Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | HYEIN HO | -- | 4954 | 2024-03-12 04:58:00 | | | |
| 2 | Catherine Yang | Meta information modification | 4954 | 2024-03-12 06:17:40 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ho, H.; Park, C.; Yoo, K.; Kim, N.; Hwang, S. Factors Affecting Akinete Germination and the Ranges of Tolerances. Encyclopedia. Available online: https://encyclopedia.pub/entry/56142 (accessed on 07 February 2026).
Ho H, Park C, Yoo K, Kim N, Hwang S. Factors Affecting Akinete Germination and the Ranges of Tolerances. Encyclopedia. Available at: https://encyclopedia.pub/entry/56142. Accessed February 07, 2026.
Ho, Hye-In, Chae-Hong Park, Kyeong-Eun Yoo, Nan-Young Kim, Soon-Jin Hwang. "Factors Affecting Akinete Germination and the Ranges of Tolerances" Encyclopedia, https://encyclopedia.pub/entry/56142 (accessed February 07, 2026).
Ho, H., Park, C., Yoo, K., Kim, N., & Hwang, S. (2024, March 12). Factors Affecting Akinete Germination and the Ranges of Tolerances. In Encyclopedia. https://encyclopedia.pub/entry/56142
Ho, Hye-In, et al. "Factors Affecting Akinete Germination and the Ranges of Tolerances." Encyclopedia. Web. 12 March, 2024.
Copy Citation
Eutrophic freshwater ecosystems are vulnerable to toxin-producing cyanobacteria growth or harmful algal blooms. Cyanobacteria belonging to the Nostocales order form akinetes that are similar to the seeds of vascular plants, which are resting cells surrounded by a thick membrane. They overwinter in sediment and germinate when conditions become favorable, eventually developing into vegetative cells and causing blooms.
cyanobacteria
life cycle
akinete
germination
formation
1. Introduction
Cyanobacteria are known to be the primary source of various toxins and odorous substances in freshwater ecosystems [1][2]. Bloom of cyanobacteria pose a critical threat to water resources management by causing harm to the ecosystem and human health, and thus impairing water use [3][4][5].
Some cyanobacteria that cause harmful algal blooms have a survival strategy that enables multi-year blooms that allow them to overcome periods of unfavorable growth by forming specialized cells called akinete within their life cycle [6][7]. The akinete-forming cyanobacteria are filamentous cyanobacteria that mostly belong to the Nostocales order, of which the genera Anabaena, Aphanizomenon, Cylindrospermopsis, Nodularia, Gloeotrichia, Nostoc, Nostochopsis, and Westiellopsis are known, while the genera of Hapalosiphon and Stigonema of the Stigonematales order have also been reported [8][9]. Compared to vegetative cells, akinetes are larger in size [9] and surrounded by a thick cell wall, which can protect them from adverse environmental conditions [10]. Akinetes also contain large amounts of energy stores and nucleic acids, which allow them to grow quickly through germination when the surrounding environmental conditions are favorable. In this way, akinetes serve as seeds for the initial growth of vegetative cells [11]. Akinetes endow an ecological advantage because they can survive unfavorable growth conditions, and thus, cyanobacteria can produce akinetes to enable perennial blooms [12][13].
The life cycle of akinete-forming cyanobacteria can be divided into vegetative cell and akinete stages [14]. These two stages have ecological significance by bridging the water column and the sediment [15]. Akinetes formed from vegetative cells remain dormant in the sediment when the growth conditions are unfavorable, but when these environmental conditions improve, they germinate and are recruited to the water column to grow into vegetative cells [11][16][17][18]. Therefore, it is very important to have information on the environmental factors involved in the formation and germination of akinetes and their tolerances to understand the overall life cycle of cyanobacteria, including their death and development, and such information can provide basic guidance for harmful algal bloom management.
2. Temperature
In aquatic ecosystems, temperature is one of the critical environmental factors that drive the seasonal succession of algae [19]. In particular, many eutrophic lakes and streams in temperate climate regions experience cyanobacteria blooms during the summer because such blooms prefer high water temperatures [2]. The germination of akinetes is strongly influenced by temperature. Several experimental studies have shown that the range of temperatures that induce akinete germination is quite wide, where the optimum temperature for the initiation and triggering of germination is species-specific. Nodularia spumigena akinetes began to germinate at 15 °C, and the germination rate increased with increasing temperature, with the highest germination rate (80%) seen at 24 °C (experimental condition: temperature 12–24 °C, light 25 μmol m−2s−1, BG-11 medium) (Figure 1. ⑨) [20]. Akinetes of Dolichospermum circinale germinated over a wide range of water temperatures (12–25 °C) but with very narrow optimal temperature ranges [11]. Park [11] reported that akinetes of D. circinale isolated from the sediment of a reservoir started germination at 12 °C and showed a low germination rate (<10%) until 17 °C but then germinated explosively (>80%) at 19–20 °C (experimental condition: temperature 12–25 °C, light 30 μmol m−2 s−1, filtered lake water in chamber) (Figure 1. ①). The optimal germination temperature (19–20 °C) coincided with the average reservoir water temperature in June when there was an increased density of vegetative cells, and explosive akinete germination led to the development of vegetative cells in the water column. These results provide important information that can inform the proactive identification and control of D. circinale blooms in the field. Other data indicate that D. circinale akinetes may have a wider range of germination temperatures (5–38 °C) depending on the characteristics of the water body in which they reside and that temperatures above 20 °C (20–25 °C) are required to trigger germination (Figure 1. ⑤). However, multiple studies have shown that very high temperatures (>30 °C) lead to a tendency for no germination or reduced germination rates [10][21][22]. Anabaena vaginicola akinetes have been shown to germinate over a relatively wider temperature range (5–45 °C). The highest germination rate occurred at 25 °C (94%), while it decreased to less than 5% at temperatures > 35 °C (experimental condition: temperature 5–45 °C, light 32.4 μmol m−2s−1, Basal medium) [23] (Figure 1. ⑥). Meanwhile, the akinetes of Cylindrospermopsis raciborskii have been found to germinate in the range of 15–25 °C, and the optimum germination temperature has been found to be 22–24 °C (Figure 1. ⑩) [24][25][26].
