Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alessandro Sciahbasi | -- | 1800 | 2024-03-11 12:26:17 | | | |
| 2 | Mona Zou | Meta information modification | 1800 | 2024-03-12 09:31:24 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Sciahbasi, A.; Mazza, T.M.; Pidone, C.; Samperi, S.; Cittadini, E.; Granatelli, A. Drug-Coated Balloons. Encyclopedia. Available online: https://encyclopedia.pub/entry/56110 (accessed on 07 February 2026).
Sciahbasi A, Mazza TM, Pidone C, Samperi S, Cittadini E, Granatelli A. Drug-Coated Balloons. Encyclopedia. Available at: https://encyclopedia.pub/entry/56110. Accessed February 07, 2026.
Sciahbasi, Alessandro, Tiziano Maria Mazza, Chiara Pidone, Simona Samperi, Edoardo Cittadini, Antonino Granatelli. "Drug-Coated Balloons" Encyclopedia, https://encyclopedia.pub/entry/56110 (accessed February 07, 2026).
Sciahbasi, A., Mazza, T.M., Pidone, C., Samperi, S., Cittadini, E., & Granatelli, A. (2024, March 11). Drug-Coated Balloons. In Encyclopedia. https://encyclopedia.pub/entry/56110
Sciahbasi, Alessandro, et al. "Drug-Coated Balloons." Encyclopedia. Web. 11 March, 2024.
Copy Citation
Drug-coated balloons (DCB) are a well-established option for treating in-stent restenosis endorsed by European Guidelines on myocardial revascularization. However, in recent years, a strategy of “leaving nothing behind” with DCB in de novo coronary stenosis has emerged as an appealing approach.
drug-coated balloon
percutaneous coronary interventions
paclitaxel
sirolimus
1. Drug-Coated Balloon Components
There are many DCBs on the market characterized by a different combination of drugs, carriers, and polymers. Each component of the device is essential to the acute and long-term efficacy of the DCB.
1.1. The Drug
Among all the different drugs tested on the DCB, paclitaxel, and sirolimus are those that obtained the best results and are the most utilized.
- -
-
Paclitaxel-coated balloons. Paclitaxel is an anti-neoplastic, cytotoxic drug that binds to microtubules in mitosis, inhibiting cell division and proliferation [1]. Paclitaxel is the most widely used drug in the setting of DCB due to its physiochemical properties and, in particular, due to the lipophilicity of the drug that allows it to rapidly penetrate the cell membrane of smooth muscle cells and to support a long-lasting antiproliferative action even after a brief, single-dose application [2]. The drug has a narrow therapeutic window, and the dosage utilized on DCB ranges between 2 and 3.5 µg/mm2.
- -
-
Sirolimus-coated balloons. Sirolimus is a cytostatic drug that inhibits smooth muscle cell proliferation and migration by blocking cell-cycle progression at the G1/S transition [3]. Compared to Paclitaxel, sirolimus has a wider therapeutic range (1–7 µg/mm2), greater anti-restenotic and anti-inflammatory effects, slower tissue absorption, and short tissue retention. However, a major disadvantage of the drug is its non-lipophilic nature, which makes tissue absorption and elution more difficult, requiring a dedicated carrier to allow diffusion and penetration in the vessel wall [4]. Moreover, the drug should be continuously delivered over time, and dedicated technologies are necessary to maintain a constant release.
1.2. Carriers and Polymers
Carriers and polymers are, in general, essential to allow the retention of the drug during the vascular transit, to provide adhesion of the drug to the vessel wall during the inflation, and to ensure a rapid and homogenous drug transfer and deposition in the tissue. Because of their bioadhesive surface, these coating materials guarantee an easy penetration into the arterial wall, reduce mechanical trauma, and increase the antiproliferative effectiveness of the drugs used, keeping them adhesive locally into the vessel wall and avoiding drug dilution. For the first time in 2003, Sheller et al. [5] demonstrated that the association of Paclitaxel with ipopromide obtained a complete inhibition of proliferation of smooth muscle cells, and the effect was superior compared to Paclitaxel alone. Since then, for Paclitaxel-coated DCB, other different additives such as urea, Acetyl tributyl citrate, or n-Butyryl citrate have been tested with good results [1].
