1. Introduction
Oil has been a primary source of energy for the last centuries. Along with technological advancement, energy consumption has increased over the years. Based on the current energy consumption growth rate, the energy demands will be estimated to be around 260 peta watthours by 2050, nearly 50% higher than the current consumption
[1]. Recently, many non-conventional and renewable energy sources have been used to reduce the dependency on environmentally harmful resources. Nonetheless, due to the inherently low efficiency and sluggish development of renewable energy technologies, conventional oils are expected to be researchers' primary energy source for the foreseeable future. To meet the increasing energy demands, the recovery rate of conventional oils needs to be increased because only 10% of oils out of total deposits can be recovered from the reservoir via traditional extraction methods
[2]. Therefore, several enhanced oil recovery (EOR) techniques have been developed and implemented in the oil recovery process. There are three main stages to completing oil extraction processes: primary, secondary, and tertiary oil recovery
[3]. The primary oil recovery process is the first stage of hydrocarbon production, achieved by the reservoir’s natural driving forces, initially present in the reservoir due to rock and fluid expansion, solution gas, water influx, gas cap, and gravity drainage
[4]. From the primary process, nearly 10% of oil within the reservoir can be recovered
[2]. This small percentage of oil recovery causes the secondary and tertiary processes to extract additional oil from the reservoir. In the secondary recovery process, external fluids like water and gas are injected to recover more oil via a volumetric sweep of the original oil in place (OOIP) while maintaining additional pressure in the reservoir
[5]. Water or immiscible gas injections are the most common techniques used in this process
[6]. Despite primary and secondary recovery implementation, around 70% of oil remains in the reservoirs
[7].
The tertiary recovery method targets immobile oil remaining within the reservoir after the secondary recovery process is completed because primary and secondary oil recovery processes mainly target mobile oil in the reservoir
[8]. Tertiary recovery is generally completed by injecting chemicals, gasses, and thermal energy
[9]. The term “enhanced oil recovery (EOR)” was adopted in place of tertiary recovery because the chronological term “tertiary” does not suitably describe certain unconventional recovery operations that are applied as a secondary recovery method
[9]. In EOR processes, injected fluids interact with the rock, oil, and brine system within the reservoir, altering the reservoir’s rock and fluid properties. Using the appropriate method of injection of gases, thermal energy, and chemicals can significantly impact reservoir oil production efficiency
[9].
An appropriate EOR method should be selected to maximize recovery efficiency based on the wide range of reservoir properties like temperature, depth, the permeability of porous structure, and the type of rocks. In the past few decades, EOR projects have been accomplished mainly based on thermal methods
[10]. However, recent target reservoirs for oil recovery are located at higher depths as the old reservoirs are depleted, which makes the thermal method unsuited as this method is not economically efficient at deeper depths
[11]. In contrast, chemical EOR methods show great potential in deep reservoirs due to their technical and economic feasibility
[11]. Over decades of research, wettability alteration, interfacial tension reduction (IFT), and mobility ratio have been identified as primary governing mechanisms in these methods. In EOR, the wettability alteration is preferably conducted to make the rock reservoir surface water-wet (hydrophilic) from oil-wet (hydrophobic), leading to easy oil extraction. In chemical EOR, wettability alteration is mostly achieved by chemical flooding, which includes surfactant flooding, injection of polymer solutions, alkaline flooding, and nanoparticles suspended in fluids termed nanofluid flooding (also known as nano-assisted flooding).
