Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Wensuo Jia | -- | 2292 | 2024-03-12 03:15:35 | | | |
| 2 | Jessie Wu | + 10 word(s) | 2302 | 2024-03-12 03:29:16 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Jin, J.; Wang, W.; Fan, D.; Hao, Q.; Jia, W. MAPK Signaling in Fruit Ripening and Quality Formation. Encyclopedia. Available online: https://encyclopedia.pub/entry/56139 (accessed on 07 February 2026).
Jin J, Wang W, Fan D, Hao Q, Jia W. MAPK Signaling in Fruit Ripening and Quality Formation. Encyclopedia. Available at: https://encyclopedia.pub/entry/56139. Accessed February 07, 2026.
Jin, Juan, Wei Wang, Dingyu Fan, Qing Hao, Wensuo Jia. "MAPK Signaling in Fruit Ripening and Quality Formation" Encyclopedia, https://encyclopedia.pub/entry/56139 (accessed February 07, 2026).
Jin, J., Wang, W., Fan, D., Hao, Q., & Jia, W. (2024, March 12). MAPK Signaling in Fruit Ripening and Quality Formation. In Encyclopedia. https://encyclopedia.pub/entry/56139
Jin, Juan, et al. "MAPK Signaling in Fruit Ripening and Quality Formation." Encyclopedia. Web. 12 March, 2024.
Copy Citation
Fleshy fruit ripening is a unique biological process that involves dramatic changes in a diverse array of cellular metabolisms. The regulation of these metabolisms is essentially mediated by cellular signal transduction of internal (e.g., hormones) and external cues (i.e., environmental stimuli). Mitogen-activated protein kinase (MAPK) signaling pathways play crucial roles in a diverse array of biological processes, such as plant growth, development and biotic/abiotic responses. Accumulating evidence suggests that MAPK signaling pathways are also implicated in fruit ripening and quality formation.
MAPK
signal transduction
fruit ripening
postharvest quality
1. Roles of Mitogen-Activated Protein Kinase Signaling in Fruit Development and Ripening as Revealed by Loss-of-Function and Gain-of-Function Studies
Loss of function and gain of function are basic strategies for the functional identification of genes. They can be realized using several technologies. As far as fruit studies are concerned, the most commonly used technologies are gene knockdown/out and overexpression. Loss-of-function and gain-of-function studies strongly suggest that a number of mitogen-activated protein kinase (MAPK) signaling components, modules and pathways play crucial roles in the regulation of a diverse assay of biological processes in fruit ripening and quality formation, as summarized and discussed below.
1.1. Mitogen-Activated Protein Kinase Signaling Mediates Ripening-Associated Metabolisms
An early study of tomatoes showed that MAPK pathway inhibitor U0126 treatment resulted in a reduction in fruit ET, proline production, fruit firmness and cold tolerance. Moreover, in response to cold and ethephon treatment, the ET content, the activity of ACS and ACO and the expression of LeACS2, LeACO1 and LeMAPK4 increased, indicating that MAPK signaling was involved in the regulation of ET production and a variety of ripening-associated metabolisms [1]. More recently, a study by Shu et al. (2023) [2] reported that cold treatment promoted SlMAPK3 expression in tomato fruit in an ET-dependent manner; meanwhile, the overexpression of SlMAPK3 promoted ET production and the expression of ET-, cold- and heat-responsive genes in tomato fruits [2]. In strawberries, a study by Mao et al. (2021) [3] reported that low temperatures inhibited anthocyanin metabolism. The results of their study showed that FvMPK3 could phosphorylate and enhance the degradation of FvMYB10, a key TF controlling anthocyanin accumulation. As the activity of FvMPK3 could be induced by low temperature, the authors proposed that the low temperature-induced inhibition of anthocyanin accumulation resulted from the activation of FvMPK3. Noteworthily, many MAPKs have been commonly reported to be activated transcriptionally or post-transcriptionally in different plant species [4][5][6][7][8][9]. In particular, FvMPK6, a MAPK with a similar structure and function to FvMPK3, was reported to be sensitively activated by high temperatures in strawberries. This has raised the question of whether high temperature-induced anthocyanin accumulation might be related to FvMPK3. To clarify this, the pattern of FvMPK3 responses to high-temperature stress needs to be investigated. In Arabidopsis, AtMPK3 was reported to be activated by ABA [10][11]. Given that ABA is a key regulator of strawberry fruit ripening, it is important to elucidate whether ABA-induced anthocyanin accumulation is correlated to FvMPK3 in strawberry fruit ripening.
