1. Introduction
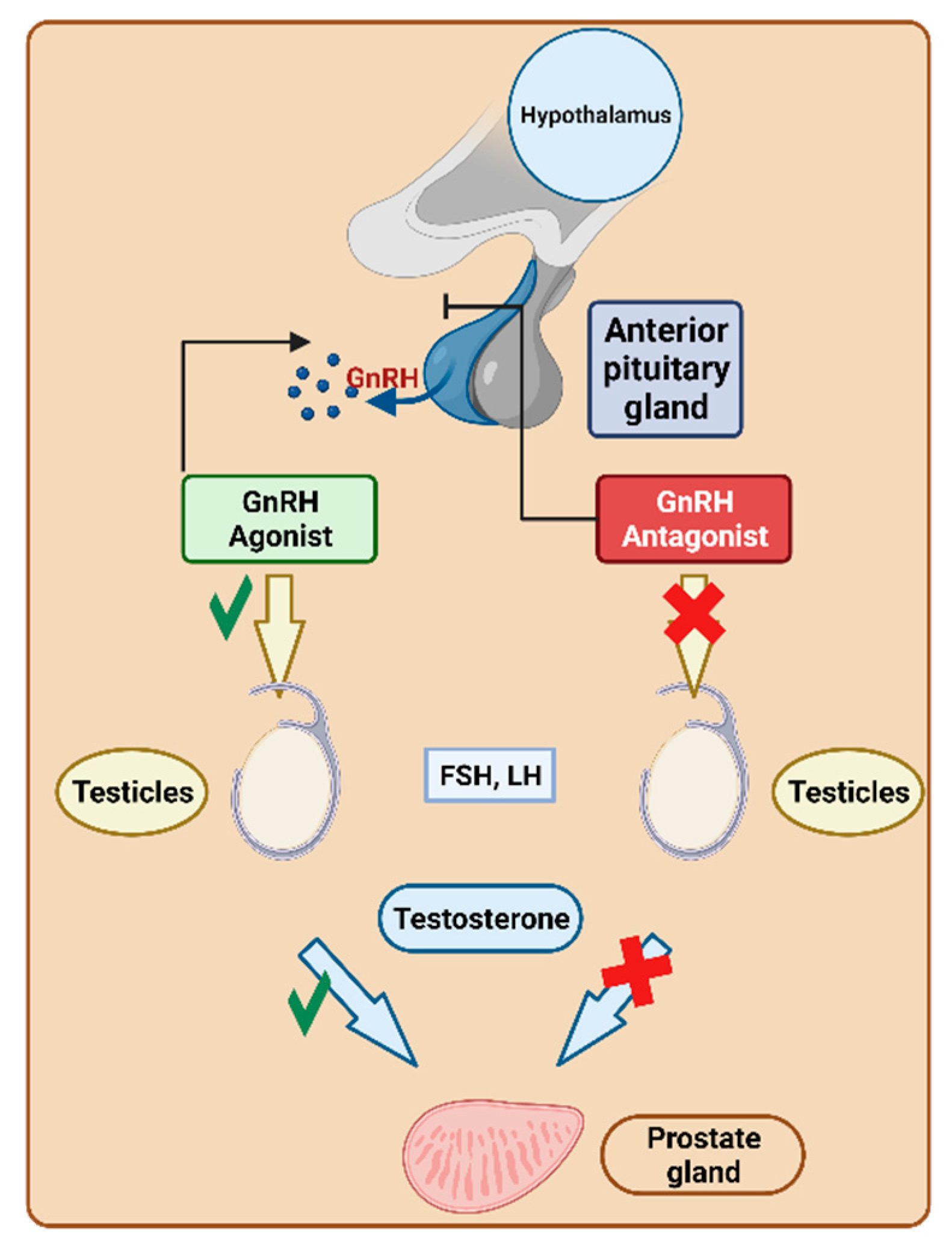
Gonadotropin-releasing hormone (GnRH) triggers the pituitary secretion of luteinising hormone (LH) and follicle-stimulating hormone (FSH)
[1][2]. As a result, GnRH regulates the hormonal and reproductive functions of the gonads
[1]. The overexpression of GnRH has been detected in various human cancerous cells, including both reproductive and nonreproductive organs
[3]. Manipulating the activation or suppression of this functionality is crucial for fulfilling various requirements. For instance, it is essential in preventing luteinization during assisted reproduction or for treating disorders dependent on sex hormones (
Figure 1).
Figure 1. Gonadotropin-releasing hormone (GnRH) mechanism of action. LH, luteinizing hormone; FSH, follicle-stimulating hormone.
Although numerous non-peptide GnRH antagonists are available
[4], peptides have also established their significance in this important field
[5]. They are used to treat prostate cancer by reducing testosterone levels, which are responsible for stimulating the growth of various forms of prostate cancer. This reduction in testosterone contributes to the shrinkage of prostate cancer. Notably, their mechanism avoids hypoestrogenic side effects, flare-ups, or the extended down-regulation period associated with agonists
[6]. They preferentially bind to pituitary GnRH receptors and can either enhance or inhibit their release within hours. The FDA has granted approval to nine peptide-based drugs targeting GnRH (
Table 1).
Table 1. FDA-approved gonadotropin-releasing hormone (GnRH) peptides.