Many reviews have been published on a specific aspect of the wettability alteration. For example, there is a comprehensive review of theoretical and experimental advancement in surfactant-induced EOR with published literature, mainly from 1970 to 2010
[12]. In this review, the evolution of reservoir wettability and its effect on oil recovery was discussed. Additionally, based on the experimental results of over a half-decade, it was discussed that the effectiveness of surfactants depends not only on the wetting condition of the reservoir but also on other conditions like capillary pressure, rock mineralogy, and porosity. In addition, the chemicals used in wettability alteration and their feasibility were summarized
[13]. Based on more than 100 experimental research publications, they found that chemical-based EOR is most effective when conducted in light oil reservoirs. They discussed the need for screening criteria for the use of surfactants. Furthermore, there is a thorough review of the factors affecting surfactant flooding, including the effect of phase behavior, adsorption, and structure–property relationship on wettability alteration
[14]. It was concluded that the wettability alteration of sandstone reservoirs requires more attention due to its varying parameters and challenging implementation. Another critical review summarizes the experimental work on nanofluid-assisted wettability alteration
[15]. Based on many experimental results, they summarized the mechanisms and factors affecting wettability alteration by nanofluid flooding. Despite the available work for reviews, concerns about a lack the understanding of the wettability alteration mechanisms in various reservoir conditions still exist. Fortunately, an emerging technology, microfluidics associated with microfabrication and reservoir micromodels, has been implemented over the last decades to better understand wettability alteration mechanisms in EOR. The microfluidic technologies enable real-time in situ visualization and various contactless measurements to assess the geochemical changes during the EOR to further understand the underlying mechanism like wettability alteration, which was not possible in the core-based experiments.