In apples (Malus domestica), a study by Sun et al. (2022) [12] reported that low-nitrogen conditions could induce anthocyanin synthesis in apple callus and promote the expression of mitogen-activated protein kinase 9 (MdMKK9). CRISPR/Cas9 mutation of MdMKK9 compromised low-nitrogen-induced anthocyanin biosynthesis. Conversely, the overexpression of MdMKK9 induced anthocyanin synthesis and a set of anthocyanin metabolism-associated genes, suggesting that MdMKK9 was implicated in the modification of color metabolism via nitrogen availability. HY5 plays crucial roles in light-induced anthocyanin accumulation. A more recent study reported that the mitogen-activated protein kinase MdMPK6 could interact with and phosphorylate MdHY5 in response to light signaling. Light-activated MdMPK6 phosphorylated MdHY5, leading to the anthocyanin accumulation of related genes in apple fruit [13]. In the banana (Musa acuminata), Wu et al. (2019) [14] identified a basic leucine zipper (bZIP) TF, MabZIP93, and showed that MabZIP93 acted to control the expression of a set of genes implicated in cell wall metabolisms, such as MaPL2, MaPE1, MaXTH23 and MaXGT1. Transient overexpression of MabZIP93 in banana fruit promoted the expression of MaPL2, MaPE1, MaXTH23 and MaXGT1. The mitogen-activated protein kinase MaMPK2 could interact with and phosphorylate MabZIP93. These observations suggest that the MaMPK2-MabZIP93 signaling module functions in the regulation of cell wall metabolism during banana fruit ripening [14].
1.2. Mitogen-Activated Protein Kinase Signaling Targets Ripening-Associated Transcription Factors
In the banana (Musa acuminata), through phosphoproteomic analysis during fruit ripening, Wu et al. (2022) [15] identified MabZIP21, a basic leucine zipper TF 21, which could be phosphorylated by MaMPK6-3. Transient overexpression of the phosphomimetic form of MabZIP21 accelerated banana fruit ripening. These results suggested that the MaMPK6-3-MabZIP21 signaling module played a role in the regulation of banana fruit ripening [15]. Subsequently, the authors demonstrated that MaMKK1 was implicated in the regulation of banana fruit ripening. Transient overexpression or silencing of MaKK1 in fruit, respectively, accelerated and delayed banana fruit ripening. MaMKK1 could interact with and phosphorylate MaMPK6-3 and MaMPK11, thereby activating the MPK kinases, and MaMPK11-4 could in turn phosphorylate MabZIP21 to regulate its transcription activity. As such, the authors identified the signaling pathway of the MaMPK6-3/11-4-MabZIP21 module that was implicated in the regulation of banana fruit ripening [16].
