Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ronald B. Brown | -- | 1923 | 2024-03-11 13:48:57 | | | |
| 2 | Peter Tang | Meta information modification | 1923 | 2024-03-12 02:18:54 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Brown, R.B. Myopia, Sodium Chloride, and Vitreous Fluid Imbalance. Encyclopedia. Available online: https://encyclopedia.pub/entry/56116 (accessed on 07 February 2026).
Brown RB. Myopia, Sodium Chloride, and Vitreous Fluid Imbalance. Encyclopedia. Available at: https://encyclopedia.pub/entry/56116. Accessed February 07, 2026.
Brown, Ronald B.. "Myopia, Sodium Chloride, and Vitreous Fluid Imbalance" Encyclopedia, https://encyclopedia.pub/entry/56116 (accessed February 07, 2026).
Brown, R.B. (2024, March 11). Myopia, Sodium Chloride, and Vitreous Fluid Imbalance. In Encyclopedia. https://encyclopedia.pub/entry/56116
Brown, Ronald B.. "Myopia, Sodium Chloride, and Vitreous Fluid Imbalance." Encyclopedia. Web. 11 March, 2024.
Copy Citation
Myopia prevalence is low in native people consuming traditional diets lacking in sodium chloride, and nutritional epidemiological evidence supports the association of rising myopia prevalence with dietary sodium intake. East Asian populations have among the highest rates of myopia associated with high dietary sodium. Similar associations of sodium and rising myopia prevalence were observed in the United States in the late 20th century.
axial myopia
sodium chloride
vitreous humor
nutritional epidemiology
grounded theory
osmolality
low-salt diet
1. Introduction
In a 2005 study of native Amazon tribes of northwestern Brazil, Thorn et al. noted that most of the people in the study were illiterate and “remarkably free of myopia” [1]. The researchers implied an association of nearsightedness with near work (e.g., reading), but more recent epidemiological findings linking near work with myopia are inconsistent [2]. Other theories have associated myopia with environmental factors, such as “use of mobile devices, spending more time indoors or less time outdoors, or lack of exposure to sunshine”, yet researchers note that a consensus has not been reached confirming the causality of these factors [3]. Notably, among the Amazon tribes whom Thorn et al. described as “remarkably free of myopia”, the traditional diet of the Yanomamo tribe is also remarkably free of sodium chloride [4].
Similarly, artic explorer Vilhjalmur Stefansson noted the absence of dietary salt in the traditional diet of indigenous people within the Artic regions, “in whose language the word mamaitok, meaning ‘salty’, is synonymous with ‘evil-tasting’” [5]. In 1969, Young et al. reported that myopia prevalence was low among older indigenous people of Artic regions who consumed a traditional diet, compared to an unusually high 58% myopia prevalence among indigenous offspring [6]. Increased myopia in Artic regions parallels a transition to a salty Western diet and the consumption of “processed and packaged products” [7]. Recently, refractive errors have reached 78% prevalence among northern Canadian indigenous children [8].
Studying the beneficial effects of diet on hypertension, Dr. Walter Kempner published findings in 1948 describing how patients consuming a no-salt diet of rice and fruit reversed advanced retinopathy [9], a degenerative condition associated with severe myopia [10]. Follow-up experimental studies based on Kempner’s clinical findings were conducted in 1948 by Stocker, Holt, and Clower [11]. The researchers found that patients without glaucoma who followed the no-salt rice diet “showed a striking and persistent reduction of intraocular tension.”


2. Myopia History and Pathophysiology
Descriptions of eye blurriness appeared around 1550 BC in ancient Egypt [12], and the ancient Egyptians had discovered how to preserve meat with salt [13]. “Blindness and varying degrees of visual impairment were widespread in the ancient Greco-Roman world” [14], and Roman emperor Nero was said to be nearsighted [15]. Salt was a highly valued commodity at the time, and the ancient Greeks described the value of a slave with the expression, “not worth his salt”, while the word “salary” is derived from the salt paid to Roman soldiers [16]. In ancient China, salt was used to preserve food as early as 5000 years ago [16], and “interest in diseases of the eyes (which were probably rampant in antiquity) is evident in early medical writings from the Middle East, India and China” [17]. Greek philosopher Aristotle is credited with coining the word myopia in 350 BC [15]. In his Book of Problems, Aristotle wrote, “the eye is moist above all parts of the body, and of a waterish nature; and as the water is clear and smooth, so likewise is the eye” [18].