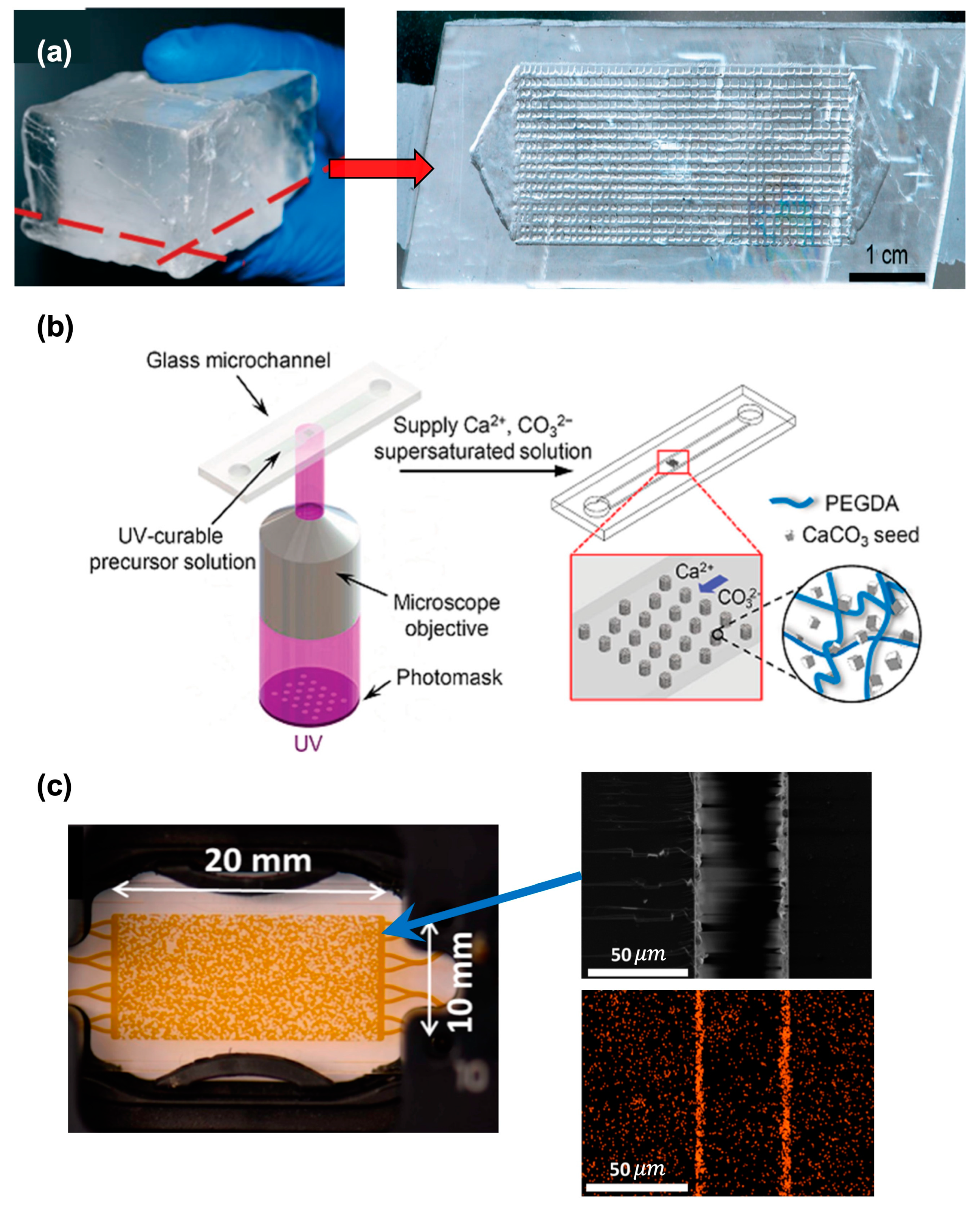
Before discussing the new development in understanding the underlying EOR mechanism, the microfluidic reservoir technologies and how they closely mimic the actual reservoir conditions must be clarified. In the initial phase, the microfluidic platforms of reservoir porous structures were mostly fabricated from glass, silicon, and polymeric materials like PMMA, with well-documented methods like acid etching, lithography, and laser cutting. These models were not able to mimic certain properties of the reservoir, such as different wetting orders and the geochemistry of carbonate or sandstone rocks. However, the new fabrication techniques have removed these limitations. Firstly, actual calcite rock was used to fabricate a 2D porous network by acid itching (
Figure 1a)
[16]. It enabled the assessment of the geochemical effect of reservoir rock on EOR, along with flow visualization. Later, the in situ growth of CaCO
3 crystals in the micromodels was visualized by providing Ca
2+ and CO
32− ion-rich solutions to the targeted zones in the channels. This process enables the fabrication of synthetic carbonate rock reservoir surfaces in the micromodels (
Figure 1b)
[17]. A coated CaCO
3 nanocrystal layer on the borosilicate surface of the microchannel with a controlled thickness of 1–2 μm (
Figure 1c) was used to mimic the reservoir structure
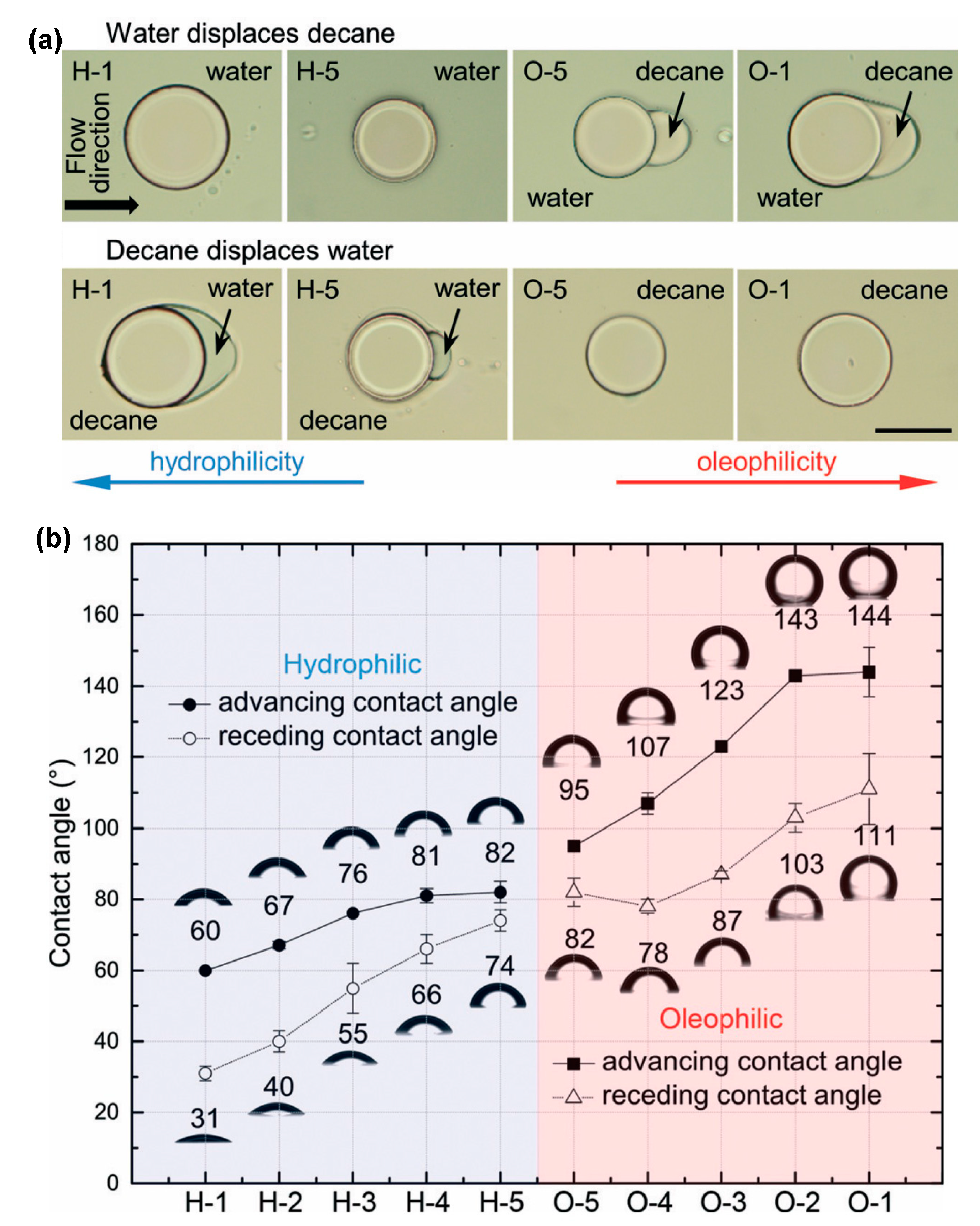
[18]. Furthermore, well-established methods like salinization, UV-ozone treatment, and UV-initiated polymer grafting are used to achieve different wetting orders into the micromodels. An article about the tailored wetting order in the same pore network was published. In their study, different polymeric crosslinkers with different ratios of hydrophobic and hydrophilic reactive groups were polymerized via UV exposure, modulating a wide range from 60° to 144° contact angle (
Figure 2)
[19].