In the apple (Malus domestica), a study by Wei et al. (2023) [17] reported that the activity of MdMAPK3 was activated by ET. MdMAPK3 could interact with and phosphorylate MdNAC72, a NAM-ATAF1/2-CUC2 72 TF, which acted to repress the expression of the cell-wall-degradation-related gene POLYGALACTURONASE1 (MdPG1). Moreover, the protein stability of MdNAC72 was modulated by MdMAPK3-targeted phosphorylation in response to ET signaling via an E3 ubiquitin ligase pathway. Transient overexpression of MdMAPK3 promoted fruit ripening and, conversely, transiently silenced MdMAPK3 in fruit showed the phenotype opposite to MdMAPK3-OE fruit during storage. These results demonstrated that the MdMAPK3-MdNAC72 signaling module plays an important role in apple fruit ripening [17]. A study by Yang et al. (2022) [18] showed that the protein level of the TF MaMYB4 decreased along with banana fruit ripening, which was coupled with ET production and a decline in firmness. MaMYB4 could bind to the promoters of a series of ripening-associated genes, including ET biosynthetic and cell-wall-modifying genes. In addition, the authors found that the protein levels of the two RING finger E3 ligases MaBRG2/3 increased with fruit ripening; moreover, they could interact with and ubiquitinate MaMYB4 to promote its degradation. Collectively, these results suggest that MaMYB4 negatively modulated banana fruit ripening [18]. A further study suggested that transient overexpression of MaMPK14 and MaMYB4 delayed fruit ripening. MaMYB4 represses the expression of the genes involved in ET biosynthesis and fruit softening, such as MaACS1, MaXTH5, MaPG3 and MaEXPA15. Moreover, MaMPK14 was shown to be able to phosphorylate MaMYB4 at Ser160 to reduce the interaction with MaMPK14 [19].
Yang et al. (2021) [20] reported that the expression of two MdMPK4 genes was induced by light. MdMPK4 could interact with MdMYB1, and the overexpression of MdMPK4 and MdMYB1 promoted anthocyanin accumulation in apple (Malus domestica) fruit peels, suggesting that the MdMPK4-MdMYB1 signaling module is implicated in light-induced anthocyanin accumulation [20].
In summary, it has been increasingly suggested that the loss of function or gain of function of some members of MAPs kinases may be able to alter the progress of fruit ripening. However, it should be noted that fruit ripening is orchestrated by dramatic changes in diverse cellular metabolisms. Most of the studies on MAPK signaling in relation to fruit ripening have focused on the effects of loss of function and gain of function in MAPs kinase on the accumulation of anthocyanin. To comprehensively demonstrate the role of MAPK signaling in fruit ripening, studies on other related metabolisms, such as sugar, acid, texture, flavor, etc., are required. Also, owing to the lack of stable transgenic techniques for most FFPs, many loss-of-function and gain-of-function studies of fruit have used unreliable transient expression systems to draw associated conclusions. Regardless, current studies have provided strong evidence for the involvement of MAPK signaling in the regulation of fruit ripening.
2. Roles of Mitogen-Activated Protein Kinase Signaling in the Regulation of Fruit Disease Resistance as Demonstrated by Loss-of-Function and Gain-of-Function Studies
Fruit postharvest quality is determined by changing patterns of cellular metabolisms during fruit ripening, such as sugar, acid, color, flavor, texture, etc. Besides these common quality parameters, fruit storage quality is especially important because of the huge economic loss caused by fruit rot worldwide. Although MAPK signaling is involved in the regulation of a diverse array of biological processes, a major role of MAPK signaling is in mediating plant defense responses [21][22][23][24]. Accordingly, it is likely that MAPK signaling plays a crucial role in the formation of fruit storage quality. In support of this speculation, a number of studies have provided evidence for the important role of MAPK signaling in fruit disease resistance. For example, a pharmacological study showed that the application of acibenzolar-S-methyl (ASM), a functional analog of salicylic acid, and the mitogen-activated protein kinase (MAPK) inhibitor PD98059 to apple fruit provided resistance to infection by Penicillium expansum. ASM treatment inhibited lesion growth and the activities of a series of ROS-scavenging enzymes, resulting in the elevation of H2O2 content. Meanwhile, ASM treatment promoted the expression of MdMAPK4, MdMAPK2 and MdMAPKK1, whereas it suppressed the expression of MdMAPK3. PD98059 +ASM treatment increased CAT activity. These findings indicate that MAPK cascades are implicated in ASM-induced apple fruit resistance to Penicillium expansum [25].