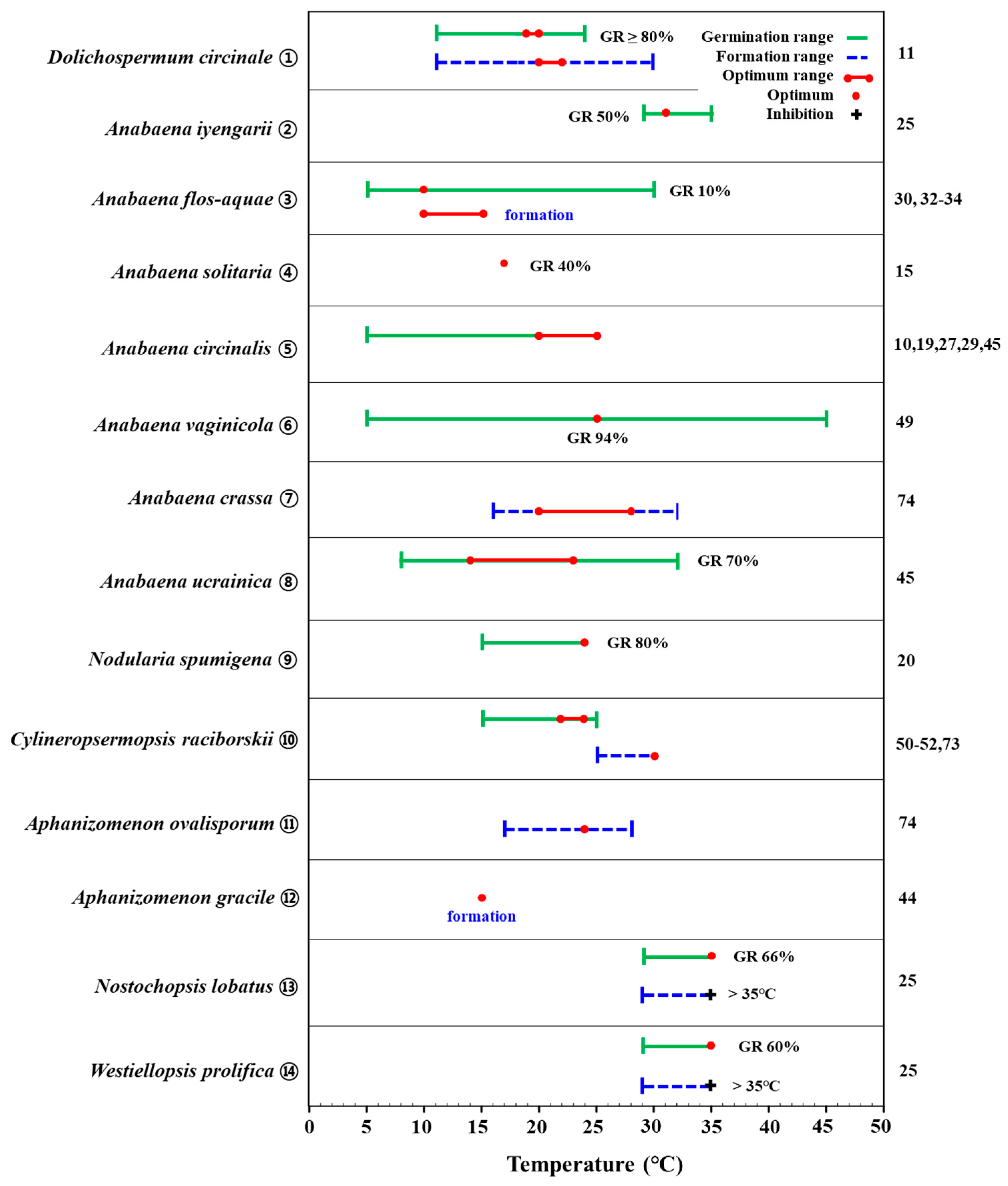
Figure 1. Graphic summary of temperature-dependent akinete germination and formation of cyanobacterial species from the Nostocales order. Numbers appearing on the right column are those in accordance with the list in the references. GR: akinete germination rate.
Taken together, these results suggest that akinetes of some species of cyanobacteria have a similar range of germination and triggering temperatures. On the other hand, the germination of akinetes also seems to be related to the temperature regime of the specific environment(s) in which they live and the temperature tolerances of the cyanobacteria. In Sweden, Anabaena solitaria akinetes showed the highest germination rate (40%) at 17 °C (experimental condition: temperature 7–17 °C, light 100 μmol m−2s−1, filtered lake water) (Figure 1. ④) [15]. Anabaena ucrainica akinetes started germination at 8 °C, with a high germination rate of 70% in the range from 14 to 23 °C (experimental condition: temperature 5–32 °C, light 70 μmol m−2s−1, CT medium) (Figure 1. ⑧); the temperature range of germination was 8–32 °C, but the germination rate decreased (<40%) above 26 °C [27]. Unlike other cyanobacteria in the genus Anabaena, Anabaena flos-aquae akinetes have shown high germination rates at low temperatures (5 °C: 49%, 10 °C: 51%) (experiment condition: temperature 5–30 °C, light 30 μmol m−2 s−1, filtered lake water) (Figure 1. ③). However, akinete germination was significantly reduced (<2%) at temperatures above 20 °C. The high germination rate of this species at low temperatures coincided with the water temperature range (<10 °C) during the season (Oct-Nov), during which the cell density of A. flos-aquae spiked in the field [28]. By contrast, in India, Anabaena iyengarii did not germinate at all at <29 °C, while it predominantly germinated (>40%) at 29–35 °C (experimental condition: temperature 29–41 °C, light 0–40.5 μmol m−2s−1, BG-11 medium) [29]. Akinetes of Nostochopsis lobatus and Westiellopsis prolifica have also shown high germination rates at high temperatures (29–35 °C: >30%), where the highest germination rates were 66% and 60%, respectively, at 35 °C (Figure 1. ⑬, ⑭) [29].
The literature clearly indicates that temperature is a critical factor in the germination of akinetes. However, temperature tolerances, including the temperature at which germination begins and the optimum temperature for germination, vary among different species of cyanobacteria. Differences in laboratory culture conditions (temperature, light, nutrients, etc.) may affect the germination of akinetes of the target species, but another factor may be the adaptation of the cyanobacteria in the field to the local environment (and the temperature regime in particular) or laboratory culture conditions. For example, the difference between Anabaena flos-aquae, which has a high germination rate at low temperatures, and Anabaena iyengarii, which has a high germination rate at high temperatures, is understood to reflect differences in water temperature in the environments in which these two species occur [28][29]. D. circinale has also been shown to have different germination temperature ranges in several studies [11][21][22]. These results suggest the possibility of the occurrence of ecotypes, by which even the same species shows different germination responses in terms of temperature when inhabiting different environments [30][31][32]. In conclusion, although the germination of akinetes in cyanobacteria can occur at low temperatures, the optimal germination temperature at which a high proportion of germination is triggered is similar to the temperature range at which vegetative cells grow actively, suggesting that the germination of akinetes and the development of vegetative cells in the aquatic environment may be closely linked in time.
3. Light
Prior studies have demonstrated that light is a critical resource that allows akinetes to germinate [20][28][33]. Anabaena circinalis has been found to germinate in the light intensity range of 5–100 μmol m−2s−1, with a maximum germination light intensity of 30 μmol m−2s−1 (Figure 2. ③) [22][34]. According to van Dok and Hart [34], A. circinalis akinetes did not germinate in dark conditions without light, and an increase in light intensity in the range of 15–50 μmol m−2s−1 did not significantly induce an increase in germination rate, thus showing similar results (germination rate: 17–23%) (experimental condition: temperature 25 °C, light 0–50 μmol m−2s−1, ASM-1 medium). Park et al. [22] also showed that akinetes of A. circinalis did not germinate under dark conditions, with a germination rate of 45% at 5–10 μmol m−2s−1 light conditions, and the highest germination rate of 60 % at 30 μmol m−2s−1. On the other hand, at light intensity exceeding 50 μmol m−2s−1, the germination rate decreased (10%) (experimental condition: temperature 25 °C, light 0–100 μmol m−2s−1, filtered lake water). Anabaena cylindrica akinetes also germinated in a wide light intensity range of 2–60 μmol m−2s−1 (experimental condition: temperature 27 °C, light 2–60 μmol m−2s−1, Detmer medium) (Figure 2. ④) [33].