Figure 1. Evolution of microfluidic fabrication to mimic reservoir rock. (a) Microchannel made out of real calcite rock by acid etching process; (b) in situ growth of CaCO3; (c) nanocrystal layer of CaCO3 in microchannel surface (left—actual microchannel image; right—electron image and electron diffraction spectroscopic image of calcium).
Figure 2. Demonstration of tailored wetting order in microchannel surface. (a) Actual image of hydrophilic and oleophilic surfaces with H-1 being most hydrophilic and O-1 being most oleophilic (scale bar is 100 µm long). (b) The graph shows the image of different contact angles obtained via different polymeric crosslinkers.
2. Wettability Characteristics and Its Effect on Oil Recovery
2.1. Wettability Characteristics
Wettability is defined as the ability of a fluid to stay in contact with a solid surface preferentially to another immiscible fluid present due to intermolecular interactions
[20]. The cohesive and adhesive forces describe the intermolecular interaction between solids and liquids. Cohesive force attracts similar molecules; adhesive force attracts different molecules. The balance of adhesive and cohesive forces of the oil–water–rock phase defines the wettability conditions of reservoir rocks
[20]. The wettability condition of reservoir rocks is one of the most critical factors influencing fluid flow in porous media, subsequently impacting the overall hydrocarbon recovery efficiency
[21]. Based on their wettability characteristics, oil reservoirs are divided into water-wet, oil-wet, mixed-wet, and intermediate-wet.
Many studies claim that the reservoirs were originally saline reservoirs before oil migration
[13][22]. In some reservoirs, when oil invades the water-occupied porous media, a thin water film is conserved between the rock surface and oil throughout the evolution of the reservoir. These reservoirs exist as water-wet reservoirs. In some other reservoirs, the water films collapsed throughout the development of the multiphase flow, and the reservoirs became oil-wet
[23][24]. The water film’s stability depends on the crude oil’s pH and asphaltenes. A detailed investigation of this phenomenon is provided in the literature published by Hirasaki
[25], Kovscek et al.