2. Gonadotropin-Releasing Hormone Agonists
2.1. Goserelin (Zoladex)
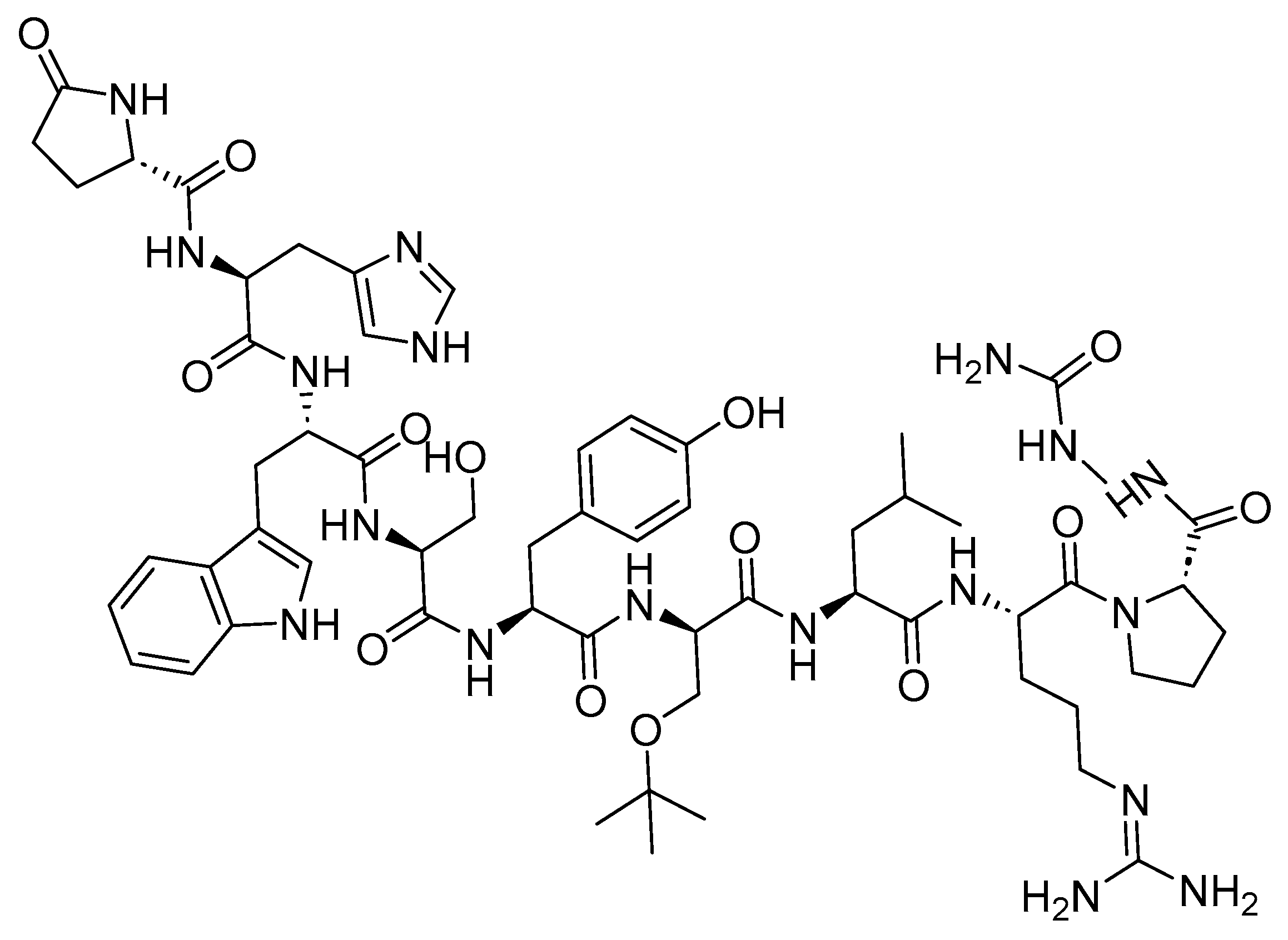
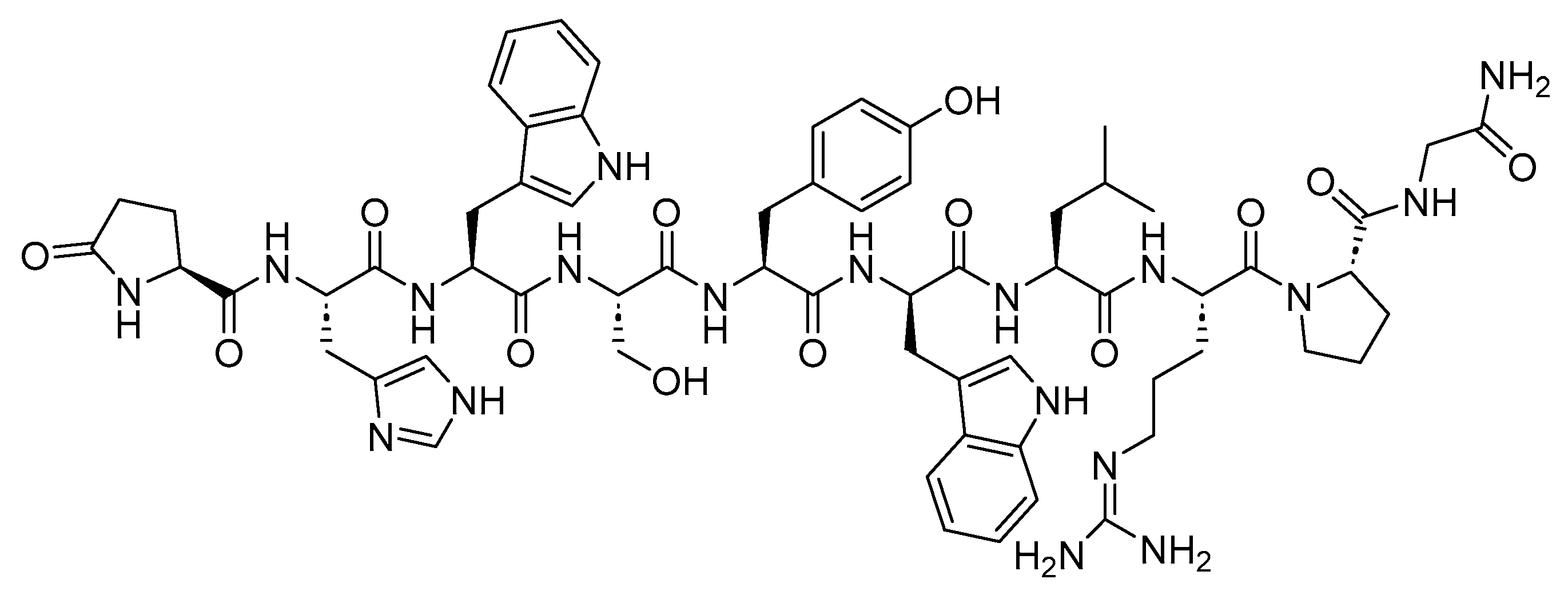
Goserelin is a 10-mer peptide and GnRH agonist (
Figure 2). It is used in conjunction with flutamide for the management of locally confined carcinoma of the prostate, as well as for the palliative treatment of advanced carcinoma of the prostate. The utilization of both goserelin and flutamide in combination resulted in an increased rate of response and a faster normalization of elevated levels of prostatic acid phosphatase and prostatic-specific antigen compared to the use of goserelin alone in
[7]. Additionally, it is utilized in the management of endometriosis, as an endometrial-thinning agent before endometrial ablation for dysfunctional uterine bleeding, and in the palliative treatment of advanced breast cancer in pre- and perimenopausal women
[8].