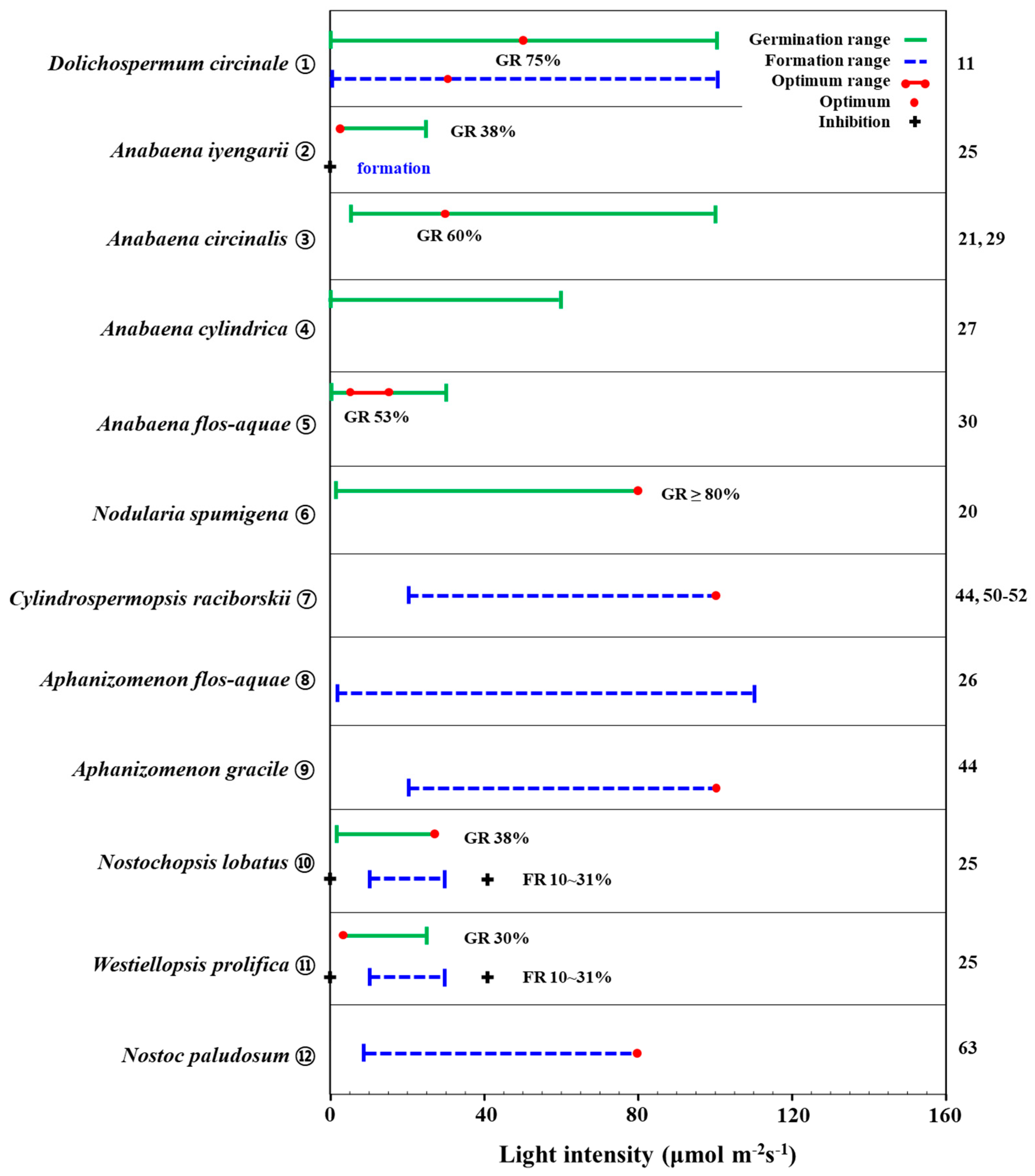
Figure 2. Graphic summary of light-dependent akinete germination and formation of some cyanobacterial species from the Nostocales order. Numbers appearing on the right column are those in accordance with the list in the references. GR: akinete germination rate. FR: akinete formation rate.
Several studies have demonstrated that light is an essential resource for akinete germination, but some authors have also observed germination in the dark. However, the observed effect has been very small. In Park [11], Dolichospermum circinale akinetes were shown to be germinated in the dark, albeit very weakly (germination rate: 3%) (experimental condition: temperature 20 °C, light 0–100 μmol m−2s−1) (Figure 2. ①). Further, Kim et al. [28] showed that Anabaena flos-aquae germinated under dark conditions (germination rate: 4%) (Figure 2. ⑤). As light is an essential resource for photosynthesis, then even if germination can occur under dark conditions, such conditions still cannot promote vegetative cell growth, so akinete germination under dark conditions observed in the above studies may not be a common phenomenon, particularly in the conditions that are common in the field. The observed positive correlation between chlorophyll-a concentration and germination rate in akinetes of Anabaena circinalis suggests that their germination rate is related to photosynthesis [10].
In general, the light intensity required for akinete germination is not high [20][29][34], but the range of light intensities for germination and the optimum light intensity varies among different species of cyanobacteria. Further, in certain light intensity ranges, akinete germination is promoted by increasing light intensity [9][35]. Anabaena flos-aquae akinetes were found to germinate at light intensity 0–30 μmol m−2s−1 and showed a high germination rate (>53%) at 5–15 μmol m−2s−1 (experimental condition: temperature 10 °C, light 0–30 μmol m−2s−1, filtered lake water) [28]. Anabaena iyengarii akinetes also germinated under low light conditions (experimental condition: temperature 29–41 °C, light 0–40.5 μmol m−2s−1) [29]. They germinated more than 20% in the range of light intensity 4.05–27 μmol m−2s−1, while they showed the maximum germination rate (38%) at light intensity 4.05 μmol m−2s−1. On the other hand, no germination of A. iyengarii akinetes occurred under very low and high light intensity (0–1.35 μmol m−2s−1 and 40.5 μmol m−2s−1, respectively), nor did Nostochopsis lobatus and Westiellopsis prolifica akinetes germinate under the same experimental conditions (Figure 2. ⑩, ⑪). For N. lobatus, the akinete germination rate increased with increasing light intensity within the range of 4.05–27 μmol m−2s−1, and it was highest (38%) at 27 μmol m−2s−1. W. prolifica akinete started to germinate at 4.05 μmol m−2s−1 (30% germination rate), followed by germination rates of 14% at 6.75 μmol m−2 s−1 and 28 % at 27 μmol m−2s−1 [29]. On the other hand, Nodularia spumigena germinated at a rate of 29% under very low light conditions of 0.5 μmol m−2 s−1, and the germination rate increased with increasing light intensity (experimental condition: temperature 21 °C, light 0–99 μmol m−2 s−1) (Figure 2. ⑥) [20]. This species did not germinate under dark conditions, with a germination rate of 51% at 9 μmol m s−2−1 and a rate of more than 80% at 80 μmol m−2s−1. Notably, germination was triggered at low light levels, ranging from 0.5 to 9 μmol m−2s−1, after which it continuously up to 80 μmol m−2s−1.
Several previous studies have shown that akinete germination occurs over a wide range of light intensities, although with generally higher germination rates at lower light intensities than the corresponding rates at higher light intensities. This suggests that akinetes do not require high light levels to germinate. Although some cyanobacterial akinetes have been shown to germinate in the dark, albeit to a very small extent, in most experiments, akinetes did not germinate in the dark. Given that the germination of akinetes in the field begins in the sediment, which is subject to dilution and extinction of light through the water column, it is ecologically plausible to assume that germination occurs under low light conditions [36]. Further, within the range of low light intensities, an increase in light intensity is linked to an increase in photosynthesis in germinated cells, which may facilitate akinete germination. Compared to water temperature, changes in light intensity are relatively less seasonal, so the effect of light on akinete germination in the field is likely to be more dependent on spatial differences (water column versus sediment) than seasonal differences. In particular, for akinetes that are distributed in shallow sediments, the availability of light is likely to initiate and trigger germination [37].