Refractive errors in myopia are categorized as mild (−0.5 to −4 diopters), moderate (−4 to −8 diopters), and severe high (>−8 diopters) [19]. The risk of eye diseases, such as glaucoma, cataract, retinal detachment, and myopic macular degeneration, increases in high myopia [20]. Among people over 70 years of age with high myopia, pathological myopia prevalence is 65%, a leading cause of blindness worldwide [21]. Axial myopia, the most common type of myopia or nearsightedness, is caused by the increased axial length of the eye, which increases the distance between the retina in the posterior segment of the eye and the focus of light coming through the cornea and lens, as shown in Figure 1 [22]. The retina is part of the central nervous system and transforms light into electrical impulses, which are transmitted to the optic nerve. Less commonly, refractive myopia shortens the focus of light in front of the retina, due to structural or location changes in the cornea and lens [23]. Interestingly, Figure 1 shows that the length of focused light in axial myopia is generally comparable to normal vision, implicating the displaced location of the retina due to eye elongation, indicated by the blue arrows in the figure. The retina lines the vitreous humor in the posterior chamber, which is filled with a gel and water that provides shape to the eye. Logically, changes in the ocular fluid volume are potentially related to axial elongation in myopia.

Figure 1. Normal eye and myopia, based on the National Eye Institute, National Institutes of Health (NIE/NIH) [22]. The blue arrows indicate the direction of axial elongation.
3. Nutritional Epidemiology of Myopia and Dietary Sodium
Myopia is projected to affect about half of the global population (49.8%) by 2050 [24], and reducing myopia prevalence is a “high research priority” [23]. Global annual loss of productivity from uncorrected vision impairment in myopia was estimated at USD 244 billion in 2015 [25], and higher myopic refractive error in patients tends to adversely affect quality of life [20]. Although asthenopia (visual fatigue) may accompany myopia, studies show that asthenopia can occur without refractive errors [26].
Myopia is associated with genetic factors, and “more than 400 associated gene loci have been mapped for myopia and refractive errors” [27]. Additionally, a strong role is played by environmental and lifestyle factors. For example, the prevalence of myopia in immigrant children arriving in Israel at an older age was lower than in children born in Israel or children immigrating at an earlier age [28]. Migrant studies demonstrate how health in people immigrating to a new country can decline as immigrants adjust to life in their adopted country, “perhaps caused by changes in nutrition, customs and lifestyle” and other factors [29].
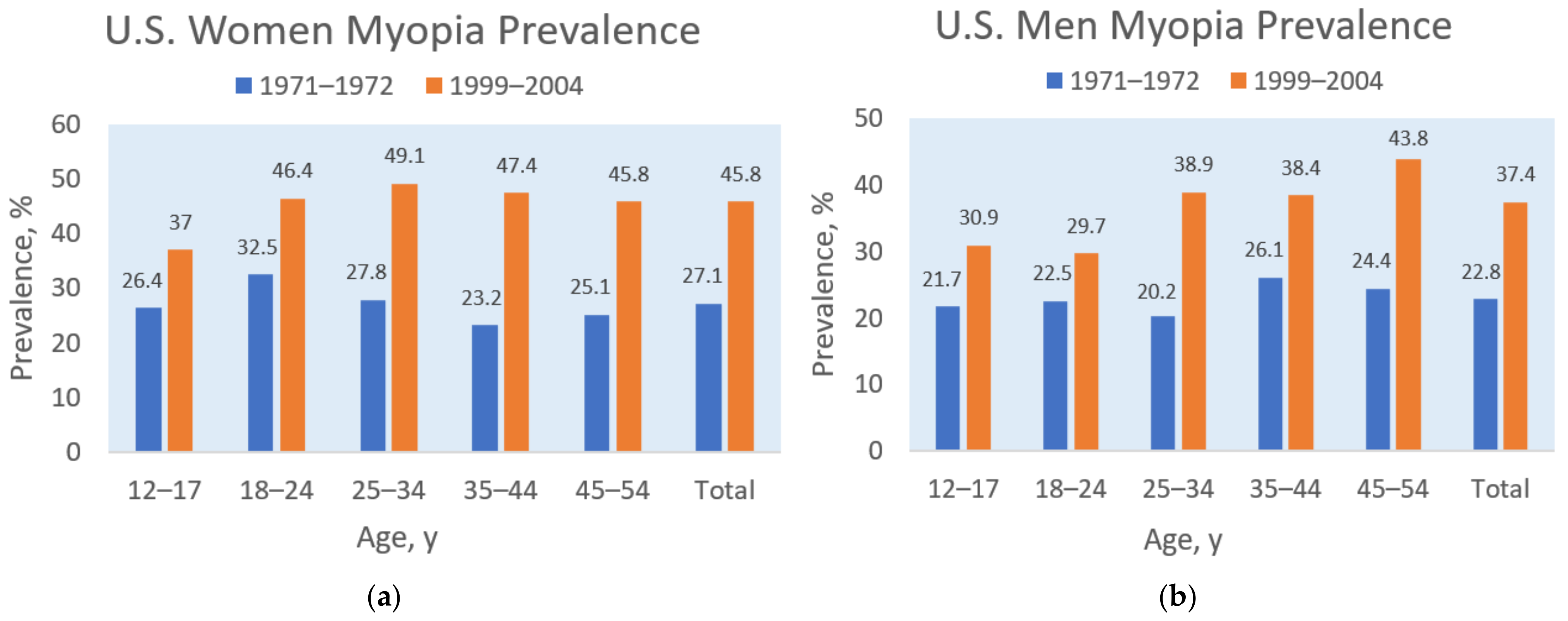
