Precision cancer medicine primarily aims to identify individual patient genomic variations and exploit vulnerabilities in cancer cells to select suitable patients for specific drugs. These genomic features are commonly determined by gene sequencing prior to therapy, to identify individuals who would be most responsive. This precision approach in cancer therapeutics remains a powerful tool that benefits a smaller pool of patients, sparing others from unnecessary treatments. A limitation of this approach is that proteins, not genes, are the ultimate effectors of biological functions, and therefore the targets of therapeutics. An additional dimension in precision medicine that considers an individual’s cytokine response to cancer therapeutics is proposed. Cytokine responses to therapy are multifactorial and vary among individuals. Thus, precision is dictated by the nature and magnitude of cytokine responses in the tumor microenvironment exposed to therapy.
1. Precision Cancer Medicine
At the center of precision medicine, often interchangeably termed as ‘personalized medicine’ or ‘individualized medicine’, is the need to deliver ‘the right drug, with the right dose at the right time to the right patient’
[1][2]. Irrespective of the different interpretations of these terminologies, the overarching premise remains the recognition of individual patients’ molecular differences as the basis to guide disease management, and consideration of individual patients as independent biological units to avoid ‘group’-prescription of therapy and subsequent side-effects or non-responsiveness.
Currently, the most common application of precision cancer medicine involves pharmacogenomics, for which genomic determinants of drug metabolism (pharmacokinetics and pharmacodynamics) can be predicted from the polymorphisms and expressions of the enzymes that are involved
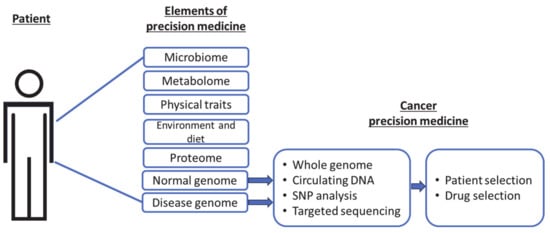
[3][4][5]. Specifically, genomic information from normal or malignant cells is used to select an appropriate drug or patient, with the intent to avoid unnecessary treatment (
Figure 1). Unlike proteomic, metabolomic, and other elements, genomic data are the least variable, making it the most stable and widely comparable across multiple platforms.
Figure 1. Current precision targets in cancer medicine as a subset of the broader elements of precision medicine.
It is practical and convenient to make clinical ‘individualization’ of patients based on the identification of genomic (DNA) or RNA signatures, and as such, to sort patients into ‘most or least likely to respond’, or ‘most likely to experience side-effects’ groups. However, precision medicine centered on DNA/RNA-based tools leaves a translational uncertainty, since proteins are the actionable targets and ultimate effectors of biological functions. This uncertainty becomes obvious when the proportions of patients ‘expected’ to respond are compared to patients who clinically ‘respond.’ Understandably, this uncertainty is multifactorial in origin and remains a challenge to identify and address the reasons for non-responsiveness, causing doubts about the entire precision approach
[6][7].
2. Cytokine Pathways as Therapeutic Targets
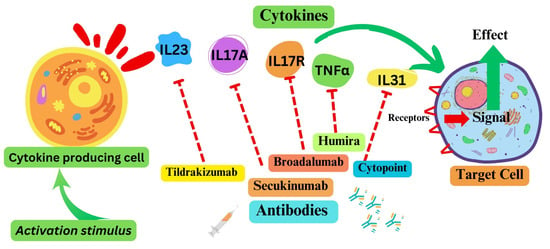
Cytokines, cytokine receptors, and associated pathways offer opportunities to target signaling pathways associated with various disease conditions. Given their roles, inflammatory cytokines and receptors, as potential therapeutic targets, have been the focus of researchers, clinicians, and pharmaceutical companies (
Figure 2). The most clinically advanced of these include antibody-based anti-psoriasis drugs targeting interleukin 23 (IL23, tildrakizumab), IL17A (secukinumab, ixekizumab), or IL17R (broadalumab). Additionally, anti-TNF antibodies have been deployed for the treatment of rheumatoid arthritis and psoriasis
[8][9][10]. Among the most widely used drugs are an anti-TNFα antibody drug for arthritis marketed for humans as Humira, and an anti-IL-31 antibody for canine atopic or allergic dermatitis used under the trade name Cytopoint
[11]. An IL6R-targeting mAb (tocilizumab) has been approved to treat a few inflammatory conditions, including rheumatoid arthritis and cytokine release syndrome after therapies
[12]. However, the utility of tocilizumab against COVID-19 could not be established unequivocally, although IL6 levels correlated with severe disease
[13][14]. IL6R antagonists, tocilizumab and sarilumab, are currently recommended for severely or critically ill COVID-19 patients (Therapeutics and COVID-19: living guideline. Geneva: World Health Organization
[15]). Two cytokines, IL2 and IFNα, exhibited moderate therapeutic effects for the treatment of a variety of malignant conditions and were thus approved by the Food and Drug Administration (FDA). IL2 is used for advanced metastatic renal cell cancer and melanoma
[16]; IFNα is indicated for hairy cell leukemia, follicular non-Hodgkins’s lymphoma, melanoma, and AIDS-related Kaposi’s sarcoma. In 2015, IL15 was approved for treatment of solid tumors
[17].