FERONIA (FER) is a receptor-like kinase that plays important roles in diverse biological processes. It has been demonstrated that FER regulates fruit ripening [26][27]. In the tomato (Solanum lycopersicum), a recent study by Ji et al. (2023) [28] showed that SlFERL, a receptor-like-kinase, interacted with the secreted virulence protein BcPG1 from Botrytis cinerea. SlFERL acts to trigger downstream signaling by phosphorylating SlMAP3K18 at Thr45, Ser49, Ser76 and Ser135. SlMAP2K2 and SlMAP2K4 were shown to be related to the immune response of tomatoes to Botrytis cinerea. Furthermore, the phosphorylation of SlMAP2K2/SlMAP2K4 by SlMAP3K18 modulated protein stability and kinase activity. Thus, this research identified a signaling cascade: BcPG1-SlFERL-SlMAP3K18-SlMAP2K2/SlMAP2K4-immuno response to Botrytis cinerea invasion. Li et al. (2021) [29] reported that the application of exogenous β-aminobutyric acid (BABA) could induce TGA1-related systemic acquired resistance (SAR), thereby alleviating Rhizopus rot in postharvest peach fruit. Upon treatment with BABA, the expressions of a set of redox-regulated genes were promoted, and the activity of PpTGA1, a key redox-controlled factor of SAR essential for the activation of priming resistance in postharvest peach fruit [30], was elevated. PpMAPKK5 was shown to interact with and regulate the DNA-binding activity of PpTGA1 for the activation of salicylic acid (SA)-responsive PR genes. The heterologous expression of PpMAPKK5 in Arabidopsis conferred resistance against the fungus Rhizopus stolonifer. These observations suggest that the PpMAPKK5-PpTGA1 module has a disease-resistance function in postharvest peach fruit [29]. In plants, MAPK cascades have a critical role in the regulation of plant immunity and disease resistance. Although the function of MAPK cascades in immunity regulation is partially conserved between different species, the mechanisms vary in different host and pathogen combinations. To date, the MAPK cascade function of woody plants in the regulation of disease resistance has seldom been reported. A study by Wang et al. (2022) [31] showed that Botryosphaeria dothidea infection induced MdMAPKKK1 expression in apple fruit. The overexpression of MdMAPKKK1 induced pathogen-independent cell death, thereby increasing fruit resistance to B. dothidea, and conversely, MdMAPKKK1 silencing reduced fruit resistance to B. dothidea. MdMAPKKK1 could interact with and be phosphorylated by MdBSK1, a brassinosteroid-signaling kinase protein. MdBSK1 silencing also reduced fruit resistance to B. dothidea, implying that the MdBSK1- MdMAPKKK1 signaling module may play an important role in apple fruit resistance to B. dothidea [31].
In summary, it has been established that MAPK signaling plays crucial roles in the defense responses of Arabidopsis and some crop plants [21][22][23][24]. Similarly, it has been increasingly reported that MAPK signaling may also play an important role in FFP resistance to biotic stresses as discussed above. Fruit shelf life constitutes an essential part of fruit quality. While reports on the role of MAPK signaling in FFP disease resistance are accumulating, studies on the role of MAPK signaling in fruit shelf life are still very limited. Furthermore, fruit shelf life is not only determined by fruit disease resistance but also by the ripening progress. Given that MAPK signaling may play an important role in FFP disease resistance and fruit ripening, to decipher the mechanism of MAPK signaling in relation to fruit shelf life, a profound analysis of the distinct roles of MAPK signaling in fruit disease resistance and ripening is required.