[26], and Kaminsky and Radke
[27]. In some cases, the reservoir’s wetting condition is heterogeneous, meaning some part of the reservoir is oil-wet, and others are water-wet, defined as mix-wet
[28][29]. In some literature, this condition is further classified into two cases: mixed-wet large and mixed-wet small. Mixed-wet large is considered when the large pores of the mixed-wet reservoir are oil-wet, and small pores are water-wet, and vice versa for the mixed-wet small
[30]. The mixed-wet order is mostly found in the carbonate reservoirs
[28]. Lastly, there is a particular case where reservoir rock does not have a strong affinity for either of the fluids. In other words, the reservoir rock has tendencies for oil and water to adhere to the rock pore surface. This case is classified as intermediate-wet
[31]. It is important to note that the fundamental difference between intermediate-wet and mixed-wet is that intermediate-wet lacks a wetting preference or strong affinity for water or oil. In contrast, mixed-wet entails an array of wetting preferences encompassing intermediate wetting conditions. A study analyzes a large group of mixed-wet reservoirs and provides an understanding of mixed-wet reservoirs with theoretical and experimental studies
[30].
2.3. Effect of Wettability Parameters on Oil Recovery
2.3.1. Relative Permeability
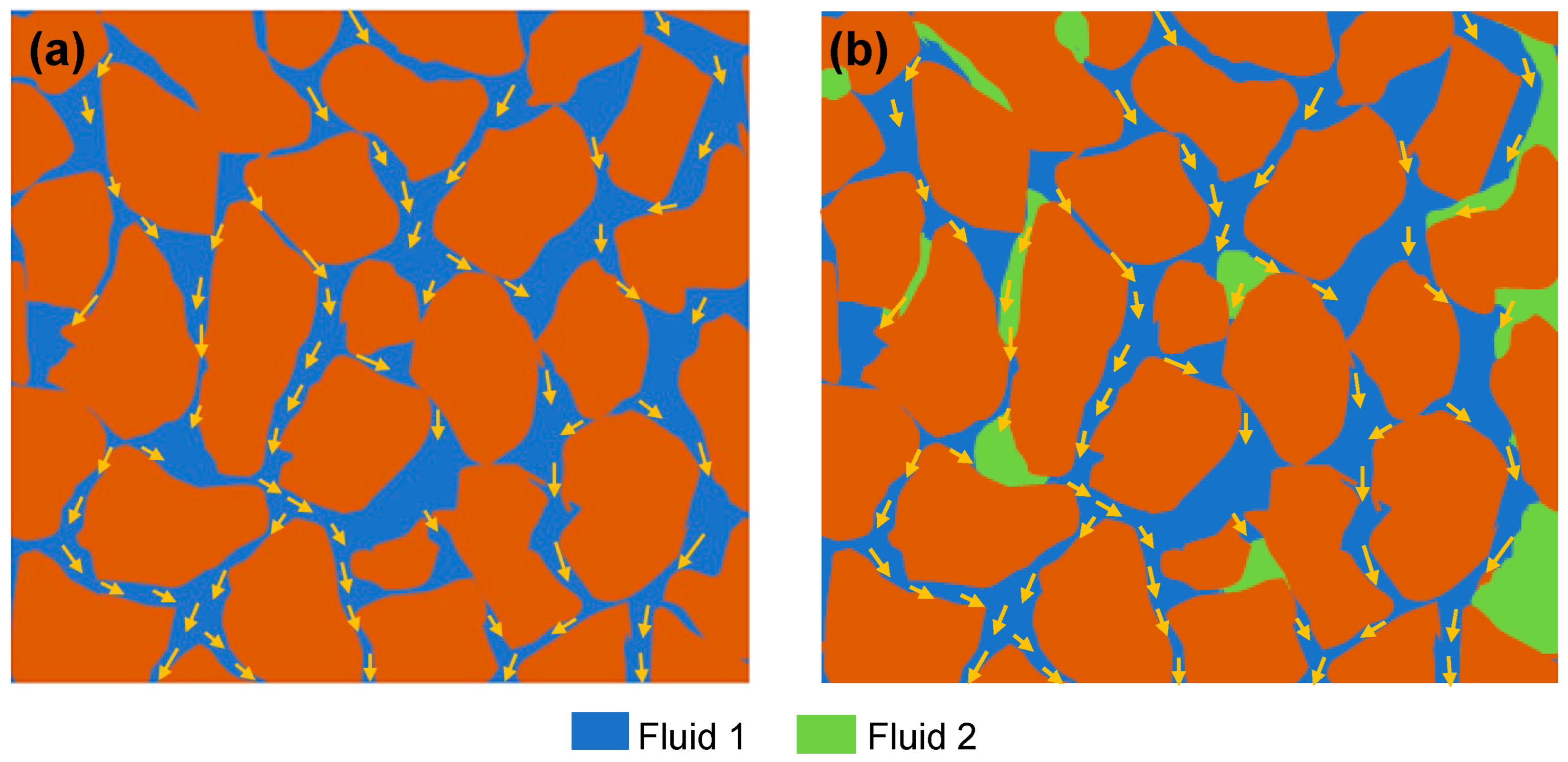
Permeability is a measure of the ability of a porous media to allow a single fluid to flow through it
[32]. The permeability of one fluid in the matrix of multiple fluids is called the effective permeability of that fluid. Naturally, the porous media of the reservoir is saturated by fluid (most likely water), which cannot be removed from the pores due to the inherent complexity of the porous system. The volume of this fluid is termed irreducible saturation of the fluid. In the presence of this irreducible saturated fluid, the ability of other fluids to flow through porous media is called base (absolute) permeability (
Figure 3). The relative permeability is the ratio of effective permeability to base permeability
[33].
Figure 3. Illustration of permeability. (a) Base permeability of fluid 1. (b) Relative permeability of fluid 1 (permeability of fluid 1 in the presence of fluid 2).