A similar relationship between dietary sodium and myopia prevalence appeared in the United States. For example, Figure 2 shows that myopia prevalence increased in the U.S. population during the last three decades of the 20th century, rising, on average, from 27.1% to 45.8% in women and from 22.8% to 37.4% in men [30]. Simultaneously, dietary sodium intake increased in the adult U.S. population during the same period, as shown in Figure 3 [31]. A possible mediating factor explaining the association of rising myopia prevalence with increasing salt intake could be the rapid increase in consumption of ultra-processed food in North America, rising in Canada from “24 to 61.7% of total calories” during the late 20th century [32]. Increased consumption of ultra-processed food with added sodium, sugar, and fat could also mediate the rising prevalence of myopia associated with increasing rates of obesity [28][33], and more research is needed in this area.

Figure 2. Myopia prevalence for women (a) and men (b) by age groups in the 19711972 and 1999–2004 U.S. National Health and Nutrition Examination Surveys, based on Vitale et al. [30].

Figure 3. Mean daily dietary sodium intake in U.S. men and women aged 18–74 in the 1971 to 2010 National Health and Nutrition Examination Surveys [31]. Courtesy of New York State Department of Health.
4. Sodium Chloride, Osmolality, and Myopia
In cellular volume homeostasis, water flows across a cell membrane into a solution with a higher solute concentration, creating an osmotic gradient, and the number of solute particles in a solution determines the attraction of water, also known as the solution’s osmolality, by mass (solute particles per kg solvent) or osmolarity by volume (solute particles per L solvent) [34]. Hypertonic solutions have high osmolality, and hypotonic solutions have low osmolality. “All cells face constant challenges to their volume either through changes in intracellular solute content or extracellular osmolality” [34]. In ocular tissue, intravenous injections of hypertonic saline (containing higher concentrations of sodium chloride than in the blood) were observed to draw fluid out of the ocular vitreous and temporarily lower intraocular pressure, likely by increasing “the osmotic gradient between tissues and the blood, which pulls fluid from interstitial spaces to the intravascular space” [35].
5. Vitreous Fluid Accumulation
The apical barrier of the RPE—the endothelial cells of the retinal blood vessels—has also been hypothesized to break down from hyperosmolar stress, leading to fluid and solute leakage from the retina and choroid (the vascular layer of the eye), with “massive water accumulation that can affect vision” [36]. For example, Cases et al. found that the liquid vitreous fraction in mice with high myopia was at least eight times higher than in control mice, while the sodium and chloride concentrations and osmolality in the myopic vitreous were slightly lower [37], possibly due to the diluting effect of excessive water volume. Additionally, experimental induction of myopia through vision deprivation (form-deprivation myopia) increased the volume of the liquid vitreous in neonatal chicks [38][39]. Marshall and Crewther posited that “water efflux across the RPE to the choroid has to be responsible for the concomitant change in the shape of the eye and changes in the fluid volume of the vitreous humor” [40]. Notably, “The fenestrated capillaries in the choroid are very permeable to low molecular weight substances; sodium permeability in the choroid is probably 50 times that in skeletal muscle” [41].