As summarized in Figure 1, a number of MAPs kinases have been identified to be involved in fruit ripening and quality formation. Moreover, some specific signaling modules and pathways of MAPs kinases have been elucidated. As most of them were identified via loss-of-function and gain-of-function studies, it can be concluded that MAPK signaling plays essential roles in the regulation of fruit ripening and quality formation. Nevertheless, current information in this research area is still very limited, and the profound mechanisms remain largely unknown. Reseachers have proposed several points and questions that should be given special consideration in future studies.
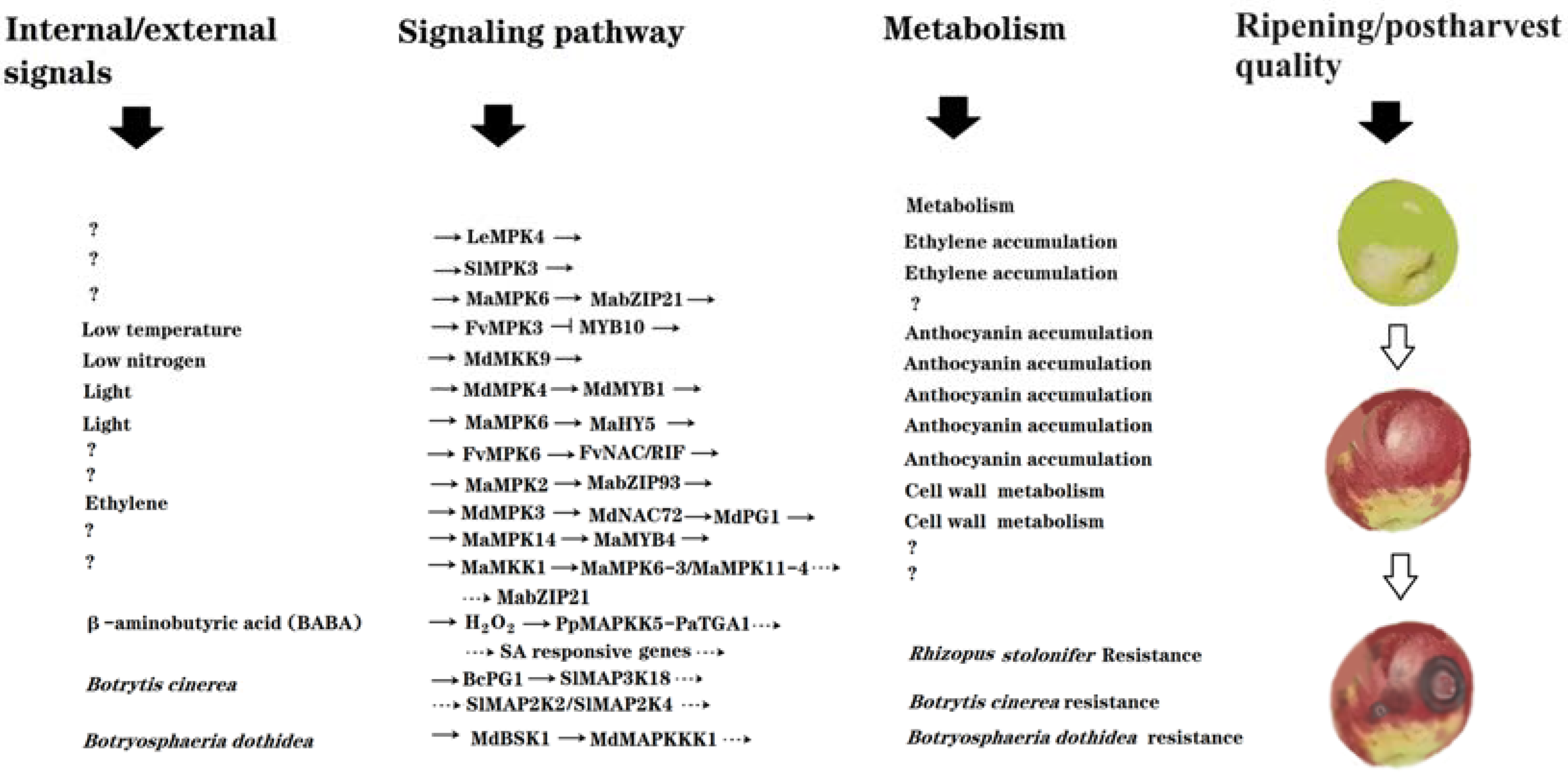
Figure 1. Diagram showing the updated signaling components and pathways of MAPs kinases identified as being implicated in the regulation of fruit ripening and postharvest quality. Fruit ripening and postharvest quality are determined by internal and external signals or, alternatively, by hormonal and environmental signals. Fruit ripening and quality are essentially determined by complicated cellular metabolisms, such as color, sugar, acid, flavor, texture, etc. In addition, anti-pathogenic activity is also an important part of fruit quality, as it determines the postharvest shelf life of fruits. MAPK signaling mediates the internal/external signal-triggered regulation of fruit ripening and quality-associated metabolisms. ‘?’ denotes currently unidentified components/biological events; ‘→’ denotes activation; and a horizontal ‘⊥’ denotes inhibition. Plant species are as follows: ‘Sl’ for tomato ‘Solanum lycopersicum’; ‘Le’ for tomato ‘Lycopersicon esculentum’; ‘Ma’ for banana ‘Musa acuminata’; ‘Fv’ for strawberry ‘Fragaria vesca’; ‘Md’ for apple ‘Malus domestica’.