Figure 2. Some cytokine pathways as therapeutic targets. Note: The dotted red arrow represents the inhibition of the respective cytokine. Green arrows represent the activation signal.
Chemokines form a sub-group of cytokines characterized as molecules mediating the trafficking of immune cells
[18]. Although several drugs targeting chemokine pathways have been developed, only a few have been approved for a disease condition. As an example, maraviroc, a CCR5 co-receptor antagonist that interferes with HIV entry into CD4 T cells, was approved in 2007 for treatment of HIV patients, in combination with other antiviral medications
[19][20].
Currently, the primary goal in cytokine-directed therapy is to block the interaction of these molecules with the corresponding receptors, or to dampen the pathways downstream of the receptors. The impetus to advance the inhibitory approaches is because of the role of most of the targeted cytokines in enhancing disease processes, commonly inflammation. Unlike inhibition, an approach to enhance the expression or functions of cytokines for cancer therapy is still experimental, or is expected to be more challenging than inhibition, because of the need to fine-tune the activity of cytokines. For example, IL2, a potent T-cell mitogen, enhances antitumor responses of the immune system, but high doses can be detrimental to patients because of excessive cytokine release and activity
[21].
3. Cells of Cytokine Origin
Most cell types within the tumor microenvironment, including tumor cells, immune cells such as macrophages, dendritic cells, and T cells, as well as stromal cells such as fibroblasts and endothelial cells produce cytokines in response to chemotherapy or radiation. Thus, tumor cells are involved in their own response to treatment. Macrophages, which are involved in the immune response, also produce cytokines in response to chemotherapy and radiation. Dendritic cells, another type of immune cells, also produce cytokines
[22][23]; their role in cytokine production may involve the presentation of tumor antigens to T-cells, leading to an immune response. Antigen-stimulated CD4+ T-cells
[24], along with natural killer T cells
[25], CD8+ T-cells
[26], mast cells
[27], and dendritic cells
[28] are the primary producers of IL2. Endogenous IL2 therapy increases the expression of CD25 and IL2 receptor, with subsequent proliferation of CD8+ T-cells
[29]. IL2 enhances the expression of LAMP-1
[30] on the surface of CD8+ T-cells while decreasing the expression of PD-1
[31], an inhibitory receptor, thereby facilitating CD8+ T-cell cytotoxicity.
4. Cytokine Responses in Solid-Tumors Therapy: An Emerging Theme in Cancer Precision Medicine
As mentioned above, the concept of targeting cytokines or cytokine pathways to control specific diseases or processes is not new. After the role of receptors in viral entry was established, these pathways have even been targeted for infectious diseases such as HIV (CXCR4 and CCR5)
[32][33][34]. Although most primary solid or intraepithelial tumors can be treated with several types of chemotherapy and radiation, adaptive resistance of tumor cells and recurrence of cancer have hampered durable success of these therapies. The central theme of cancer precision medicine has been to identify patients who will favorably respond to a current therapy module, avoiding the side-effects of therapy on those who are least likely to respond
[7][35].
For colorectal cancers, the clinical trials and applications of precision medicine have been narrowly focused on microsatellite status and the MAPK pathway proteins downstream of EGFR and HER2 receptors
[36]; these are used for targeted therapeutics. Although the results and ultimate impact of these genomics-based approaches are still to be determined, to benefit patients, additional targets for precision medicine need to be identified. Microsatellite instability (MSI), defined by its genomic signatures and pro-mutagenic characteristics, is an appropriate tumor characteristic to identify colon cancer patients better suited for certain chemotherapy regimens, and especially for immunotherapy. However, a substantial proportion of the MSI tumors do not respond to current immunotherapy regimens
[37]. Within the multifactorial background of ‘non-responsiveness’, which may involve added gene alterations, the role of signaling proteins may be substantial. After initiation of standard cancer therapy, a panel of targetable proteins unique to a patient could constitute a framework for precision medicine. Since the type and intensity of protein-response by malignant and normal cells in the tumor microenvironment will vary among individuals, the development and inclusion of ‘therapy-induced cytokine signature’ is emerging as a new dimension of precision medicine
[38][39][40].
In malignant cells, cancer therapy using drugs or radiation elicits ‘danger’ signaling pathways, which have, primarily, a protective nature. These signals could be mediated by cytokines, and some cytokine pathways, such as transforming growth factor-beta (TGF-β), may have dual roles of inhibition or acceleration of tumor growth
[41][42]. Other cytokine pathways may drive cell death, attract or deflect immune cells, or induce a senescence-like state to remain dormant through the stress period
[43][44][45][46]. Therefore, an analysis of active cytokines in an individual’s cancer microenvironment or tissues during chemotherapy, especially in comparison to the pre-intervention levels or activities, could reveal information about the biological mechanisms operating in the tumor under therapy stress. Such cytokine readouts may correlate with patient clinical outcomes
[47][48][49][50]. Since cytokines are readily detectable in tissue fluids, this approach to precision medicine has an added value for clinical monitoring and decision making.