Figure 2. Chemical structure of goserelin.
Goserelin functions by inhibiting pituitary gonadotropin secretion, leading to an initial rise in serum LH and FSH levels. Subsequently, there are subsequent increases in serum testosterone levels
[8].
AstraZeneca developed Zoladex, which received FDA approval in 1989
[9]. Zoladex is administered subcutaneously, and it may lead to adverse effects including hot flashes, sexual dysfunction, decreased erections, and lower urinary tract symptoms
[8].
2.2. Leuprolide (Lupron)
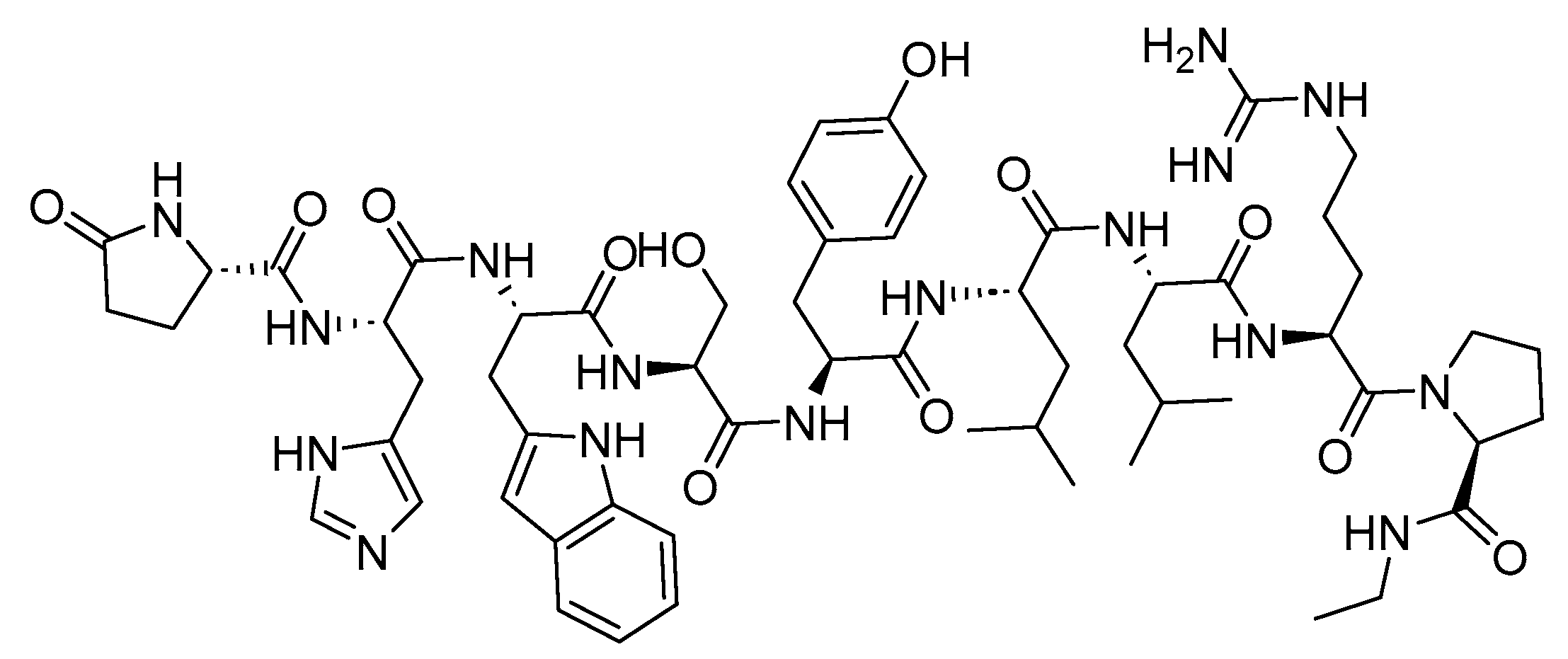
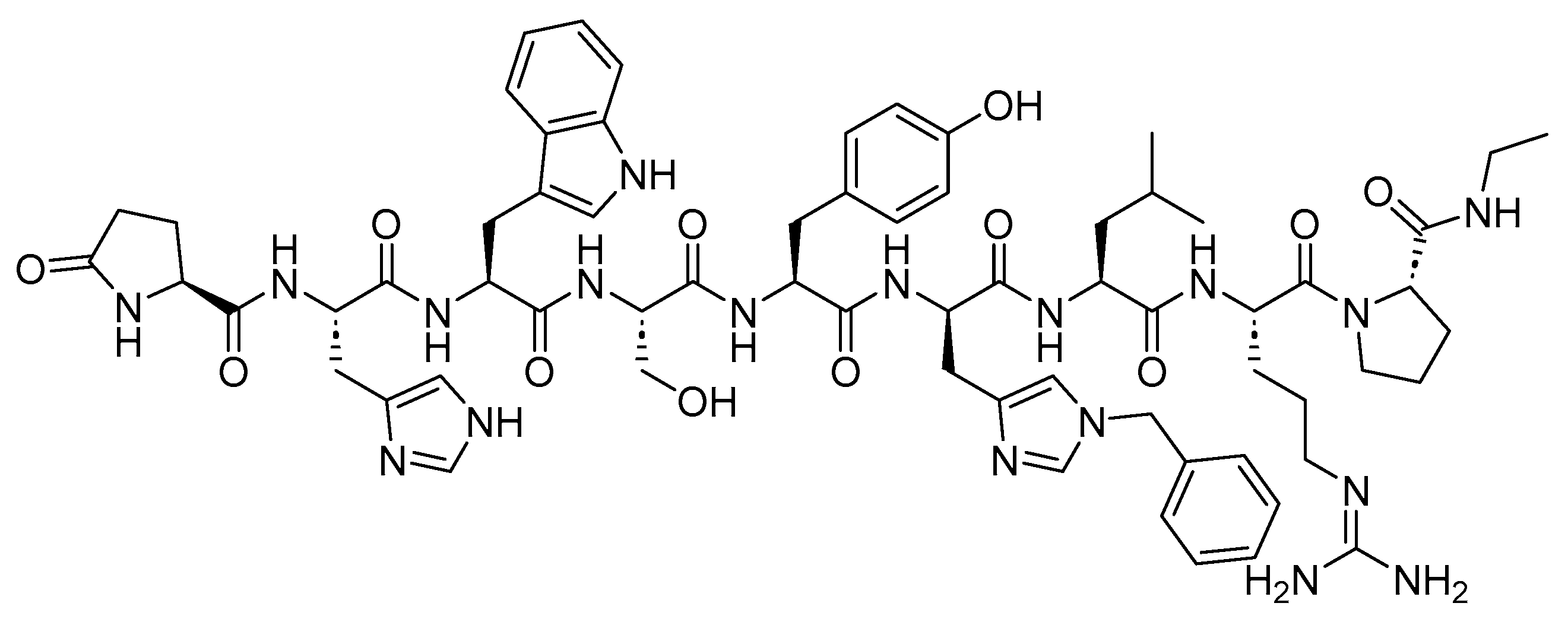
Leuprolide is a 9-mer acid peptide with agonist activity against GnRH (
Figure 3). It is used for the palliative treatment of prostate cancer, uterine leiomyomata, endometriosis, and central precocious puberty
[10].
Figure 3. Chemical structure of leuprolide.