For Sirolimus-coated DCB, carriers, and polymers are even more important in order to obtain a prolonged release of the drug after balloon inflation. Dedicated technologies have been tested: nanocarriers in the form of nanoparticles containing Sirolimus [6], spherical micro-reservoirs [7], or crystalline coatings of Sirolilmus [8] are currently the most promising formulations.
2. A New Frontier: Large Vessel Coronary Artery Disease
Based on the encouraging results reported for the use of DCB in small-vessel disease, continuous efforts have been made to investigate the role of this promising technology in large native vessels. Most of the feasibility data of DCB PCI in large vessels come from sub-analysis of larger observational retrospective or prospective studies with few dedicated studies. Data obtained from observational studies [9][10][11][12][13][14][15][16] are summarized in Table 1, whereas randomized studies comparing DCB and stent are presented in Table 2 and Table 3 [17][18][19][20][21][22][23].
Table 1. Prospective and Retrospective Observational studies.
| First Author | Year | Design | Patients or Lesions (n) |
Clinical Presentation ACS, n |
RVD, mm |
Type DCB |
FU (Months) |
MACE, n (%) |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DCB | Stent | DCB | Stent | DCB | Stent | DCB | Stent | |||||
| Nijhoff et al. [9] | 2015 | P | 40 | 49 | 40 | 49 | 2.8 ± 0.5 | 2.8 ± 0.5 | Pac | 12 | 5 (12.5) | 1 (2) |
| Lu et al. [11] | 2019 | P | 92 | -- | 47 | -- | >2.75 | -- | Pac | 11.4 ± 1.6 | 4 (4.3) | -- |
| Yu et al. [10] | 2019 | R | 200 | -- | 183 | -- | 3.2 ± 2.8 | -- | Pac | 10 | 0 (0) | -- |
| Rosenberg et al. [24] | 2019 | P | 134 | -- | 44 | -- | >2.75 | -- | Pac | 9 | 7 (5.2) | -- |
| Iwasaki et al. [12] | 2021 | R | 69 | 88 | 2 | 2 | 3 ± 0.5 | 3 ± 0.4 | Pac | 12 | 6 (8.7) | 4 (4.5) |
| Merinopoulos et al. [13] | 2023 | R | 544 | 693 | 0 | 0 | >2.75 | >3.0 | Pac | 42 | 33 (5) TLR | 32 (4.6) TLR |
| Nakamura et al. [14] | 2023 | R | 73 | 81 | 0 | 0 | 3 ± 0.4 | 3.3 ± 0.4 | Pac | 14 | 8 (11) | 21 (26) |
| Gunawarden et al. [15] | 2023 | R | 41 | 107 | 28 | 57 | 3.8 ± 0.3 | 4.3 ± 0.6 | Pac | 33 | 5 (12.2) | 23 (21.5) |
| Gitto et al. [16] | 2023 | R | 139 | 139 | 0 | 0 | 3.1 ± 0.5 | 3.1 ± 0.4 | Pac + Sir | 24 | 4 (2.9) | 24 (17.3) |
ACS: acute coronary syndrome; DCB: Drug-coated Balloon; P: Prospective; Pac: Paclitaxel; R: Retrospective; RVD: reference vessel diameter; MACE: major adverse cardiovascular events (cardiovascular death, myocardial infarction, target lesion revascularization); Sir: Sirolimus.