Earlier experiments in rabbits demonstrated that hypertonicity inside the vitreous barrier, subsequent to serum injections of urea, osmotically induced “an increase in the size of the vitreous body from an excess uptake of water to compensate for the existing hypertonicity” [42]. The researchers concluded that the vitreous volume “is not stable but is dependent, i.e., on the condition of the blood”. Furthermore, Kinsey described the ocular movement of sodium and chloride ions from the blood across the ciliary epithelium (iris blood vessels) into the posterior chamber and the diffusion of the ions between the posterior chamber and the vitreous [43]. In addition to affecting the vitreous fluid, a case of osmotic swelling in the aqueous humor-filled anterior chamber of a patient with hypernatremia was induced by sodium shifted into the lens, causing lens fiber damage and the rapid onset of myopic error [44].
Abnormal concentrations of sodium chloride in the vitreous of postmortem human cases were associated with antemortem hyponatremia and hypernatremia in the deceased patients, often in conditions with dysnatremia, such as community-acquired pneumonia [45]. Choroid thickening in patients is associated with increased blood plasma volume from water retention in hyponatremia [46], also known as hypervolemic hyponatremia [47]. Conversely, choroid thinning occurs in myopia as the ocular axis elongates [48], which stretches and thins the choroid [49], sclera [50], and retina [51]. Evidence suggests that extensibility of ocular tissue in myopia may play a role in helping to downregulate intraocular pressure through volume expansion. For example, intraocular pressure is lower in patients with medium myopia, compared to high myopia [52]. Importantly, thinning of ocular tissue from biomechanical changes in axial elongation should be differentiated from thinning that occurs due to neurodegeneration in conditions such as retinal endothelial cell apoptosis [53]. Coincidently, high dietary salt intake in a rat model of retinal ischemia/reperfusion exacerbated neurodegeneration of the retina, causing cell apoptosis and retinal thinning [54].
Age-related deterioration of the vitreous gel (liquefaction) is associated with the loss of viscoelasticity of the eye [55]. As ocular tissue loses elasticity, the inability to stretch and compensate for increasing pressure from fluid retention may increase the risk of ocular hypertension, which damages the retina and optic nerve in diseases like glaucoma [56]. Loss of viscoelastic biomechanical properties was found in an examination of human donor eyes with glaucoma, which were stiffer and less responsive to changes in intraocular pressure [57]. The interaction of impaired tissue extensibility with increased fluid volume and hypertension induced by high concentrations of sodium chloride could help explain the association of salt intake with open-angle glaucoma [58].
References
- Thorn, F.; Cruz, A.A.; Machado, A.J.; Carvalho, R.A. Refractive status of indigenous people in the northwestern Amazon region of Brazil. Optom. Vis. Sci. 2005, 82, 267–272.
- Fu, Q.; Zhang, Y.; Chen, L.; Dong, M.; Tang, W.; Chen, S.; Qu, J.; Zhou, X.; Zhi, Z. Near work induces myopia in Guinea pigs. Exp. Eye Res. 2022, 224, 109202.
- Pärssinen, O.; Kauppinen, M. Associations of near work time, watching TV, outdoors time, and parents’ myopia with myopia among school children based on 38-year-old historical data. Acta Ophthalmol. 2022, 100, e430–e438.
- Oliver, W.J.; Cohen, E.L.; Neel, J.V. Blood pressure, sodium intake, and sodium related hormones in the Yanomamo Indians, a “no-salt” culture. Circulation 1975, 52, 146–151.
- Heinbecker, P. Not by bread alone. By Vilhjalmur Stefansson. The MacMillan Company, New York, xvi + 339 pp., 1946. ($3.50). Am. J. Phys. Anthropol. 1947, 5, 104–106.