Leuprolide binds to GnRH receptors, triggering the downstream secretion of LH and FSH. This sustained stimulation leads to the substantial and prolonged suppression of both hormones, causing tissues and functions reliant on gonadal steroids to become quiescent
[10][11][12][13].
Tap Pharmaceuticals developed Lupron, and it received FDA approval in 1995
[14]. Lupron is administered intramuscularly and may cause side effects such as itching, rashes, or hives; difficulty breathing or swallowing; pain in the arms, back, chest, neck, or jaw; slow or difficult speech; dizziness or fainting; weakness; numbness or an inability to move an arm or leg; and bone pain
[10].
2.3. Nafarelin (Synarel)
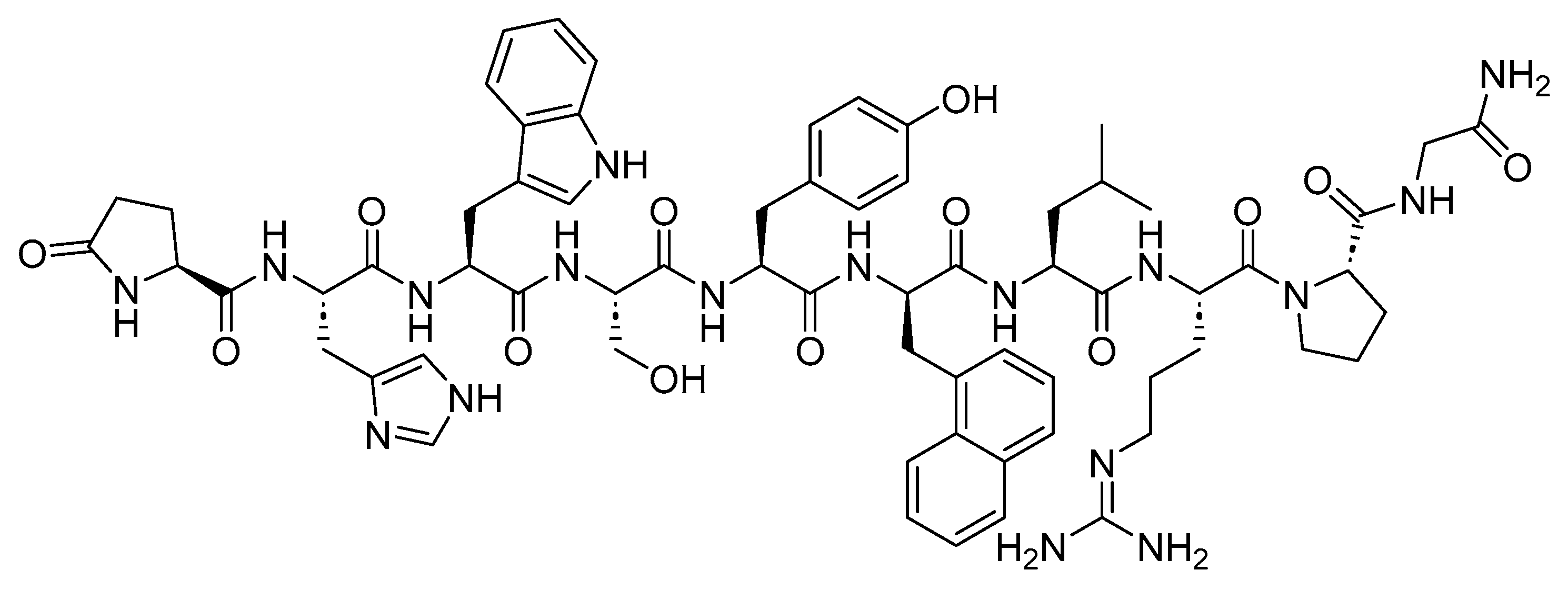
Nafarelin is a 10-mer peptide amide and GnRH agonist (
Figure 4). It is used to manage endometriosis, providing pain relief and reducing endometriotic lesions
[15].