Table 2. Randomized trials in large vessels.
| First Author | Year | Total Patients, n | Clinical Presentation ACS n (%) |
RVD, (mm) |
Type DCB |
Follow-Up Duration (months) |
MACE n, (%) |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DCB | Stent | DCB | Stent | DCB | Stent | DCB | Stent | ||||
| Nishiyama et al. [17] | 2016 | 27 | 33 | 27 (100) | 33 (100) | 2.9 ± 0.6 | 2.7 ± 0.6 | Paclitaxel | 8 | 0 (0) | 2 (6.1) |
| Gobic et al. [18] | 2017 | 38 | 37 | 38 (100) | 37 (100) | 2.6 ± 0.5 | 3.0 ± 0.5 | Paclitaxel | 6 | 2 (5.26) | 2 (5.40) |
| Vos et al. [21] | 2019 | 59 | 61 | 59 (100) | 61 (100) | 3.3 ± 0.5 | 3.2 ± 0.5 | Paclitaxel | 9 | 2 (3.39) | 1 (1.63) |
| Rissanen et al. [19] | 2019 | 102 | 106 | 47 (46) | 49 (46) | 2.5–4 | 2.5–4 | Paclitaxel | 9 | 1 (1.02) | 15 (14.1) |
| Shin et al. [20] | 2019 | 20 | 20 | 6 (30) | 8 (40) | >2.8 | >2.8 | Paclitaxel | 12 | 0 (0) | 3 (15) |
| Yu X et al. [22] | 2022 | 84 | 79 | 76 (91) | 69 (87.4) | 2.8 (2.5–3.3) | 3.0 (2.7–3.4) | Paclitaxel | 12 | 2 (2.38) | 4 (5.06) |
| Wang et al. [23] | 2022 | 92 | 92 | 92 (100) | 92 (100) | 3.31 ± 0.56 | 3.4 ± 0.5 | Paclitaxel | 12 | 5 (5.7) | 6 (7.1) |
ACS: acute coronary syndrome. DCB: Drug-coated Balloon; MACE: major adverse cardiovascular events; RVD: reference vessel diameter.
Table 3. Angiographic characteristics and target vessel revascularizations in randomized clinical trials.
| Author | Total Patients, n |
Lesion Location (LAD) (%) |
p | Lesion Length (mm) |
p | Late Lumen Loss | p | TLR (%) |
p | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DCB | Stent | DCB | Stent | DCB | Stent | DCB | Stent | DCB | Stent | |||||
| Nishiyama [17] | 27 | 33 | 44 | 52 | 0.699 | 16 ± 5 | 18 ± 7 | 0.241 | 0.25 ± 0.25 | 0.37 ± 0.40 | 0.185 | 0 | 6.1 | 0.193 |
| Gobic [18] | 38 | 37 | NA | NA | - | NA | NA | - | −0.09 ± 0.09 | 0.10 ± 0.19 | <0.05 | NA | NA | - |
| Vos [21] | 59 | 61 | 32 | 40 | 0.50 | NA | NA | - | 0.05 | 0.00 | 0.51 | 3 | 2 | 1.00 |
| Rissanen [19] | 102 | 106 | 40 | 38 | 0.94 | NA | NA | NA | NA | 0 | 6 | 0.015 | ||
| Shin [20] | 20 | 20 | 30 | 40 | 0.507 | 21 ± 3 | 19 ± 3 | 0.052 | 0.2 ± 0.3 | 1.2 ± 0.8 | <0.001 | 0 | 15 | 0.106 |
| Yu X [22] | 84 | 79 | 57 | 44 | 0.101 | 18 | 20 | 0.202 | −0.19 ± 0.49 | 0.03 ± 0.64 | 0.019 | 1.2 | 3.8 | 0.361 |
| Wang [23] | 92 | 92 | 55 | 57 | 0.836 | 31 ± 10 | 34 ± 13 | 0.095 | 0.24 ± 0.39 | 0.31 ± 0.38 | 0.266 | 2.3 | 2.4 | 1.00 |
DCB: Drug-coated Balloon; LAD: Left Anterior Descending; NA: Not Available; TLR: Target vessel revascularization.