4. Nutrients
Unlike external stimuli, such as temperature and light, nutrients can potentially affect akinete germination by determining the physiological state inside the akinete cell; i.e., nutrients are involved in the synthesis of energy materials and nucleic acids that akinetes need to activate germination and cell division [38][39]. Several studies have demonstrated the potential impact of nitrogen and phosphorus on akinete germination [20][23][34][40]. It has also been shown that different types of nutrients affect germination in unique ways and that the concentration of nutrients necessary to trigger germination differs not only among different species but also within the same species [11][41].
Two studies on Nodularia spumigena showed the existence of different dose-response relationships between akinete germination and nutrient (nitrogen and phosphorus) concentrations (Figure 3. ⑧; Table 1) [20][42]. In Huber [20], N. spumigena akinetes germinated more than 80% evenly in a wide concentration range (0–54.3 mgL−1) when nitrate was added, regardless of the concentration difference, but this did not occur when ammonium was added (0.14–2.28 mgL−1), as the germination rate, in that case, was highest (>80%) at 0.14 mgL−1, and germination was inhibited at higher concentrations (experimental condition: temperature 21 °C, light 25 μmol m−2s−1, N and P addition in BG-11 medium). On the other hand, overall, more than 60% of akinetes germinated under the conditions of phosphorus addition (0.113–28.212 mgL−1), but they did not germinate at all when phosphorus was deficient. Huber [20] showed that nitrate had no effect on germination and that a very low phosphorus concentration was required for germination. However, Myers et al. [42] showed that N. spumigena akinete germination rates were positively correlated with both nitrate and phosphorus concentrations (experimental condition: temperature 21 °C, light 40, 100 μmol m−2s−1, N and P addition in MLA medium). The addition of phosphorus in the concentration range of 0–2.5 mgL−1 showed a maximum germination rate of 20% at a concentration of 2.5 mgL−1. The overall germination rate as a function of phosphorus concentration was shown to be affected by light intensity, with the germination rates observed at 40 μmol m−2s−1 being higher than those observed at 100 μmol m−2s−1. In the range of nitrate concentrations from 0 to 3 mgL−1, the highest germination rate was 25% at 3 mg/L, and contrary to the case of phosphorus, the germination rate was higher at 100 μmol m−2s−1.
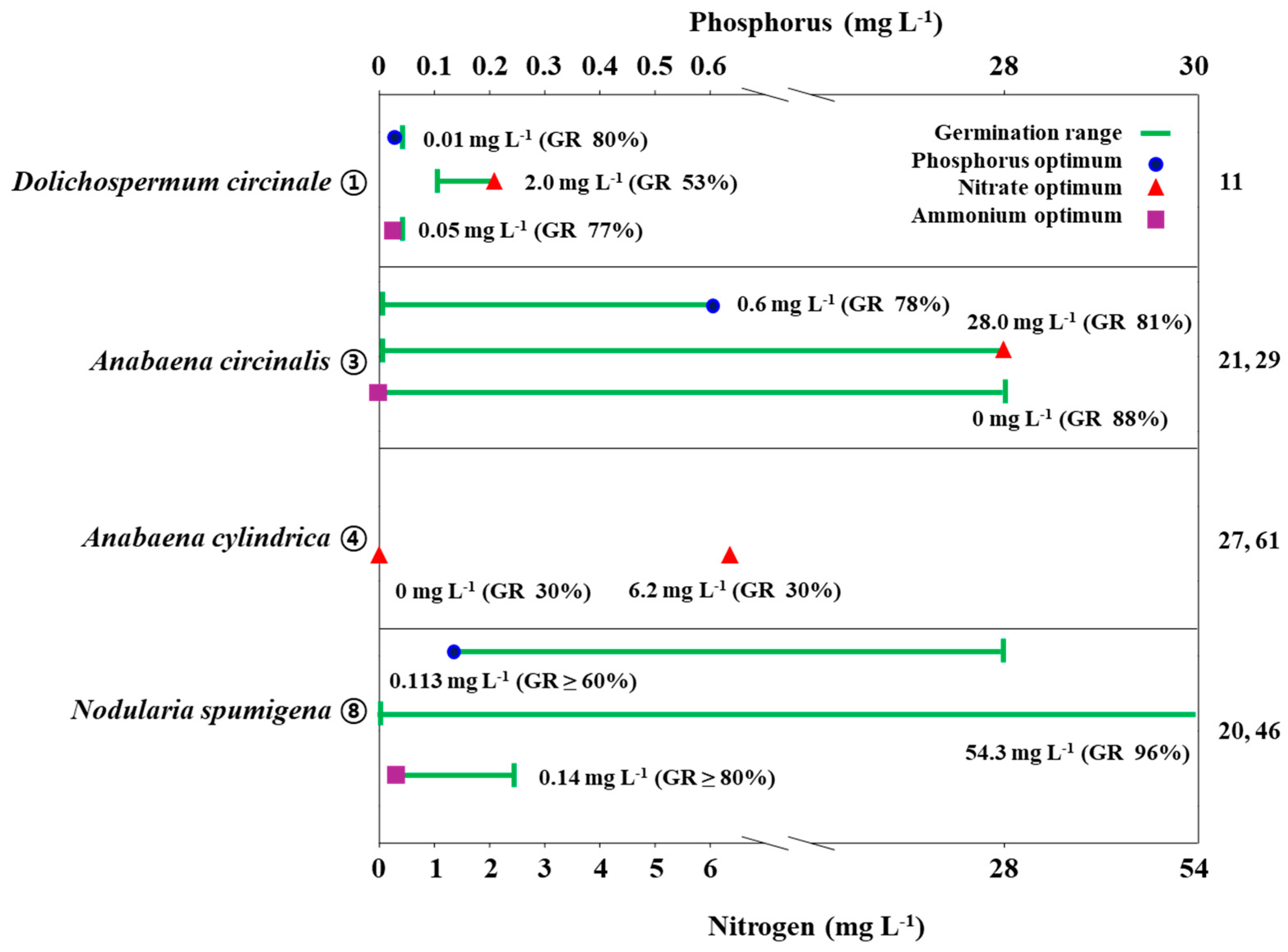
Figure 3. Graphic summary of nutrient-dependent akinete germination and formation of some cyanobacterial species from the Nostocales order. Numbers appearing on the right column are those in accordance with the list in the references. GR: akinete germination rate.
The results of some prior studies indicated that nitrogen has an unclear effect on akinete germination (Figure 3. ③, ④; Table 1) [33][34]. In Van Dok and Hart [34], Anabaena circinalis akinetes germinated at a high rate even without nitrogen addition. The germination rate was increased with increasing nitrate concentration; meanwhile, ammonium addition inhibited akinete germination. On the other hand, the germination rate increased by more than three times with the addition of phosphorus (experiment condition: temperature 25 °C, light 30 μmol m−2s−1, N and P addition in ASM-1 medium). In Yamamoto [33], the addition of nitrate had no effect on A. cylindrica akinete germination; instead, organic carbon (acetate) accelerated germination. By contrast, Rai and Pandey [23] reported that nitrate had a greater effect on akinete germination than phosphorus, based on the results indicating that Anabaena vaginicola showed a high germination rate of 96% in nitrate-supplemented conditions whereas it showed a germination rate of 43% in phosphorus-deficient conditions (experimental condition: temperature 25 °C, light 32.4 μmol m−2s−1, basal medium) (Table 1).