Figure 4. Chemical structure of nafarelin.
Nafarelin stimulates the release of pituitary gonadotropins, LH, and FSH, causing a temporary increase in gonadal steroidogenesis. With repeated dosing, the stimulatory effect on the pituitary gland diminishes, resulting in a decreased secretion of gonadal steroids. Consequently, tissues and functions relying on gonadal steroids for maintenance become quiescent
[15]. Searle developed Synarel, and it obtained FDA approval in 1998
[16].
Synarel is administered as a nasal solution, and it may lead to adverse effects such as shortness of breath, chest pain, urticaria, rash, and pruritus
[15].
2.4. Trelstar (Triptorelin)
Triptorelin is a 10-mer peptide amide and GnRH agonist (
Figure 5). It is used for the palliative treatment of advanced prostate cancer
[17].
Figure 5. Chemical structure of triptorelin.
Triptorelin initially induces a surge in the circulating levels of LH, FSH, testosterone, and estradiol. However, with continuous administration, there is a consistent decline in the secretion of LH and FSH, coupled with a notable decrease in testicular steroidogenesis. This leads to a lowering of serum testosterone concentration, reaching levels typically seen in surgically castrated men. As a result, tissues and functions reliant on these hormones for maintenance enter a quiescent state
[17][18].
Watson Laboratories developed Triptorelin, and it received FDA approval in 2000
[19]. Triptorelin is administered intramuscularly and has been associated with side effects such as hot flushes, skeletal pain, impotence, headache, edema in legs, leg pain, erectile dysfunction, and testicular atrophy
[17].
2.5. Histrelin (Supprelin LA)
Histrelin is a 9-mer peptide amide and GnRH agonist (
Figure 6). It is used for the treatment of central precocious puberty (CPP) in children
[20].
Figure 6. Chemical structure of histrelin.
Histrelin, as a GnRH agonist, acts as a gonadotropin inhibitor when administered continuously. This leads to an initial rise in LH and FSH, subsequently elevating gonadal steroids such as testosterone and dihydrotestosterone in males and estrone and estradiol in premenopausal females
[21]. Continuous administration eventually results in the down-regulation of GnRH receptors in the pituitary gland and the desensitization of pituitary gonadotropes. This leads to decreased levels of LH and FSH
[20]. Histrelin has demonstrated 100-fold higher activity than native GnRH in stimulating the release of LH hormone from monolayers of dispersed rat pituitary cells, and it exhibits a 20-fold higher potency in displacing
125I-GnRH from pituitary receptor sites
[17].
Indevus Pharmaceuticals developed Supprelin LA, and it obtained FDA approval in 2007
[22]. Supprelin LA is administered subcutaneously and may lead to adverse effects such as implant-site reactions and the suppression of endogenous sex steroid secretion
[20].