In a large retrospective study conducted by Yu et al. [10], over a total of 595 de novo lesions treated using DCB, 222 lesions were in large vessels (diameter greater than 2.8 mm). Over an average clinical follow-up period of 10.1 months, the large vessel group had no MACE nor target lesion revascularization. Subsequently, Rosenberg et al. [24] analyzed data from the international, multicenter DCB-only registry that prospectively enrolled patients in 8 European and 6 Malaysian centers treated with paclitaxel-iopromid-coated DCB: of the 686 patients with de novo lesions, 234 were propensity matched stratified into small and large vessel coronary artery disease (cut off ≥2.75 mm). The study confirmed that DCB-only PCI in large coronary arteries with de novo lesions was safe with a 6.1% rate of MACE that was similar to that in the small vessel group (5.7%, p = 0.903) despite almost half of the included coronary lesions were considered as complex. Probably, the low event rate in the study is mostly explained by the strict compliance of all investigators with predilation (more than 90% of procedures) using uncoated balloons having a balloon to-vessel ratio of 0.8–1.0. Moreover, the length of the DCB was chosen to be at least 2 to 3 mm longer than the lesion ends, and DCB PCI was performed only in the absence of flow-limiting dissections (C–E) or significant recoil.
The largest randomized trial including large vessels is the DEBUT Trial [19], which included 208 patients (83% with vessel diameter > 2.75 mm) assigned to DCB PCI (n = 102) or bare metal stent (n = 106). Exclusion criteria were in-stent restenosis, flow-limiting dissection or substantial recoil (>30%) of the target lesion after predilation. At 9 months, MACE had occurred in one patient (1%) in the DCB group and in 15 patients (14%) in the stent group. An important point was that in the stent group, there were two definitive stent thrombosis events, whereas no acute vessel closures were observed in the drug-coated balloon group. Further studies confirmed these results [17]: Nishiyama et al. performed a single-center randomized clinical study including 60 patients (30 randomized to DCB and 30 patients to stents) with stable coronary artery disease, excluding lesions severely calcified, in the left main trunk and chronic total occlusions. No acute coronary syndromes or restenotic lesions were enrolled. There were no significant differences between the two groups in major risk factors such as age, gender, hypertension, dyslipidemia, diabetes mellitus, smoking, chronic kidney disease, and family history of ischemic heart disease. At eight months of follow-up, target lesion revascularization rates were very low and similar in the two groups: zero patients (0.0%) in the DCB group and two (6.1%) in the DES group (p = 0.193). It should be noted that the use of intravascular ultrasound before DCB application and the short follow-up probably influenced the results. Recently, a meta-analysis of 7 randomized clinical trials comparing DCB and stents in large de novo coronary artery disease [25] included 770 patients (384 randomized to DCB and 386 to stent). The mean age of patients ranged from 49 to 77 years, with the majority of patients (512 patients, 66%) undergoing PCI for acute coronary syndromes. The length of follow-up ranged from 6 to 12 months, and 248 patients (from 2 different studies) used a bare metal stent as a control. All the DCBs used in the studies included in the meta-analysis were paclitaxel-coated balloons. The study showed that the MACE rate was significantly reduced by 52% in the DCB group compared to the stent group (12 vs. 28 events, p = 0.04 respectively, Figure 1). The target lesion revascularization rate was also reduced in the DCB group (10 events) compared to the stent group (24 events). Differently, the rate of myocardial infarction that was reported in 4 trialswas not reduced by the use of DCB compared with stents. Major limitations of this analysis were the small sample size and the use in some studies of bare metal stents rather than DES, which are devices no longer utilized in the majority of the centers.