References
- Zhao, R.; Xie, H.; Lv, S.; Zheng, Y.; Yu, M.; Shen, L.; Sheng, J. LeMAPK4 participated in cold-induced ethylene production in tomato fruit. J. Sci. Food Agric. 2013, 93, 1003–1009.
- Shu, P.; Li, Y.; Sheng, J.; Shen, L. SlMAPK3 Positively Regulates the Ethylene Production of Postharvest Tomato Fruits and Is Involved in Ethylene-Mediated Cold Tolerance. J. Agric. Food Chem. 2023, 71, 6003–6013.
- Mao, W.; Han, Y.; Chen, Y.; Sun, M.; Feng, Q.; Li, L.; Liu, L.; Zhang, K.; Wei, L.; Han, Z.; et al. Low temperature inhibits anthocyanin accumulation in strawberry fruit by activating FvMAPK3-induced phosphorylation of FvMYB10 and degradation of Chalcone Synthase 1. Plant Cell 2022, 34, 1226–1249.
- Ahlfors, R.; Macioszek, V.; Rudd, J.; Brosché, M.; Schlichting, R.; Scheel, D.; Kangasjärvi, J. Stress hormone-independent activation and nuclear translocation of mitogen-activated protein kinases in Arabidopsis thaliana during ozone exposure. Plant J. Cell Mol. Biol. 2004, 40, 512–522.
- Mo, S.; Qian, Y.; Zhang, W.; Qian, L.; Wang, Y.; Cailin, G.; Ding, H. Mitogen-activated protein kinase action in plant response to high-temperature stress: A mini review. Protoplasma 2021, 258, 477–482.
- Wang, T.; Liu, M.; Wu, Y.; Tian, Y.; Han, Y.; Liu, C.; Hao, J.; Fan, S. Genome-Wide Identification and Expression Analysis of MAPK Gene Family in Lettuce (Lactuca sativa L.) and Functional Analysis of LsMAPK4 in High- Temperature-Induced Bolting. Int. J. Mol. Sci. 2022, 23, 11129.
- Wang, Z.; Wan, Y.; Meng, X.; Zhang, X.; Yao, M.; Miu, W.; Zhu, D.; Yuan, D.; Lu, K.; Li, J.; et al. Genome-Wide Identification and Analysis of MKK and MAPK Gene Families in Brassica Species and Response to Stress in Brassica napus. Int. J. Mol. Sci. 2021, 22, 544.