3. Gonadotropin-Releasing Hormone Antagonists
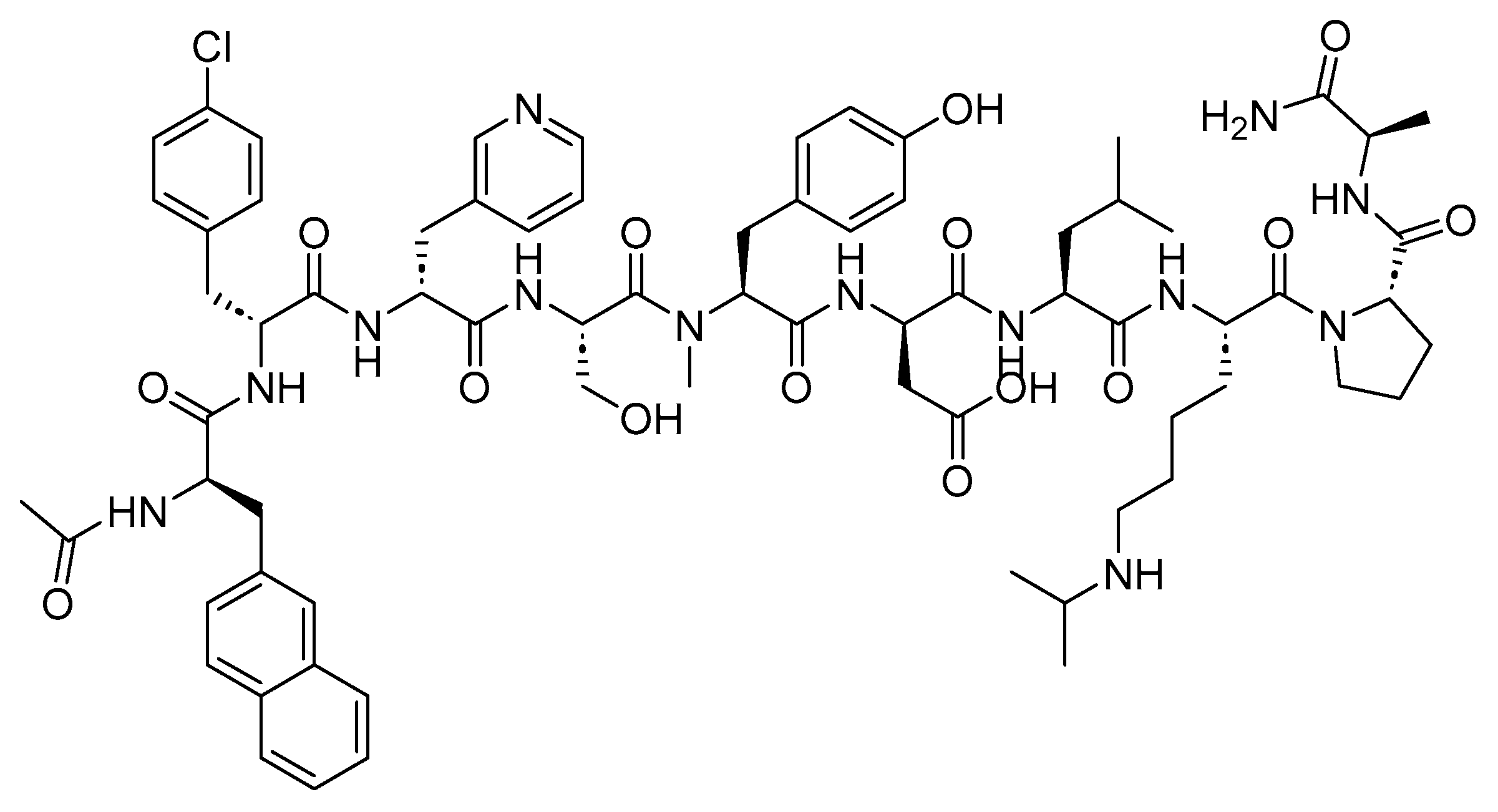
3.1. Ganirelix (Antagon)
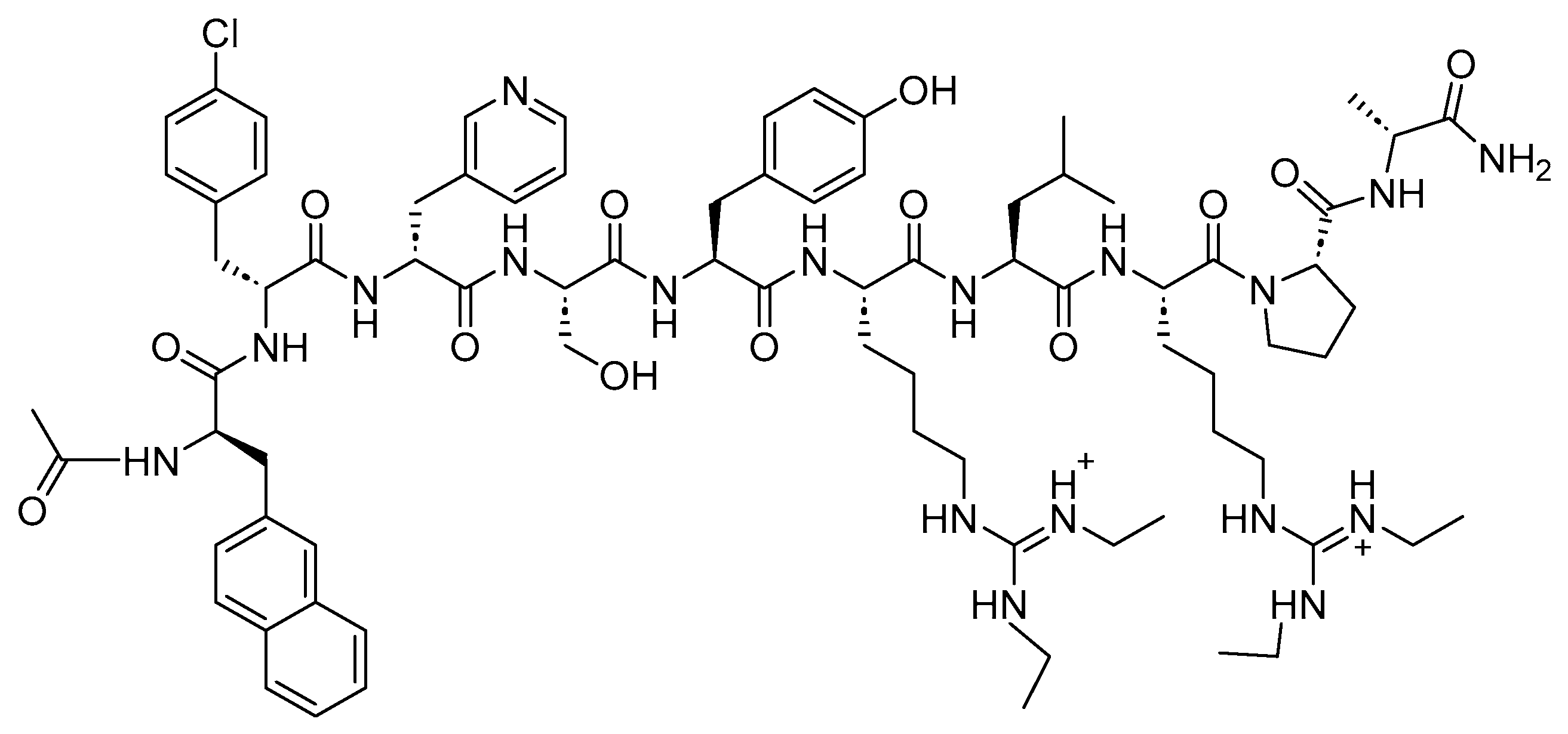
Ganirelix is a 10-mer peptide amide with antagonistic activity against GnRH. Ganirelix shares the same sequence as the native GnRH (Ac-pEHWSYGLRPGNH
2), with the exception of amino acids, 1, 2, 3, 6, 8, and 10 (
Figure 7)
[23][24]. It is used as a fertility medicine to prevent premature LH surges or ovulation in women undergoing fertility treatment involving controlled ovarian hyperstimulation
[24].