- Young, F.A.; Leary, G.A.; Baldwin, W.R.; West, D.C.; Box, R.A.; Harris, E.; Johnson, C. The transmission of refractive errors within eskimo families. Am. J. Optom. Arch. Am. Acad. Optom. 1969, 46, 676–685.
- Lougheed, T. The changing landscape of arctic traditional food. Environ. Health Perspect. 2010, 118, A386–A393.
- Sabri, K.; Jindani, Y.; De Melo, M.; Innes, E.; Kioke, S. Current Visual Acuity and Refractive Errors among Indigenous Children in Northern Canada. Investig. Ophthalmol. Vis. Sci. 2022, 63, 3368-A0155.
- Kempner, W. Treatment of hypertensive vascular disease with rice diet. Am. J. Med. 1948, 4, 545–577.
- Zhang, T.; Wei, Y.T.; Huang, W.B.; Liu, R.J.; Zuo, Y.J.; He, L.W.; Zhong, L.T.; Zhang, S.C. Prevalence and characteristics of peripheral myopic retinopathy in Guangzhou office workers. Int. J. Ophthalmol. 2018, 11, 1390–1395.
- Stocker, F.W.; Holt, L.B.; Clower, J.W. Clinical experiments with new ways of influencing intraocular tension; effect of rice diet t. Arch. Ophthalmol. 1948, 40, 46–55.
- Medow, N. Ancient Egyptian Records Provide Clues to Ophthalmic Care. Ophthalmology Times. Available online: https://www.ophthalmologytimes.com/view/ancient-egyptian-records-provide-clues-ophthalmic-care (accessed on 5 October 2023).
- Ikram, S. Meat Preservation in Ancient Egypt. In Encyclopaedia of the History of Science, Technology, and Medicine in Non-Western Cultures; Selin, H., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 1442–1443.
- Trentin, L. Exploring Visual Impairment in Ancient Rome; Brill: Leiden, The Netherlands, 2013.
- De Jong, P. Myopia: Its historical contexts. Br. J. Ophthalmol. 2018, 102, 1021–1027.
- Durack, E.; Alonso-Gomez, M.; Wilkinson, M.G. Salt: A review of its role in food science and public health. Curr. Nutr. Food Sci. 2008, 4, 290–297.
- Retief, F.; Stulting, A.; Cilliers, L. The eye in antiquity. S. Afr. Med. J. 2008, 98, 697–700.
- Salmon, W. The Works of Aristotle the Famous Philosopher; Good Press, Overdrive: Cleveland, OH, USA, 2023.
- Subudhi, P.; Agarwal, P. Myopia. Available online: https://www.ncbi.nlm.nih.gov/books/NBK580529/ (accessed on 3 September 2023).
- Chua, S.Y.L.; Foster, P.J. The Economic and Societal Impact of Myopia and High Myopia. In Updates on Myopia: A Clinical Perspective; Ang, M., Wong, T.Y., Eds.; Springer: Singapore, 2020; pp. 53–63.
- Ueta, T.; Makino, S.; Yamamoto, Y.; Fukushima, H.; Yashiro, S.; Nagahara, M. Pathologic myopia: An overview of the current understanding and interventions. Glob. Health Med. 2020, 2, 151–155.
- National Eye Institute. Myopia and Eye Development. Available online: https://www.youtube.com/watch?v=ttz6FIGhdY0 (accessed on 18 September 2023).
- Flitcroft, D.I.; He, M.; Jonas, J.B.; Jong, M.; Naidoo, K.; Ohno-Matsui, K.; Rahi, J.; Resnikoff, S.; Vitale, S.; Yannuzzi, L. IMI—Defining and Classifying Myopia: A Proposed Set of Standards for Clinical and Epidemiologic Studies. Investig. Ophthalmol. Vis. Sci. 2019, 60, M20–M30.
- Holden, B.A.; Fricke, T.R.; Wilson, D.A.; Jong, M.; Naidoo, K.S.; Sankaridurg, P.; Wong, T.Y.; Naduvilath, T.J.; Resnikoff, S. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology 2016, 123, 1036–1042.