It is intuitively understood that if the relative permeability of oil is higher in the reservoir, the overall recovery will be higher. However, the relation between the wettability and the relative permeability of the reservoir is not clear from that statement. In a water-wet reservoir, water’s affinity toward the reservoir’s surface is higher. Hence, during the water flooding, the water invading the porous media will imbibe sufficiently into small pores by replacing oil in the main and large channels. In the channels and large pores, water will create a layer or film adjacent to the reservoir’s surface, and oil will be free to flow through the pores and channels. Water flooding also creates a “snap-off point” phenomenon, where after enough water flooding, water saturation becomes high enough to block the oil flow, so the relative permeability of oil is reduced drastically toward zero because of flooding-induced oil trapping into the pores. On the other hand, if the reservoir is oil-wet, the water’s affinity toward the reservoir surface is low. Hence, during the water flooding, the water will not be able to imbibe into small pores to replace oil. Additionally, the oil will create a film or layer adjacent to the reservoir surface, and water will flow freely into the main channel. In this case, the “snap-off point” arrives earlier during water flooding. Beyond the snap-off point, water flooding is not economically viable as the oil production is very low to none. This phenomenon was established based on many core flooding experiments under different phase saturation and wetting conditions
[34][35][36][37]. By collecting these experimental data, Craig published the relation between phase saturation and its relative permeability for other wetting systems
[38], following many models that agree with Craig’s curves
[38][39][40][41].
Because of its importance in oil recovery, many experiments have been performed to determine the relative permeability of a steady-state multiphase core displacement
[42][43][44][45]. Despite the quantitative outcomes of core experiments, there are some drawbacks to the accuracy and practicality of these approaches. Firstly, the core experiment is time-consuming and expensive to accomplish the flooding in given reservoir conditions, which requires significant time and costs to cover multiple environmental conditions
[37]. Secondly, the experiment somewhat changes the core’s wetting condition and micropore structures. Hence, multiple experiments in a single core would raise the stochastic error
[42]. Lastly, the invisibility of the internal flow in conventional core experiments leaves the effect of pore structure unattained
[42].