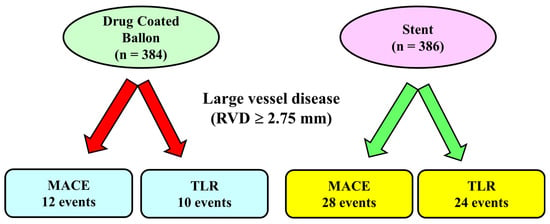
Figure 1. Main results of a meta-analysis of 7 randomized controlled trials in large vessel coronary disease.In this meta-analysis, including lesions with reference vessel diameter ≥ 2.75 mm, the number of major adverse cardiac events and the rate of target vessel revascularization was lower in the Drug-coated Balloon Group compared to the stent Group. MACE: Major adverse cardiac events, TLR: Target Lesion Revascularization.
Good results of DCB PCI have been observed even in the context of unprotected left main disease: in the SPARTAN LMS [15], 148 patients with left main stenosis have been treated by DCB (41 patients) or DES (107 patients). The 41 patients who were treated with a DCB were all treated with an iopromide paclitaxel DCB, whereas the DES group included Sirolimus, Everolimus, or Zotarolimus eluting stents. Patients treated by the DCB were older compared to the stent group and had more challenging coronary anatomy, including more than 70% of true bifurcation compared to less than 50% in the DES group (p = 0.006). DCB procedures were associated with less use of contrast media (mean 144.5 ± 41.3) mL compared with DES procedures (mean 176.5 ± 67.1 mL, p = 0.006). After an average follow-up of 33.9 ± 19.9 months, there was no significant difference in all-cause or cardiovascular mortality (4.9% for the DCB group and 6.5% for the DES group, HR 1.21 [0.31–4.67], p = 0.786) that was confirmed significant after propensity score-matched analysis [HR: 0.31 (0.05–1.930, p = 0.210]. The results of this study, although interesting, are associated with some limitations and potential bias: a retrospective design of the study, a limited number of patients enrolled, and procedures performed in a high-volume, large tertiary referral center. Consequently, larger dedicated and randomized studies are necessary to confirm these results.
Moreover, in the setting of ACS, in the 40 patients enrolled in the prospective, observational, DEB-AMI trial [9], a higher late lumen loss at 12 months has been observed in the DCB group (0.51 ± 0.59 mm) compared to DES (0.21 ± 0.32 mm, p < 0.01). Accordingly, the role of DCB PCI in the setting of ACS should be better evaluated in future and ongoing dedicated studies.
References
- Cortese, B.; Kalkat, H.; Bathia, G.; Basavarajaiah, S. The evolution and revolution of drug coated balloons in coronary angioplasty: An up-to-date review of literature data. Catheter. Cardiovasc. Interv. 2023, 102, 1069–1077.
- Axel, D.I.; Kunert, W.; Göggelmann, C.; Oberhoff, M.; Herdeg, C.; Küttner, A.; Wild, D.H.; Brehm, B.R.; Riessen, R.; Köveker, G.; et al. Paclitaxel inhibits arterial smooth muscle cell proliferation and migration in vitro and in vivo using local drug delivery. Circulation 1997, 96, 636–645.
- Sun, J.; Marx, S.O.; Chen, H.J.; Poon, M.; Marks, A.R.; Rabbani, L.E. Role for p27 (Kip1) in Vascular Smooth Muscle Cell Migration. Circulation 2001, 103, 2967–2972.
- di Palma, G.; Sanchez-Jimenez, E.F.; Lazar, L.; Cortese, B. Should paclitaxel be considered an old generation DCB? The limus era. Rev. Cardiovasc. Med. 2021, 22, 1323–1330.
- Scheller, B.; Speck, U.; Schmitt, A.; Böhm, M.; Nickenig, G. Addition of paclitaxel to contrast media prevents restenosis after coronary stent implantation. J. Am. Coll. Cardiol. 2003, 42, 1415–1420.
- Lemos, P.A.; Farooq, V.; Takimura, C.K.; Gutierrez, P.S.; Virmani, R.; Kolodgie, F.; Christians, U.; Kharlamov, A.; Doshi, M.; Sojitra, P.; et al. Emerging technologies: Polymer-free phospholipid encapsulated sirolimusnanocarriersfor the controlled release of drug from a stent-plus-balloon or a stand-alone balloon catheter. EuroIntervention 2013, 9, 148–156.