On the other hand, both nitrogen and phosphorus appear to have a synergistic effect on akinete germination (Figure 3. ③; Table 1) [22][34]. Park et al. [22] found that Anabaena circinalis akinete germination was highest (55%) in conditions involving the addition of both N and P, while the germination rate was lowest (10%) in conditions where both of them were deficient. However, when N and P were treated separately, nitrogen induced a higher germination rate (50%) than phosphorus (<25%) (experimental condition: temperature 25 °C, light 30 μmol m−2s−1, -N-P, +N-P, -N+P, +N+P in CB medium). The same results were found in Anabaena iyengarii akinetes [39]. Specifically, Agrawal and Misra [40] reported that germination was highest (50%) in conditions where both nitrate and phosphorus were added; meanwhile, in conditions where nitrate and phosphorus were deficient, germination decreased to 30% and 40%, respectively. The germination rate was lowest (20%) in the condition where both N and P were deficient (experiment condition: temperature 22 °C, light 40 μmol m−2s−1, -N-P, +N-P, -N+P, +N+P in basal medium) (Table 1). In Park’s [11] study, where nitrate and ammonium were used as nitrogen sources simultaneously, the main nutrients of Dolichospermum circinale akinete germination were ammonium and phosphorus, while in the nitrate condition, more than 80% of the akinetes germinated in the P-only addition regardless of N addition, thus showing the synergistic effect of N and P (experimental condition: temperature 20 °C, light 30 μmol m−2s−1, -N-P, +N-P, -N+P, +N+P in CB medium) (Figure 3. ①; Table 1). On the other hand, in the ammonium addition condition, the germination rate was highest (91%) in the [+N-P] condition, while P had an almost insignificant effect. Based on the experimental results, the optimal concentration range for D. circinale akinete germination was suggested to be NO3-N 1–3 mgL−1, NH4-N 0.05–0.2 mgL−1, and PO4-P 0.005–0.5 mgL−1 [11]. Westiellopsis prolifica akinetes were germinated at a more than five-fold increase under conditions with added nitrogen or phosphorus compared to nitrate or phosphorus deficiency [43]. The germination rate was highest at 58% under conditions where both nutrients were added simultaneously [40]. This species germinated 35% in nitrate deficiency and 26% in phosphorus deficiency, with the lowest germination rate of 20% in conditions where both were absent. Similarly, Nostochopsis lobatus showed similar germination rates (54%) to A. iyengarii and W. prolifica in conditions where both nitrate and phosphorus were added (Table 1) [40]. However, their germination rates in nitrate-deficient and phosphorus-deficient conditions were similar, at respective values of 38% and 35%, while they showed a germination rate of 24% in conditions lacking both nutrients.
Altogether, the literature on the relationship between nutrients and akinete germination suggests that nitrogen and phosphorus both play important roles in germination. However, the effects of nitrogen and phosphorus are different for akinetes of different species of cyanobacteria, and they are also different for the same species of cyanobacteria (Figure 3; Table 1). Nitrogen and phosphorus also appear to be synergistic in increasing akinete germination rates. The nutrient dose responses to germination were shown to vary among species, and even within the same species, and for nitrogen, the contribution of different nitrogen sources was different, with nitrate having extreme effects. In some cases, germination occurred even in the absence of nitrate, while in others, germination increased with higher nitrate concentration. However, in many cases, ammonium had a greater impact on increasing germination rates than nitrate.
These conflicting results across studies may be attributable to several factors, including different experimental conditions (e.g., differences in medium, field isolates, or laboratory cultures, differences in physiological status, differences in nutrient concentrations treated, differences in environmental conditions other than nutrients, etc.). They may also be due to the different physiological effects of nitrogen and phosphorus on akinete germination and growth for cyanobacterial species. In the field, nutrients may not be a critical factor limiting akinete germination, as suggested by the fact that the germination of akinetes has been observed under experimental conditions even without the addition of nutrients [7]. This may be because, within aquatic ecosystems, akinetes are present in the sediment or at the water–sediment interface, where the nutrient concentrations are typically high. Moreover, akinetes contain high concentrations of nitrogen and phosphorus inside their cells. Under these circumstances, it is unlikely that additional nutrients will be needed. However, in situations where akinete germination is already underway, the supply of additional nutrients will induce an increase in the germination rate and contribute to the development of vegetative cells. Therefore, nutrients are likely to play a greater role in the progression of germination to vegetative cells than they do in influencing the initiation of germination.
Table 1. Literature summary of nutrients effects on cyanobacterial akinete germination and formation.
| No | Cyanobacteria Species | Effects of N and P on AKINETE | Ref |
|---|---|---|---|
| ① | Dolichospermum circinale | With nitrate: Germination rate (GR) > 80% in (-N or +N)+P condition (Little effect of nitrate) With ammonium: GR 91% in +N-P condition (Little effect of P) The highest akinete formation (420 akinetes/g) in +N+P (N=NH4-N) condition |
[11] |
| ② | Anabaena iyengarii | GR 50% in +N+P (N=NO3-N); GR 20% in -N-P (N=NO3-N) Formation rate (FR) 6% in +N+P condition |
[40] |
| ③ | Anabaena circinalis | GR 55% in +N+P; GR 50% in +N-P; GR 25% in -N+P; GR 10% in -N-P Akinete formation in P (0.06mg/L) addition; no formation without N |
[22][34] |
| ④ | Anabaena cylindrica | No akinete formation in the absence of N | [44] |
| ⑤ | Anabaena vaginicola | GR 96% in NO3-N addition; GR 43% in P deprivation | [23] |
| ⑥ | Anabaena lemmermannii | Akinete formation in the absence of P | [45] |
| ⑦ | Anabaena crassa | No formation when depriving both N and P | [46] |
| ⑧ | Nodularia spumigena | High germination (>80%) under broad range of NO3-N (0–54.3 mg/L) | [20] |
| ⑨ | Cylindrospermopsis raciborskii | FR increased with increasing P conc. Maximum akinete formation (2310 akinetes/mL) in 70 μgP/L |
[47] |
| ⑩ | Nostoc palusodum | Akinete formation in the absence of P | [48] |
| ⑪ | Nostochopsis lobatus | GR 54% in +N+P; GR 38% in -N+P; GR 35% in +N-P; GR 24% in -N-P FR 55% in +N+P |
[40] |
| ⑫ | Westiellopsis prolifica | GR 58% in +N+P; GR 35% in -N+P; GR 26% in +N-P; GR 20% in -N-P FR 70% in +N+P |
[40] |
5. Hydrogen Ion (H+) Concentration
Several studies have demonstrated that hydrogen ion concentration is an environmental factor that substantially affects both akinete germination and vegetative cell growth [40][49]. The effect of pH on akinete germination has been reported in several species of the genus Anabaena. Fischerella muscicola germinated in the pH range from 6 to 10 and did not germinate at all in acidic conditions (pH < 5) (Figure 4. ①). Moreover, the germination rate increased with decreasing H+ concentration, with the maximum germination rate at pH 9 (68%), and the germination rate decreased rapidly (<50%) at pH 10 and above (experimental condition: temperature 25 °C, light 50 μmol m−2s−1, N-free chu medium, pH 5–10) [50]. In the range of pH 6–11, Anabaenopsis arnoldii akinetes had the highest germination rate at pH 7 (64%) and maintained a high germination rate up to pH 8.5 (58%). However, the germination rate decreased at pH 9 and above (experimental condition: temperature 28 °C, light 32.4 μmol m−2s−1, SSM medium, pH 4.5–11) [51].
