attenuation of BBB damage and neurodegeneration in traumatic brain injury models. Research has further highlighted the positive effects of cannabinoids on BBB integrity and permeability. CBD administration has been linked to enhanced BBB integrity, reduced levels of proinflammatory cytokines, and increased expression of tight junction proteins. The ECS exhibits immunomodulatory properties with potential implications for immune responses within the brain, particularly relevant in the context of neuroinflammatory processes associated with neurological disorders. This interaction gains significance due to its potential consequences for maintaining BBB integrity. Additionally, the ECS’s involvement in neuroprotective functions may indirectly influence BBB performance by counteracting oxidative stress and inflammatory processes, recognized contributors to BBB impairment. In this manner, the ECS might contribute to sustaining BBB integrity and overall cerebral well-being. Furthermore, the ECS exhibits implications in the intricate phenomenon of neurovascular coupling, governing the interplay between neuronal activity, blood flow regulation, and BBB functionality, thereby potentially coordinating neural activity, blood flow dynamics, and BBB functionality
[21][22][23][24][25][150,151,152,153,154].
3. Glial Regulation of Endocannabinoid Function
Glial cells actively participate in the regulation of endocannabinoid levels. Astrocytes, through the expression of enzymes like FAAH and MAGL, contribute to the breakdown of AEA and 2-AG, respectively. AEA is known for its modulation of synaptic transmission and involvement in various physiological processes. The expression of FAAH in astrocytes ensures the controlled breakdown of AEA, regulating its spatiotemporal availability within the synaptic cleft. Astrocytic expression of MAGL ensures the regulated breakdown of 2-AG, influencing its concentration and impact on cannabinoid receptors. This enzymatic control over 2-AG levels by astrocytes contributes to the dynamic modulation of ECS signaling. The controlled degradation of AEA and 2-AG by astrocytes is paramount in shaping the spatiotemporal dynamics of endocannabinoid signaling. By regulating the availability of these endocannabinoids, astrocytes exert a precise influence over cannabinoid receptor activation, impacting synaptic transmission and network activity. This fine-tuning ensures that endocannabinoid signaling is dynamic, responsive, and spatially restricted, avoiding unwarranted or prolonged receptor activation
[26][27][28][155,156,157].
The meticulous control of endocannabinoid levels by astrocytes has profound functional implications for neural circuitry, synaptic plasticity, and overall brain homeostasis. Dysregulation of ECS signaling is implicated in various neurological disorders, emphasizing the therapeutic potential of targeting astrocytic endocannabinoid enzymes. Strategies that modulate FAAH and MAGL activity in astrocytes hold promise for fine-tuning endocannabinoid levels and mitigating pathological conditions associated with ECS dysfunction. By controlling the degradation of endocannabinoids, astrocytes play a critical role in shaping the spatiotemporal dynamics of endocannabinoid signaling.
4. Sleep, Synaptic Plasticity, and the Endocannabinoid System in Memory Consolidation
Sleep is a complex physiological phenomenon that plays a crucial role in various cognitive functions, including learning and memory. One of the key processes underlying the relationship between sleep and cognitive functions is synaptic plasticity, which refers to the ability of synapses to undergo structural and functional changes in response to experience. Synaptic plasticity is a fundamental mechanism in learning and memory, and its modulation during sleep is a subject of intense scientific investigation.
At the molecular level, several key players contribute to the intricate dance between sleep and synaptic plasticity. One critical factor is the regulation of neurotransmitters, which are chemical messengers that facilitate communication between neurons. During wakefulness, neurotransmitters such as glutamate are released, promoting synaptic strength and connectivity. However, as an organism transitions from wakefulness to sleep, there is a shift in neurotransmitter balance. One of the major neurotransmitters involved in sleep–wake regulation is adenosine. Adenosine levels gradually build up during wakefulness, leading to an increased inhibitory effect on neurotransmission. This buildup of adenosine is a consequence of the energy-consuming processes occurring in the brain during waking hours. As an individual enters sleep, adenosine levels peak, contributing to the initiation and maintenance of sleep. The interaction between adenosine and its receptors, particularly the adenosine A1 receptor, has profound implications for synaptic plasticity. Activation of the adenosine A1 receptor inhibits the release of excitatory neurotransmitters, such as glutamate. This downregulation of excitatory transmission during sleep is thought to facilitate the consolidation of memories by preventing interference from new sensory input and promoting the reorganization of existing neural connections. Another essential player in the molecular mechanisms of sleep and synaptic plasticity is the ECS. Cannabinoid receptors are abundant in regions involved in memory and synaptic plasticity. The ECS is known to be modulated during sleep, and its activation has been linked to the promotion of SWS, a deep and restorative stage of the sleep cycle
[29][30][31][32][123,158,159,160].
Beyond adenosine and the ECS, other molecular players, such as growth factors and various signaling pathways, contribute to the dynamic regulation of synaptic strength and connectivity. One example is BDNF, a protein crucial for neuronal survival and growth. BDNF is involved in the processes of LTP and LTD, which are forms of synaptic plasticity associated with learning and memory. During sleep, BDNF levels have been shown to increase, supporting the consolidation of memories acquired during wakefulness
[24][33][34][35][36][94,153,161,162,163].