Over the last few decades, two-phase, real-time experiments in micromodels have been incorporated into the core flooding experiments to widen the fundamental understanding of the relative permeability of oil recovery. In fact, various micromodels have shown a different interpretation of the role of wettability in oil recovery. For example, phase saturation in the pore network was considered as the significant parameter controlling relative permeability in the reservoirs, but with the microfluidic experimental studies; it is found that pore structure and flow patterns of the fluid can have a high impact on relative permeability and, to an extent, oil recovery. In one experiment, the investigation of the oil flooding by water with the microfluidic approach with the use of deep learning and an image recognition intelligent model was conducted
[42]. Based on the artificial intelligent models, it was found that the relative permeability or oil recovery is affected by phase saturation, wetting order, and pore structure with 36.23%, 33.20%, and 30.57%, respectively. In that study, different pore diversities with the co-efficient of 0.872, 3.248, and 6.965 with three different flow rates of 50, 100, and 150 µL/min were flooded by water to displace light cured oil. The last three figures show the displacement of water by light crude oil at 100 µL/min. These many experiments were only feasible due to the microfluidic fabrication approach. In addition, relative permeability is considered a static function of saturation, which means that as saturation changes, the relative permeability instantaneously changes
[46][47][48]. This assumption suggests that changes in oil mobility are instantaneous in the wettability alteration process. However, in the microfluidic study conducted by Du et al., the oil saturation changed over time, suggesting that relative permeability is also a function of time
[49]. This sort of new information in these two micromodel studies shows that extensive research in micromodels would be beneficial to better understand the effect of relative permeability on oil recovery.
2.3.2. Capillary Pressure
Capillary pressure is the pressure difference between two immiscible fluids at the contact interface when the curved surface forms between them. In the case of oil reservoirs, when the radius of curvature is on the oil side, it is considered positive and vice versa. When the interface is not curved, the capillary pressure is zero
[50][51].
Capillary pressure (Pc) is an explicit function of the wettability condition. Positive capillary pressure suggests the rock surface is strongly water-wet as the contact angle is small and vice versa. The static effect of capillary pressure on oil recovery has been known for a long time, and many core-based experiments have been conducted to explore the effect of different capillary pressures on oil recovery. Based on extensive core experiments, the capillary pressure curve was created, a simple way to understand the oil-water displacement process inside the pores while flooding. The capillary pressure vs. phase saturation graph helps assess the capillary pressure zones at which the oil recovery would be efficient.
In the flooding process, the capillary pressure is responsible for the imbibition phenomena, contributing to oil recovery. When the capillary pressure is higher, the water imbibes the oil from small pores to the main channels, increasing the oil recovery. In contrast, when the capillary pressure is negative, the water drains from the small pores by oil, prohibiting oil recovery. In a water-wet reservoir, during the first and second stages of oil recovery, the capillary pressure is high, which causes spontaneous imbibition of oil from the small pores
[52].
As known, the pore networks are inconsistent in nature, which leads to different capillary pressures in different areas of the reservoir. Based on some core-based experimental analysis, it was found that the displacement fluid flows more dominantly from the areas where the capillary pressure is more favorable in heterogeneous pore structures due to different rates of imbibition phenomena. On the other hand, in places where the capillary pressure is less favorable, the meniscus between wetting and non-wetting fluid does not form prominently. It leads to less imbibition or no imbibition in that area. Hence, the flow of displacement fluid creates some streams of flow across the core, leaving a good portion of the core intact and resulting in less oil recovery. This phenomenon is called capillary fingering.
Additionally, there is another physical phenomenon called viscous fingering. During flooding, when the less viscous phase displaces the more viscous phase due to imbibition and drainage phenomena, it creates the flow velocity difference between invading fluid and recovering fluid (oil). Because of this difference, similar to capillary fingering, different flow streams across the core are created called viscous fingering
[53][54].