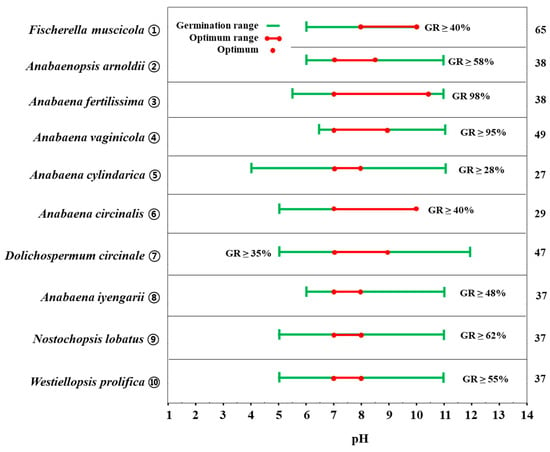
Figure 4. Graphic summary of pH-dependent akinete germination of some cyanobacterial species from the Nostocales order. Numbers appearing on the right column are those in accordance with the list in the references. GR: akinete germination rate.
By contrast, Anabaena fertilissima germinated evenly over a wide range of pH from 5.5 to 11 (Figure 4. ③). Almost all akinetes germinated at pH 7–10.5 (98%), with 75% germination observed even at pH 11. Notably, this species was found to germinate in all acidic, neutral, and alkaline conditions (experimental condition: temperature 28 °C, light 32.4 μmol m−2s−1, SSM medium, pH 4.5–11) [51]. Anabaena vaginicola started germination at pH 6 (23%), and the range in which it showed maximum germination was pH 7–9 (>95%). However, akinetes did not germinate under conditions below pH 5.5 and above pH 11 (experimental condition: temperature 25 °C, light 32.4 μmol m−2s−1, Basal medium, pH 5–12) (Figure 4. ④) [23]. Unlike other cyanobacteria in the genus Anabaena, Anabaena cylindrica akinetes even germinated under acidic conditions of pH 4. A. cylindrica akinete germinated in a very wide range from pH 4 (<5%) to pH 11 (<20%), while its germination rate was highest (28–30%) at pH 7–8. However, germination decreased below pH 6 and above pH 10 (experimental condition: temperature 27 °C, light 67.5 μmol m−2s−1, Detmer medium, pH 2–12) (Figure 4. ⑤) [33].
The decrease in akinete germination under acidic and alkaline conditions appears to be related to the destruction of akinetes under strong acidic and strong alkaline conditions. Park et al. [22] observed the destruction of the akinete cell wall in Anabaena circinalis at pH 5–6 (germination rate: 10%) and pH 11–12 (0%). High germination rates were found in the ranges of pH 7–8 (55%) and pH 9–10 (40–45%) (experimental condition: temperature 25 °C, light 30 μmol m−2s−1, CB medium, pH 5–12) (Figure 4. ⑥). Very high or low pH can alter the permeability of the cell membrane, which affects ion uptake and may also lead to a loss of soluble metabolites [52][53]. On the other hand, in Kwon et al. [54], Dolichospermum circinale akinetes were shown to germinate in strong alkaline conditions of pH 12, albeit weakly (<10%) (experimental condition: temperature 25 °C, light 30 μmol m−2s−1, CB medium, pH 5–12) (Figure 4. ⑦). However, aligning with the results of other studies, the range in which maximum germination occurred was found to be pH 7–9, with 35% germination at pH 7, 70% germination at pH 8, and 50% germination at pH 9. Anabaena iyengarii akinetes germinated in the pH 6–11 range. The highest germination rate occurred at pH 7–8 (48–50%), and the germination rate decreased by more than 40% as the pH increased from 8 to 11 (experiment condition: temperature 22 °C, light 40 μmol m−2s−1, basal medium, pH 4–11) (Figure 4. ⑧) [40].
Nostochopsis lobatus akinetes have also been shown to germinate at the highest rate (>62%) at pH 7–8 (Figure 4. ⑨) [40]. However, they showed low germination rates of < 30% at acidic conditions, pH 5–6, and weakly basic conditions, pH 9–11. Similarly, Westiellopsis prolifica exhibited the highest germination rate (>55%) at pH 7–8. At pH 5, the germination rate was 5%, and at pH >9, the germination rate started to decrease, with the lowest germination rate (6%) observed at pH 11 (Figure 4. ⑩) [40].
Most prior studies have shown that the pH tolerance for the germination of cyanobacteria akinetes broadly ranges from acidic to alkaline but that the most favored range for germination is neutral to slightly alkaline and does not seem to vary much among species. In some experiments, species have been reported to germinate in strongly acidic or strongly alkaline conditions, such as pH 4 and pH 12, but at very low or zero rates (0-< 10%). Since akinete needs to grow into a vegetative cell after germination, it is not surprising that the pH conditions for germination and cell growth are almost identical [22][40][55].
6. Dissolved Oxygen (DO), Salinity, and Sediment Disturbance (Mixing and Turnover)
The tolerance of akinetes to DO is not well understood, and there have been limited DO studies involving germination. This may be attributable to the fact that, in the field, akinetes are buried in the sediment, which makes it difficult to determine the direct effects of oxygen. Kim et al. [28] measured Anabaena flos-aquae akinete germination and DO concentration in the sediment of a reservoir; the reservoir hypolimnion was seen to remain aerobic (7–17 mg O2L−1) during the sampling period, and there was no correlation observed between akinete germination and DO concentration. However, Fay et al. [10] showed that, for the akinete of Anabaena circinalis, after germination at low light levels, the first response was oxygen uptake for respiration. This suggests that DO may not be the primary factor that induces germination but that it is associated with increased cellular activity for energy production (photosynthesis) during the early stages of germination [10]. However, low oxygen concentrations may increase akinete viability in the long term by reducing the energy required for respiration [56]. On the other hand, DO has been shown to be essential for akinete germination in Anabaena cylindrica and Nostoc PCC 7524 [33][57], and it showed a five-fold higher germination rate under aerobic conditions compared to anaerobic conditions [33].
Several prior studies have shown that different species of cyanobacteria appear to have different salinity tolerances for akinete germination. Nodularia spumigena, a brackish-water blue-green alga, showed a decreased germination rate of akinetes in response to the addition of both low concentrations [58] and high concentrations [20] of sodium chloride. Silveira and Odebrecht [59] showed that salinity had a greater effect on N. spumigena akinete germination compared to temperature. This species showed that akinete germination was highest at salinities of 7 and 15 ppm, while it was lowest at 1 ppm. Myers et al. [42] also found high germination rates between salinities 5 and 15. These results indicate that fluctuations in salinity in brackish-water conditions significantly affect the germination of this species. Baker and Bellifemine [21] showed that Anabaena circinalis is tolerant to moderate salinities. Increasing salinity to 2.5 gL−1 increased akinete germination to 27%, with a sharp decrease at >5 gL−1 and no germination at the concentration of 10 gL−1.