- Naidoo, K.S.; Fricke, T.R.; Frick, K.D.; Jong, M.; Naduvilath, T.J.; Resnikoff, S.; Sankaridurg, P. Potential Lost Productivity Resulting from the Global Burden of Myopia: Systematic Review, Meta-analysis, and Modeling. Ophthalmology 2019, 126, 338–346.
- Vilela, M.A.P.; Pellanda, L.C.; Fassa, A.G.; Castagno, V.D. Prevalence of asthenopia in children: A systematic review with meta-analysis. J. Pediatr. 2015, 91, 320–325.
- Wang, Y.M.; Lu, S.Y.; Zhang, X.J.; Chen, L.J.; Pang, C.P.; Yam, J.C. Myopia Genetics and Heredity. Children 2022, 9, 382.
- Peled, A.; Nitzan, I.; Megreli, J.; Derazne, E.; Tzur, D.; Pinhas-Hamiel, O.; Afek, A.; Twig, G. Myopia and BMI: A nationwide study of 1.3 million adolescents. Obesity 2022, 30, 1691–1698.
- Peled, A.; Afek, A.; Twig, G.; Pras, E.; Rotenstreich, Y.; Sher, I.; Derazne, E.; Tzur, D.; Gordon, B. Myopia and Childhood Migration: A Study of 607 862 Adolescents. Ophthalmology 2020, 127, 713–723.
- Vitale, S.; Sperduto, R.D.; Ferris, F.L., III. Increased Prevalence of Myopia in the United States Between 1971–1972 and 1999–2004. Arch. Ophthalmol. 2009, 127, 1632–1639.
- New York State Department of Health. The Potential Health Impact of Reducing Excess Sodium Consumption. Available online: https://www.health.ny.gov/statistics/brfss/reports/docs/1308_brfss_reduce_sodium.pdf (accessed on 10 August 2023).
- Moubarac, J.-C.; Batal, M.; Martins, A.P.B.; Claro, R.; Levy, R.; Cannon, G.; Monteiro, C. Time trends changes in the consumption of ultra-processed products during the 20th century in Canada. Can. J. Diabetes 2013, 37, S245.
- Lee, S.; Lee, H.J.; Lee, K.G.; Kim, J. Obesity and high myopia in children and adolescents: Korea National Health and Nutrition Examination Survey. PLoS ONE 2022, 17, e0265317.
- Strange, K. Cellular volume homeostasis. Adv. Physiol. Educ. 2004, 28, 155–159.
- Harju, M.; Kivelä, T.; Lindbohm, N.; Koivusalo, R.; Paloheimo, M. Intravenous hypertonic saline to reduce intraocular pressure. Acta Ophthalmol. 2013, 91, 625–629.
- Willermain, F.; Libert, S.; Motulsky, E.; Salik, D.; Caspers, L.; Perret, J.; Delporte, C. Origins and consequences of hyperosmolar stress in retinal pigmented epithelial cells. Front. Physiol. 2014, 5, 199.
- Cases, O.; Obry, A.; Ben-Yacoub, S.; Augustin, S.; Joseph, A.; Toutirais, G.; Simonutti, M.; Christ, A.; Cosette, P.; Kozyraki, R. Impaired vitreous composition and retinal pigment epithelium function in the FoxG1::LRP2 myopic mice. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1242–1254.
- Seko, Y.; Shimokawa, H.; Pang, J.; Tokoro, T. Disturbance of electrolyte balance in vitreous of chicks with form-deprivation myopia. Jpn. J. Ophthalmol. 2000, 44, 15–19.
- Pickett-Seltner, R.L.; Doughty, M.J.; Pasternak, J.J.; Sivak, J.G. Proteins of the vitreous humor during experimentally induced myopia. Investig. Ophthalmol. Vis. Sci. 1992, 33, 3424–3429.
- Marshall, A.T.; Crewther, S.G. An X-ray microanalytical method for measuring in vivo element and water concentrations, relating to osmoregulation, in cells and tissues of the posterior eye. J. Microsc. 2021, 283, 21–28.
- Törnquist, P.; Alm, A.; Bill, A. Permeability of ocular vessels and transport across the blood-retinal-barrier. Eye 1990, 4, 303–309.
- Bleeker, G.M.; van Haeringen, N.; Glasius, E. Osmotically Induced Change in the Volume of the Vitreous Body Causing Protrusion of the Ocular Diaphragm. Ophthalmologica 2010, 144, 263.