- Massaro, G.; Maffi, V.; Russo, D.; Benedetto, D.; Bonanni, M.; Chiricolo, G.; Sangiorgi, G. ‘Leave Nothing Behind’ Strategy in Coronary and Peripheral Artery Disease: An Insight into Sirolimus-Coated Balloons. EMJ Int. Cardiol. 2022, 10, 60–71.
- Clever, Y.P.; Peters, D.; Calisse, J.; Bettink, S.; Berg, M.C.; Sperling, C.; Stoever, M.; Cremers, B.; Kelsch, B.; Böhm, M.; et al. Novel Sirolimus-Coated Balloon Catheter: In Vivo Evaluation in a Porcine Coronary Model. Circ. Cardiovasc. Interv. 2016, 9, e003543.
- Nijhoff, F.; Agostoni, P.; Belkacemi, A.; Nathoe, H.M.; Voskuil, M.; Samim, M.; Doevendans, P.A.; Stella, P.R. Primary percutaneous coronary intervention by drug-eluting balloon angioplasty: The nonrandomized fourth arm of the DEB-AMI (drug-eluting balloon in ST-segment elevation myocardial infarction) trial. Catheter. Cardiovasc. Interv. 2015, 86 (Suppl. S1), S34–S44.
- Yu, X.; Ji, F.; Xu, F.; Zhang, W.; Wang, X.; Lu, D.; Yang, C.; Wang, F. Treatment of large de novo coronary lesions with paclitaxel-coated balloon only: Results from a Chinese institute. Clin. Res. Cardiol. 2019, 108, 234–243.
- Lu, W.; Zhu, Y.; Han, Z.; Sun, G.; Qin, X.; Wang, Z.; Liu, G.; Xi, W.; Wang, X.; Pan, L.; et al. Short-term outcomes from drug-coated balloon for coronary de novo lesions in large vessels. J. Cardiol. 2019, 73, 151–155.
- Iwasaki, Y.; Koike, J.; Ko, T.; Funatsu, A.; Kobayashi, T.; Ikeda, T.; Nakamura, S. Comparison of drug-eluting stents vs. drug-coated balloon after rotational atherectomy for severely calcified lesions of non small vessels. Heart Vessels 2021, 36, 189–199.
- Merinopoulos, I.; Gunawardena, T.; Corballis, N.; Bhalraam, U.; Gilbert, T.; Maart, C.; Richardson, P.; Ryding, A.; Sarev, T.; Sawh, C.; et al. Paclitaxel drug-coated balloon-only angioplasty for de novo coronary artery disease in elective clinical practice. Clin. Res. Cardiol. 2023, 112, 1186–1193.
- Nakamura, H.; Ishikawa, T.; Mizutani, Y.; Yamada, K.; Ukaji, T.; Kondo, Y.; Shimura, M.; Aoki, H.; Hisauchi, I.; Itabashi, Y.; et al. Clinical and Angiographic Outcomes of Elective Paclitaxel-Coated Balloon Angioplasty in Comparison with Drug-Eluting Stents for De Novo Coronary Lesions in Large Vessels. Int. Heart J. 2023, 64, 145–153.
- Gunawardena, T.D.; Corballis, N.; Merinopoulos, I.; Wickramarachchi, U.; Reinhold, J.; Maart, C.; Sreekumar, S.; Sawh, C.; Wistow, T.; Sarev, T.; et al. Drug-Coated Balloon vs. Drug-Eluting Stents for De Novo Unprotected Left Main Stem Disease: The SPARTAN-LMS Study. J. Cardiovasc. Dev. Dis. 2023, 10, 84.
- Gitto, M.; Sticchi, A.; Chiarito, M.; Novelli, L.; Leone, P.P.; Mincione, G.; Oliva, A.; Condello, F.; Rossi, M.L.; Regazzoli, D.; et al. Drug-Coated Balloon Angioplasty for De Novo Lesions on the Left Anterior Descending Artery. Circ. Cardiovasc. Interv. 2023, 16, e013232.