Several studies support the hypothesis that the mixing of water and sediment plays an important role in the formation of blooms of akinete-producing cyanobacteria. This hypothesis is supported by the results of higher recruitment rates of vegetative cells in shallower sediment, i.e., the shallow littoral zone, which tends to be more susceptible to disturbance than deeper sediment [37][56][60]. Moreover, data from several studies have shown that shallow sediments are important seed banks or inoculation sites for akinetes. In Gleotrichia echinulate, recruitment from the sediment bed to the water column via akinete germination was found to be significantly enhanced by mixing the sediment bed through bioturbation or physical processes [37][61]. In Lake Kinneret, the sediment in the littoral zone was found to be easily resuspended by wind-driven waves, which affected the akinete germination and recruitment of Aphanizomenon ovalisporum into the water column [62]. Therefore, it is possible that the littoral zone of shallow lakes, shallow marshes, and deep lakes represent a conducive environment for akinete germination because the mixing of sediments and continuous resuspension from the sediment into the water column can expose akinetes to the appropriate environment (e.g., light and oxygen) for germination.
References
- Dokulil, M.T.; Teubner, K. Cyanobacterial dominance in lakes. Hydrobiologia 2000, 438, 1–12.
- Paerl, H.W.; Otten, T.G. Harmful cyanobacterial blooms: Causes, consequences, and controls. Microb. Ecol. 2013, 65, 995–1010.
- Persson, P.E. Muddy odour: A problem associated with extreme eutrophication. Hydrobiologia 1982, 86, 161–164.
- WHO. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring, and Management; Taylor & Francis: London, UK; New York, NY, USA, 1999; p. 416.
- Höckelmann, C.; Jüttner, F. Off-flavours in water: Hydroxyketones and β-ionone derivatives as new odour compounds of freshwater cyanobacteria. Flavour Fragr. J. 2005, 20, 387–394.
- Barbiero, R.P.; Welch, E. Contribution of benthic blue-green algal recruitment to lake populations and phosphorus translocation. Freshw. Biol. 1992, 27, 249–260.
- Baker, P.D. Role of akinetes in the development of cyanobacterial populations in the lower Murray River, Australia. Mar. Freshw. Res. 1999, 50, 265–279.
- Dvořák, P.; Casamatta, D.A.; Hašler, P.; Jahodářová, E.; Norwich, A.R.; Poulíčková, A. Diversity of the cyanobacteria. In Modern Topics in the Phototrophic Prokaryotes; Springer International Publishing: Berlin, Germany, 2017; pp. 3–46.
- Adams, D.G.; Duggan, P.S. Tansley Review No. 107. Heterocyst and akinete differentiation in cyanobacteria. New Phytol. 1999, 144, 3–33.
- Fay, P. Viability of akinetes of the planktonic cyanobacterium Anabaena circinalis. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1988, 234, 283–301.
- Park, C.H. Study on the Akinete Life Cycle and Vegetative Cell Dynamics in a Harmful Cyanobacterium. Dolichospermum circinale (Nostocales). Ph.D Thesis, University of Konkuk, Seoul, Republic of Korea, 2018.
- Sukenik, A.; Rücker, J.; Maldener, I. Dormant cells (akinetes) of filamentous cyanobacteria demonstrate a great variability in morphology, physiology, and ecological function. In Cyanobacteria: From Basic Science to Applications; Academic Press: Cambridge, MA, USA, 2018; pp. 65–77.
- Garg, G.; Maldener, I. The formation of spore-like Akinetes; A survival strategy of filamentous cyanobacteria. Microb. Physiol. 2021, 31, 296–305.
- Suikkanen, S.; Kaartokallio, H.; Hällfors, S.; Huttunen, M.; Laamanen, M. Life cycle strategies of bloom-forming, filamentous cyanobacteria in the Baltic Sea. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2010, 57, 199–209.
- Rengefors, K.; Gustafsson, S.; Ståhl-Delbanco, A. Factors regulating the recruitment of cyanobacterial and eukaryotic phytoplankton from littoral and profundal sediments. Aquat. Microb. Ecol. 2004, 36, 213–226.
- Nichols, J.M.; Adams, D.G. Akinetes. In The Biology of Cyanobacteria; University of California Press: Berkeley, CA, USA; Los Angeles, CA, USA, 1982; pp. 387–412.
- Herdman, M. Akinetes: Structure and function. In The Cyanobacteria; Elsevier: Amsterdam, The Netherlands, 1987; pp. 227–250.
- Yema, L.; O’farrell, I.; de Tezanos Pinto, P. The sediment akinete bank links past and future blooms of Nostocales in a shallow lake. J. Plankton Res. 2020, 42, 668–679.
- Sommer, U.; Adrian, R.; De Senerpont Domis, L.; Elser, J.J.; Gaedke, U.; Ibelings, B.; Jeppesen, E.; Lurling, M.; Molinero, J.C.; Mooij, W.M.; et al. Beyond the Plankton Ecology Group (PEG) model: Mechanisms driving plankton succession. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 429–448.
- Huber, A.L. Factors affecting the germination of akinetes of Nodularia spumigena (Cyanobacteriaceae). Appl. Environ. Microbiol. 1985, 49, 73–78.
- Baker, P.D.; Bellifemine, D. Environmental influences on akinete germination of Anabaena circinalis and implications for management of cyanobacterial blooms. Hydrobiologia 2000, 427, 65–73.
- Park, C.H.; Lim, B.J.; You, K.A.; Park, M.H.; Hwang, S.-J. Effects of environmental factors on akinete germination of Anabaena circinalis (Cyanobacteriaceae) isolated from the North Han River, Korea. Korean J. Ecol. Environ. 2014, 47, 292–301.
- Rai, A.K.; Pandey, G.P. Influence of environmental stress on the germination of Anabaena vaginicola akinetes. Ann. Bot. 1981, 48, 361–370.
- Gorzó, G. Fizikai és kémiai faktorok hatása a Balatonban elöforduló heterocisztás cianobaktériumok spóráinak csírázására (The influence of physical and chemical factors on the germination of spores of heterocystic cyanobacteria in lake Balaton). Hidrológiai Közlön 1987, 67, 127–133.
- Padisák, J. Estimation of minimum sedimentary inoculum (akinete) pool of Cylindrospermopsis raciborskii: A morphology and life-cycle based method. In Phytoplankton and Equilibrium Concept: The Ecology of Steady-State Assemblages; Springer Science: Berlin, Germany, 2003; pp. 389–394.
- Hong, Y.; Steinman, A.; Biddanda, B.; Rediske, R.; Fahnenstiel, G. Occurrence of the toxin-producing cyanobacterium Cylindrospermopsis raciborskii in Mona and Muskegon Lakes, Michigan. J. Great Lakes Res. 2006, 32, 645–652.
- Tsujimura, S.; Okubo, T. Development of Anabaena blooms in a small reservoir with dense sediment akinete population, with special reference to temperature and irradiance. J. Plankton Res. 2003, 25, 1059–1067.
- Kim, B.H.; Lee, W.S.; Kim, Y.O.; Lee, H.O.; Han, M.S. Relationship between akinete germination and vegetative population of Anabaena flos-aquae (Nostocales, Cyanobacteria) in Seokchon reservoir (Seoul, Korea). Arch. Hydrobiol. 2005, 163, 49–64.
- Agrawal, S.C.; Singh, V. Vegetative survival, akinete formation and germination in three blue-green algae and one green alga in relation to light intensity, temperature, heat shock and UV exposure. Folia Microbiol. 2000, 45, 439–446.
- Bischoff, B.; Wiencke, C. Temperature ecotypes and biogeography of Acrosiphoniales (Chlorophyta) with Arctic-Antarctic disjunct and Arctic/cold-temperature distributions. Eur. J. Phycol. 1995, 30, 19–27.