Figure 7. Chemical structure of ganirelix.
Ganirelix modulates the hypothalamic–pituitary–gonadal axis by inducing a rapid, profound, and reversible suppression of endogenous gonadotropins. During controlled ovarian stimulation, it specifically suppresses LH and FSH secretion from the pituitary gland
[24]. Interestingly, unlike other GnRH agonists, ganirelix does not cause this initial increase gonadotropin levels before inhibiting premature LH surges
[25]. Ganirelix does not exhibit hypo-estrogenic side effects, flare-up, or a long down-regulation period, which are commonly associated with GnRH agonists
[6].
Organon developed Antagon, which received FDA approval in 1999
[26]. Antagon is administered subcutaneously and has shown various adverse effects including headache, hot flashes, pain, redness, or irritation at the injection site
[24].
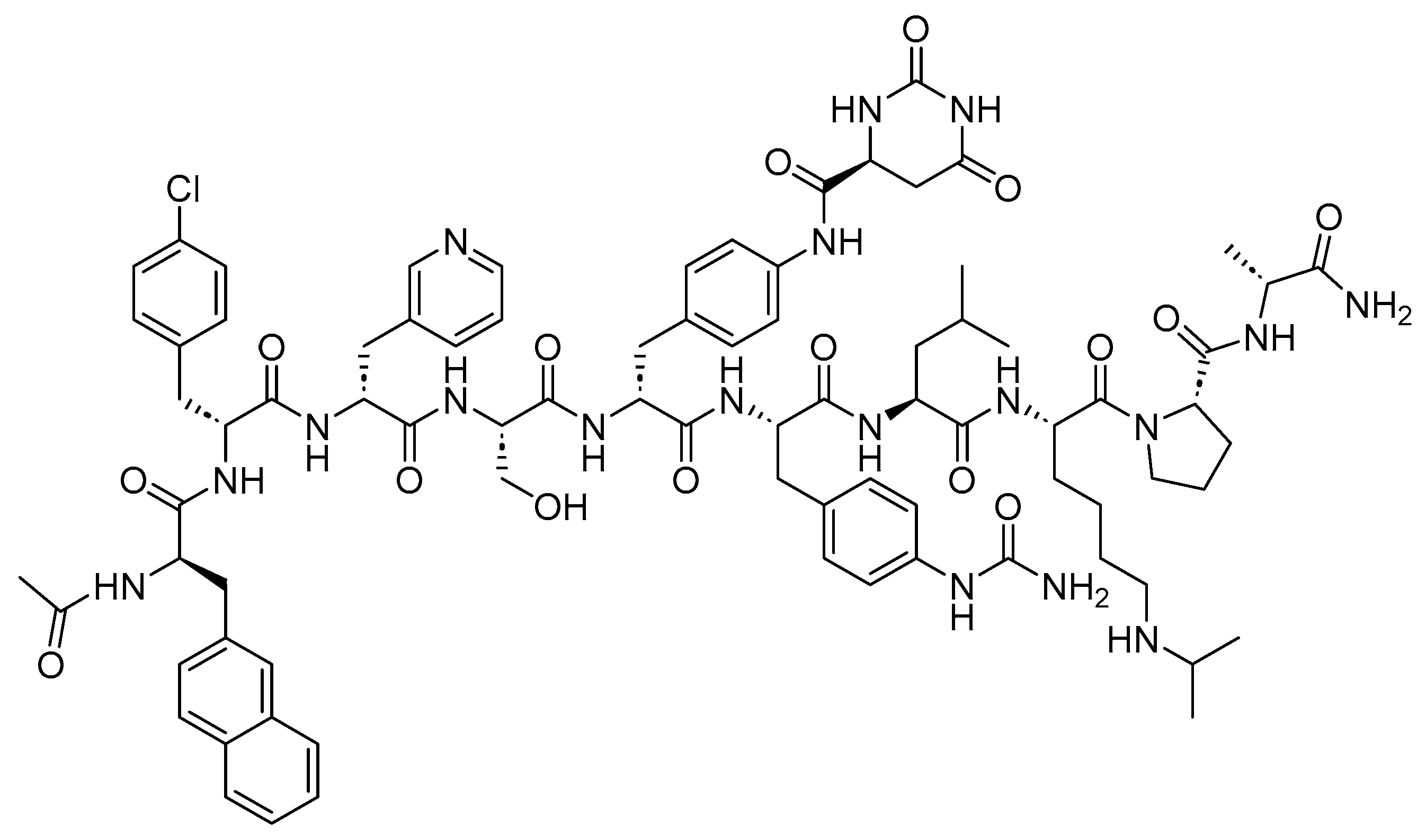
3.2. Cetrorelix (Cetrotide)
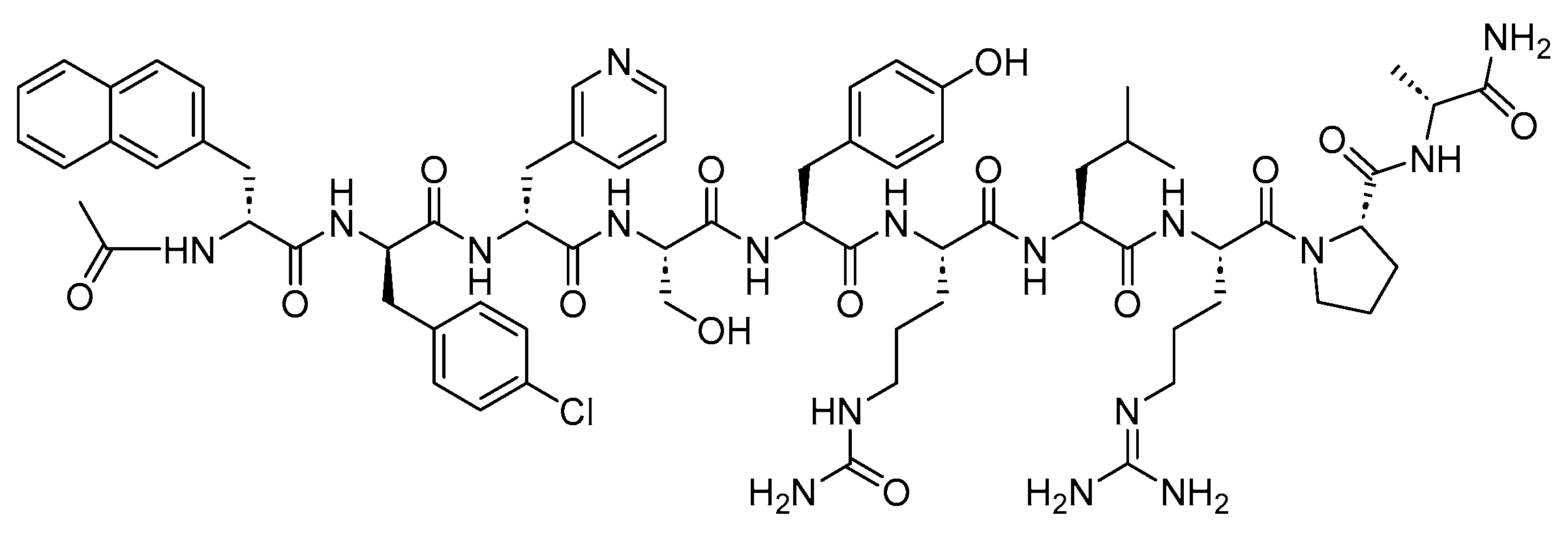
Cetrorelix is a 10-mer peptide amide with antagonistic activity against GnRH
[27]. Cetrorelix shares the same sequence as the native GnRH (Ac-pEHWSYGLRPGNH
2), with the exception of amino acids, 1, 2, 3, 6, and 10 (
Figure 8)
[23][27]. It is used to prevent premature ovulation, which refers to the early release of eggs from the ovary
[27].
Figure 8. Chemical structure of cetrorelix.
Cetrorelix binds to the GnRH receptor, inhibiting the secretion of gonadotropin and thereby controlling the release of LH and FSH in a dose-dependent manner
[27][28].
ASTA medica developed Cetrotide, which received FDA approval in 2000
[29]. Cetrotide is administered subcutaneously and has been associated with adverse effects such as stomach pain; bloating; nausea; vomiting; diarrhea; rapid weight gain (especially in the face and midsection); little or no urination; pain during breathing; rapid heart rate; and a sensation of breathlessness, particularly when lying down