- Zaynab, M.; Hussain, A.; Sharif, Y.; Fatima, M.; Sajid, M.; Rehman, N.; Yang, X.; Khan, K.A.; Ghramh, H.A.; Li, S. Mitogen-Activated Protein Kinase Expression Profiling Revealed Its Role in Regulating Stress Responses in Potato (Solanum tuberosum). Plants 2021, 10, 1371.
- Zhang, X.; Wang, X.; Zhuang, L.; Gao, Y.; Huang, B. Abscisic acid mediation of drought priming-enhanced heat tolerance in tall fescue (Festuca arundinacea) and Arabidopsis. Physiol. Plant. 2019, 167, 488–501.
- Hasan, M.M.; Liu, X.D.; Waseem, M.; Guang-Qian, Y.; Alabdallah, N.M.; Jahan, M.S.; Fang, X.W. ABA activated SnRK2 kinases: An emerging role in plant growth and physiology. Plant Signal. Behav. 2022, 17, 2071024.
- Sun, Y.; Pri-Tal, O.; Michaeli, D.; Mosquna, A. Evolution of Abscisic Acid Signaling Module and Its Perception. Front. Plant Sci. 2020, 11, 934.
- Sun, X.; Li, X.; Wang, Y.; Xu, J.; Jiang, S.; Zhang, Y. MdMKK9-Mediated the Regulation of Anthocyanin Synthesis in Red-Fleshed Apple in Response to Different Nitrogen Signals. Int. J. Mol. Sci. 2022, 23, 7755.
- Xing, Y.; Sun, W.; Sun, Y.; Li, J.; Zhang, J.; Wu, T.; Song, T.; Yao, Y.; Tian, J. MPK6-mediated HY5 phosphorylation regulates light-induced anthocyanin accumulation in apple fruit. Plant Biotechnol. J. 2023, 21, 283–301.
- Wu, C.; Shan, W.; Liang, S.; Zhu, L.; Guo, Y.; Chen, J.; Lu, W.; Li, Q.; Su, X.; Kuang, J. MaMPK2 enhances MabZIP93-mediated transcriptional activation of cell wall modifying genes during banana fruit ripening. Plant Mol. Biol. 2019, 101, 113–127.
- Wu, C.J.; Shan, W.; Liu, X.C.; Zhu, L.S.; Wei, W.; Yang, Y.Y.; Guo, Y.F.; Bouzayen, M.; Chen, J.Y.; Lu, W.J.; et al. Phosphorylation of transcription factor bZIP21 by MAP kinase MPK6-3 enhances banana fruit ripening. Plant Physiol. 2022, 188, 1665–1685.
- Wu, C.; Deng, W.; Shan, W.; Liu, X.; Zhu, L.; Cai, D.; Wei, W.; Yang, Y.; Chen, J.; Lu, W.; et al. Banana MKK1 modulates fruit ripening via the MKK1-MPK6-3/11-4-bZIP21 module. Cell Rep. 2023, 42, 112832.
- Wei, Y.; Liu, Z.; Lv, T.; Xu, Y.; Wei, Y.; Liu, W.; Liu, L.; Wang, A.; Li, T. Ethylene enhances MdMAPK3-mediated phosphorylation of MdNAC72 to promote apple fruit softening. Plant Cell 2023, 35, 2887–2909.
- Yang, Y.Y.; Shan, W.; Yang, T.W.; Wu, C.J.; Liu, X.C.; Chen, J.Y.; Lu, W.J.; Li, Z.G.; Deng, W.; Kuang, J.F. MaMYB4 is a negative regulator and a substrate of RING-type E3 ligases MaBRG2/3 in controlling banana fruit ripening. Plant J. 2022, 110, 1651–1669.