- Jia, N.; Wang, Y.; Guan, Y.; Chen, Y.; Li, R.; Yu, G. Occurrence of Raphidiopsis raciborskii blooms in cool waters: Synergistic effects of nitrogen availability and ecotypes with adaptation to low temperature. Environ. Pollut. 2021, 270, 116070.
- Piccini, C.; Aubriot, L.; Fabre, A.; Amaral, V.; González-Piana, M.; Giani, A.; Figueredo, C.C.; Vidal, L.; Kruk, C.; Bonilla, S. Genetic and eco-physiological differences of South American Cylindrospermopsis raciborskii isolates support the hypothesis of multiple ecotypes. Harmful Algae 2011, 10, 644–653.
- Yamamoto, Y. Effect of some physical and chemical factors on the germination of akinetes of Anabaena cylindrica. J. Gen. Appl. Microbiol. 1976, 22, 311–323.
- van Dok, W.; Hart, B.T. Akinete germination in Anabaena circinalis (cyanophta). J. Phycol. 1997, 33, 12–17.
- Herdman, M. Cellular differentiation: Akinetes. Methods Enzymol. 1988, 167, 222–232.
- Calomeni, A.J.; McQueen, A.D.; Kinley-Baird, C.M.; Clyde, G.A. Identification and Preventative Treatment of Overwintering Cyanobacteria in Sediments: A Literature Review; ERDC/EL TR-22-10; U.S. Army Engineer Research and Development Center: Vicksburg, MS, USA, 2022.
- Karlsson-Elfgren, I.; Brunberg, A.K. The Importance of Shallow Sediments in the Recruitment of Anabaena and Aphanizomenon (Cyanophyceae). J. Phycol. 2004, 40, 831–836.
- Simon, R.D. Inclusion bodies in the cyanobacteria: Cyanophycin, polyphosphate, polyhedral bodies. In The Cyanobacteria; Elsevier Science Publishers B.V.: Amsterdam, The Netherlands, 1987; pp. 199–225.
- Sukenik, A.; Hadas, O.; Kaplan, A.; Quesada, A. Invasion of Nostocales (cyanobacteria) to subtropical and temperate freshwater lakes–physiological, regional, and global driving forces. Front. Microbiol. 2012, 3, 86.
- Agrawal, S.; Misra, U. Vegetative survival, akinete and zoosporangium formation and germination in some selected algae as affected by nutrients, pH, metals, and pesticides. Folia Microbiol. 2002, 47, 527–534.
- Agrawal, S.C. Factors controlling induction of reproduction in algae—Review: The text. Folia Microbiol. 2012, 57, 387–407.
- Myers, J.H.; Beardall, J.; Allinson, G.; Salzman, S.; Gunthorpe, L. Environmental influences on akinete germination and development in Nodularia spumigena (Cyanobacteriaceae), isolated from the Gippsland Lakes, Victoria, Australia. Hydrobiologia 2010, 649, 239–247.
- Agrawal, S.C.; Sharma, U.K. Sporulation and spore germination in Westiellopsis prolifica JANET in various culture conditions. Phykos 1994, 33, 31–38.
- Hori, K.; Ishii, S.I.; Ikeda, G.; Okamoto, J.I.; Tanji, Y.; Weeraphasphong, C.; Unno, H. Behavior of filamentous cyanobacterium Anabaena spp. in water column and its cellular characteristics. Biochem. Eng. J. 2002, 10, 217–225.
- Olli, K.; Kangro, K.; Kabel, M. Akinete production of Anabaena lemmermannii and A. cylindrica (cyanophyceae) in natural populations of N-and P-limited coastal mesocosms. J. Phycol. 2005, 41, 1094–1098.
- Li, R.; Watanabe, M.; Watanabe, M.M. Akinete formation in planktonic Anabaena spp. (Cyanobactefua) by treatment with low temperature. J. Phycol. 1997, 33, 576–584.
- Moore, D.; O’donohue, M.; Shaw, G.; Critchley, C. Potential triggers for akinete differentiation in an Australian strain of the cyanobacterium Cylindrospermopsis raciborskii (AWT 205/1). Hydrobiologia 2003, 506, 175–180.
- Dextro, R.B.; Moutinho, F.H.M.; Nordi, C.S.F. Growth and special structures production of Nostoc paludosum (Nostocaceae, Cyanobacteria) under nutrient starvation and different light intensities. Rev. Ambiente Água 2018, 13, 1–16.
- Lopez-Archilla, A.I.; Moreira, D.; López-García, P.; Guerrero, C. Phytoplankton diversity and cyanobacterial dominance in a hypereutrophic shallow lake with biologically produced alkaline pH. Extremophiles 2004, 8, 109–115.
- Mishra, B.N.; Kaushik, M.S.; Abraham, G.; Singh, P.K. Physico-chemical factors influencing spore germination in cyanobacterium Fischerella muscicola. J. Basic Microbiol. 2018, 58, 679–685.
- Reddy, P.M. Influence of pH on Sporulation, Spore Germination and Germling Survival in Blue-green Algae. Acta Hydrochim. Hydrobiol. 1984, 12, 411–417.
- Holm-Hansen, O. Ecology, physiology, and biochemistry of blue-green algae. Annu. Rev. Microbiol. 1968, 22, 47–70.
- Singh, P.K. Algicidal effect of 2, 4-dichlorophenoxy acetic acid on blue-green alga Cylindrospermum sp. Arch. Microbiol. 1974, 97, 69–72.
- Kwon, D.; Kim, K.; Jo, H.; Lee, S.D.; Yun, S.M.; Park, C. Environmental factors affecting akinete germination and resting cell awakening of two cyanobacteria. Appl. Microsc. 2023, 53, 2.
- Agrawal, S.C. Factors affecting spore germination in algae. Folia Microbiol. 2009, 54, 273–302.
- Brunberg, A.K.; Blomqvist, P. Recruitment of Microcystis (Cyanophyceae) from lake sediments: The importance of littoral inocula. J. Phycol. 2003, 39, 58–63.
- Chauvat, F.; Corre, B.; Herdman, M.; Joset-Espardellier, F. Energetic and metabolic requirements for the germination of akinetes of the cyanobacterium Nostoc PCC 7524. Arch. Microbiol. 1982, 133, 44–49.
- Pandey, R.K.; Talpasayi, E.R.S. Factors affecting germination of spores in a blue-green alga Nodularia spumigena. Acta Bot. Indica 1981, 9, 35–42.
- Silveira, S.B.; Odebrecht, C. Effects of salinity and temperature on the growth, toxin production, and akinete germination of the cyanobacterium Nodularia spumigena. Front. Mar. Sci. 2019, 6, 339.
- Faithfull, C.L.; Burns, C.W. Effects of salinity and source of inocula on germination of Anabaena akinetes from a tidally influenced lake. Freshw. Biol. 2006, 51, 705–716.
- Ståhl-Delbanco, A.; Hansson, L.A. Effects of bioturbation on recruitment of algal cells from the “seed bank” of lake sediments. Limnol. Oceanogr. 2002, 47, 1836–1843.
- Hadas, O.; Pinkas, R.; Delphine, E.; Vardi, A.; Kaplan, A.; Sukenik, A. Limnological and ecophysiological aspects of Aphanizomenon ovalisporum bloom in Lake Kinneret, Israel. J. Plankton Res. 1999, 21, 1439–1453.
More
Information
Subjects:
Environmental Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
621
Revisions:
2 times
(View History)
Update Date:
12 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