[27].
3.3. Abarelix (Plenaxis)
Abarelix is a 10-mer linear peptide amide with potent antagonistic activity against GnRH (
Figure 9). It is used in the palliative treatment of advanced prostate cancer
[30].
Figure 9. Chemical structure of abarelix.
Abarelix inhibits gonadotropin by competitively blocking GnRH receptors in the pituitary, thereby suppressing LH and FSH. This results in a reduction in the secretion of testosterone by the testicles, leading to the slowing of the growth and reduction in the size of prostate cancers
[30][31]. Most importantly, abarelix does not induce the initial testosterone surge that is typical of GnRH agonists, a characteristic that could potentially exacerbate the situation
[31].
Praecis Pharmaceuticals developed Plenaxis, and it received FDA approval in 2003
[32]. Plenaxis is administered intramuscularly and may result in adverse effects such as hot flashes, sleep disturbances, and breast enlargement or pain
[30].
3.4. Degarelix (Firmagon)
Degarelix is a 10-mer linear peptide amide with potent antagonistic activity against GnRH (
Figure 10). It is used to treat advanced prostate cancer
[33].
Figure 10. Chemical structure of degarelix.
Degarelix inhibits GnRH receptors in the pituitary, thereby suppressing LH and FSH. This suppression leads to a reduction in the secretion of testosterone, which, in turn, slows the growth and reduces the size of prostate cancers
[34]. Notably, degarelix does not induce the initial testosterone surge that is characteristic of GnRH agonists
Ferring Pharmaceuticals developed Firmagon, and it received FDA approval in 2008
[34]. Firmagon is administered subcutaneously and may lead to side reactions such as injection-site reactions, hot flashes, weight gain, and an increase in the serum levels of transaminases and γ-glutamyl transferase (GGT)
[33].