- Yang, Y.; Wu, C.; Shan, W.; Wei, W.; Zhao, Y.; Kuang, J.; Chen, J.; Jiang, Y.; Lu, W. Mitogen-activated protein kinase 14-mediated phosphorylation of MaMYB4 negatively regulates banana fruit ripening. Hortic. Res. 2023, 10, uhac243.
- Yang, T.; Ma, H.; Li, Y.; Zhang, Y.; Zhang, J.; Wu, T.; Song, T.; Yao, Y.; Tian, J. Apple MPK4 mediates phosphorylation of MYB1 to enhance light-induced anthocyanin accumulation. Plant J. 2021, 106, 1728–1745.
- Hamel, L.P.; Nicole, M.C.; Duplessis, S.; Ellis, B.E. Mitogen-activated protein kinase signaling in plant-interacting fungi: Distinct messages from conserved messengers. Plant Cell 2012, 24, 1327–1351.
- Sun, T.; Zhang, Y. MAP kinase cascades in plant development and immune signaling. EMBO Rep. 2022, 23, e53817.
- Pitzschke, A.; Schikora, A.; Hirt, H. MAPK cascade signalling networks in plant defence. Curr. Opin. Plant Biol. 2009, 12, 421–426.
- Thulasi Devendrakumar, K.; Li, X.; Zhang, Y. MAP kinase signalling: Interplays between plant PAMP- and effector-triggered immunity. Cell. Mol. Life Sci. 2018, 75, 2981–2989.
- Cheng, Y.; Li, C.; Hou, J.; Li, Y.; Jiang, C.; Ge, Y. Mitogen-Activated Protein Kinase Cascade and Reactive Oxygen Species Metabolism are Involved in Acibenzolar-S-Methyl-Induced Disease Resistance in Apples. J. Agric. Food Chem. 2020, 68, 10928–10936.
- Jia, M.; Ding, N.; Zhang, Q.; Xing, S.; Wei, L.; Zhao, Y.; Du, P.; Mao, W.; Li, J.; Li, B.; et al. A FERONIA-Like Receptor Kinase Regulates Strawberry (Fragaria × ananassa) Fruit Ripening and Quality Formation. Front. Plant Sci. 2017, 8, 1099.
- Jia, M.; Du, P.; Ding, N.; Zhang, Q.; Xing, S.; Wei, L.; Zhao, Y.; Mao, W.; Li, J.; Li, B.; et al. Two FERONIA-Like Receptor Kinases Regulate Apple Fruit Ripening by Modulating Ethylene Production. Front. Plant Sci. 2017, 8, 1406.
- Ji, D.; Liu, W.; Cui, X.; Liu, K.; Liu, Y.; Huang, X.; Li, B.; Qin, G.; Chen, T.; Tian, S. A receptor-like kinase SlFERL mediates immune responses of tomato to Botrytis cinerea by recognizing BcPG1 and fine-tuning MAPK signaling. New Phytol. 2023, 240, 1189–1201.
- Li, C.; Wang, K.; Huang, Y.; Lei, C.; Cao, S.; Qiu, L.; Xu, F.; Jiang, Y.; Zou, Y.; Zheng, Y. Activation of the BABA-induced priming defence through redox homeostasis and the modules of TGA1 and MAPKK5 in postharvest peach fruit. Mol. Plant Pathol. 2021, 22, 1624–1640.
- Li, C.; Wang, K.; Zheng, Y. Redox status regulates subcelluar localization of PpTGA1 associated with a BABA-induced priming defence against Rhizopus rot in peach fruit. Mol. Biol. Rep. 2020, 47, 6657–6668.
- Wang, N.; Liu, Y.; Dong, C.; Zhang, Y.; Bai, S. MdMAPKKK1 Regulates Apple Resistance to Botryosphaeria dothidea by Interacting with MdBSK1. Int. J. Mol. Sci. 2022, 23, 4415.
More
Information
Subjects:
Horticulture
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
555
Revisions:
2 times
(View History)
Update Date:
12 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