- Nishiyama, N.; Komatsu, T.; Kuroyanagi, T.; Fujikake, A.; Komatsu, S.; Nakamura, H.; Yamada, K.; Nakahara, S.; Kobayashi, S.; Taguchi, I. Clinical value of drug-coated balloon angioplasty for de novo lesions in patients with coronary artery disease. Int. J. Cardiol. 2016, 222, 113–118.
- Gobić, D.; Tomulić, V.; Lulić, D.; Židan, D.; Brusich, S.; Jakljević, T.; Zaputović, L. Drug-Coated Balloon Versus Drug-Eluting Stent in Primary Percutaneous Coronary Intervention: A Feasibility Study. Am. J. Med. Sci. 2017, 354, 553–560.
- Rissanen, T.T.; Uskela, S.; Eränen, J.; Mäntylä, P.; Olli, A.; Romppanen, H.; Siljander, A.; Pietilä, M.; Minkkinen, M.J.; Tervo, J.; et al. Drug-coated balloon for treatment of de-novo coronary artery lesions in patients with high bleeding risk (DEBUT): A single-blind, randomised, non-inferiority trial. Lancet 2019, 394, 230–239.
- Shin, E.S.; Lee, J.M.; Her, A.Y.; Chung, J.H.; Eun Lee, K.; Garg, S.; Nam, C.W.; Doh, J.H.; Koo, B.K. Prospective randomized trial of paclitaxel-coated balloon versus bare-metal stent in high bleeding risk patients with de novo coronary artery lesions. Coron. Artery Dis. 2019, 30, 425–431.
- Vos, N.S.; Fagel, N.D.; Amoroso, G.; Herrman, J.R.; Patterson, M.S.; Piers, L.H.; van der Schaaf, R.J.; Slagboom, T.; Vink, M.A. Paclitaxel-Coated Balloon Angioplasty Versus Drug-Eluting Stent in Acute Myocardial Infarction: The REVELATION Randomized Trial. JACC Cardiovasc. Interv. 2019, 12, 1691–1699.
- Yu, X.; Wang, X.; Ji, F.; Zhang, W.; Yang, C.; Xu, F.; Wang, F. A Non-inferiority, Randomized Clinical Trial Comparing Paclitaxel-Coated Balloon versus New-Generation Drug-Eluting Stents on Angiographic Outcomes for Coronary De Novo Lesions. Cardiovasc. Drugs Ther. 2022, 36, 655–664.
- Wang, Z.; Yin, Y.; Li, J.; Qi, W.; Yu, B.; Xu, Z.; Zhu, W.; Yang, F.; Cao, M.; Zhang, H. New Ultrasound-Controlled Paclitaxel Releasing Balloon vs. Asymmetric Drug-Eluting Stent in Primary ST-Segment Elevation Myocardial Infarction—A Prospective Randomized Trial. Circ. J. 2022, 86, 642–650.
- Rosenberg, M.; Waliszewski, M.; Krackhardt, F.; Chin, K.; Wan Ahmad, W.A.; Caramanno, G.; Milazzo, D.; Nuruddin, A.A.; Liew, H.B.; Maskon, O.; et al. Drug Coated Balloon-Only Strategy in De Novo Lesions of Large Coronary Vessels. J. Interv. Cardiol. 2019, 2019, 6548696.
- Ma, Z.; Liu, K.; Hu, Y.; Hu, X.; Wang, B.; Li, Z. Comparison between Drug-Coated Balloon and Stents in Large De Novo Coronary Artery Disease: A Systematic Review and Meta-Analysis of RCT Data. Cardiovasc. Drugs Ther. 2024.
More
Information
Subjects:
Cardiac & Cardiovascular Systems
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
468
Revisions:
2 times
(View History)
Update Date:
12 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