- Kinsey, V.E. Ion movement in the eye. Circulation 1960, 21, 968–987.
- Horng, C.T.; Lee, Y.L.; Wu, H.C.; Lai, H.Y.; Hsu, J.Y.; Hsu, C.W.; Chien, K.J.; Kuo, W.H. High salt diet induced the rapid myopic shift of cataract formation. Life Sci. J. 2014, 11, 396–399.
- Zilg, B.; Alkass, K.; Berg, S.; Druid, H. Interpretation of postmortem vitreous concentrations of sodium and chloride. Forensic Sci. Int. 2016, 263, 107–113.
- Kang, H.M. Transient Choroidal Thickening Associated with Hyponatremia. J. Retin. 2018, 3, 92–96.
- Fortune, B.E.; Garcia-Tsao, G. Hypervolemic hyponatremia: Clinical significance and management. Clin. Liver Dis. 2013, 2, 109.
- Muhiddin, H.S.; Mayasari, A.R.; Umar, B.T.; Sirajuddin, J.; Patellongi, I.; Islam, I.C.; Ichsan, A.M. Choroidal Thickness in Correlation with Axial Length and Myopia Degree. Vision 2022, 6, 16.
- Teberik, K.; Kaya, M. Retinal and Choroidal Thickness in Patients with High Myopia without Maculopathy. Pak. J. Med. Sci. 2017, 33, 1438–1443.
- Metlapally, R.; Wildsoet, C.F. Scleral Mechanisms Underlying Ocular Growth and Myopia. Prog. Mol. Biol. Transl. Sci. 2015, 134, 241–248.
- Wu, P.C.; Chen, Y.J.; Chen, C.H.; Chen, Y.H.; Shin, S.J.; Yang, H.J.; Kuo, H.K. Assessment of macular retinal thickness and volume in normal eyes and highly myopic eyes with third-generation optical coherence tomography. Eye 2008, 22, 551–555.
- Patel, A.; Patel, D.; Prajapati, V.; Patil, M.S.; Singhal, D. A Study on the Association Between Myopia and Elevated Intraocular Pressure Conducted at a Tertiary Care Teaching Hospital in Gujarat, India. Cureus 2022, 14, e28128.
- Abcouwer, S.F.; Shanmugam, S.; Muthusamy, A.; Lin, C.M.; Kong, D.; Hager, H.; Liu, X.; Antonetti, D.A. Inflammatory resolution and vascular barrier restoration after retinal ischemia reperfusion injury. J. Neuroinflamm. 2021, 18, 186.
- Li, Q.; Fang, W.; Hu, F.; Zhou, X.; Cheng, Y.; Jiang, C. A high-salt diet aggravates retinal ischaemia/reperfusion injury. Exp. Eye Res. 2019, 188, 107784.
- Schulz, A.; Wahl, S.; Rickmann, A.; Ludwig, J.; Stanzel, B.V.; von Briesen, H.; Szurman, P. Age-Related Loss of Human Vitreal Viscoelasticity. Transl. Vis. Sci. Technol. 2019, 8, 56.
- Davis, B.M.; Crawley, L.; Pahlitzsch, M.; Javaid, F.; Cordeiro, M.F. Glaucoma: The retina and beyond. Acta Neuropathol. 2016, 132, 807–826.
- Karimi, A.; Razaghi, R.; Padilla, S.; Rahmati, S.M.; Downs, J.C.; Acott, T.S.; Kelley, M.J.; Wang, R.K.; Johnstone, M. Viscoelastic Biomechanical Properties of the Conventional Aqueous Outflow Pathway Tissues in Healthy and Glaucoma Human Eyes. J. Clin. Med. 2022, 11, 6049.
- Tseng, V.L.; Topouzis, F.; Yu, F.; Keskini, C.; Pappas, T.; Founti, P.; Anastasopoulos, E.; Harris, A.; Wilson, M.R.; Coleman, A.L. Association Between Dietary Salt Intake and Open Angle Glaucoma in the Thessaloniki Eye Study. J. Glaucoma 2022, 31, 494–502.
More
Information
Subjects:
Ophthalmology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
870
Revisions:
2 times
(View History)
Update Date:
12 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