As more displacement fluid has been pushed into the core during the flooding, the capillary number changes and three flow regimes could be formed: stable regime, viscus fingering regime, and capillary fingering flow regime
[55]. The stable regime is achieved when the flooding fluid moves forward without any fingering effect. The oil recovery is highest in the stable regime flooding as it does not leave any portion of the core intact. Graphically, these three different regions can be understood with the plot of two non-dimensional numbers called the capillary nu number and mobility ratio, where the capillary number (Ca) is the ratio of viscous drag force, and the interfacial tension, mobility ratio (M) is the ratio of the mobility of invading phase to receding phase.
Despite many extensive core experiments, very little effort was given to understand the transition zone from viscus fingering to capillary fingering regimes due to limited dynamic analysis of the flooding in cores
[56]. The understating of the (transition) crossover zone is important as the oil recovery is minimal in this zone as both viscous and capillary fingering coexist. To avoid these phenomena, its accurate characteristics should be known. The core experiments provide a wide range of these crossover zones. However, after implementing the microfluidics approaches on flooding experiments, analysis of the effect of dynamic capillary pressure on oil recovery is possible while allowing the real-time analysis of the fluid invasion to various pore structures to quantify the fluid invasion morphology and especially the accurate identification of crossover zones.
In one of the micromodel research projects, a micromodel surface was analyzed with various capillary and viscous forces to analyze the crossover zone, and it was found that the crossover zone was ted from 15% to 25% of a water saturation zone or an invading fluid saturation zone. This research gave insight into how capillary and viscous forces determine a crossover zone
[57].
Similarly, another micromodel experiment was conducted with significant variations in mobility ratio (M) and capillary numbers (Ca) to thoroughly analyze the transition or crossover zones from viscus fingering to capillary fingering regimes. It was found that the crossover regime found in the experimental results consists of a slightly larger portion of the total flow regime compared to other published reports
[58]. As mentioned earlier, the knowledge of this crossover zone is important as it is one of the deciding factors for selecting various surfactants and nanoparticles for flooding, which could maintain the desire Ca and M. The wider crossover zone estimation would be safe to avoid less oil recovery. However, it could also lead to a more costly EOR process. The accurate and narrow crossover zones would allow wider options for Ca and M and, to an extent, more options for surfactants and nanoparticles, which could be economically beneficial. The inconsistency in different experimental results shows the need for a more thorough analysis to provide an accurate regime diagram associated with capillary pressure.
These wettability parameters are often analyzed in computational studies to simulate the chemical floodings for EOR. The microfluidic experiments could be used to validate the new theoretical results. For example, an article was published related to the modeling and simulation of surfactant–polymer flooding to analyze a new hybrid method
[59]. In this study, the effect on residual saturation of both (oil and flooding fluid) phase, relative permeability, and capillary pressure by IFT and wettability were analyzed. Similarly, in one of the modeling analyses, the simulations were performed to understand and predict the EOR as a function of wettability alteration
[60]. The impact of uncertainties in the pores network, along with other wettability parameters on EOR, was simulated. These types of theoretical results can be future validated by micromodel experiments without economic challenges to test new theories.
3. Wettability Alteration by Surfactants
More than 60% of the oil reservoirs in the world are classified as carbonate reservoirs, which are inherently oil-wet or mix-wet; therefore, the oil extraction process from these reservoirs is challenging [61][62]. This challenge arises due to the weak capillary driving force existing in the rock reservoirs, making the spontaneous imbibition process difficult. Furthermore, carbonate reservoirs are considered highly fractured, which makes the second stage recovery less efficient. Consequently, around 80% of OOIP remains in the reservoir after the second stage of recovery [63]. In this case, altering the reservoir wettability to strong water-wet is necessary. In the last few decades, extensive research has been conducted to alter the wettability by chemical flooding, especially with different surfactants and nanofluids.
Surfactants are organic compounds composed of a long molecular chain with a hydrophilic head and a hydrophobic tail that allow them to be soluble in water and organic solvents [64]. These compounds can alter the rock wettability to more water-wet, especially in the carbonate reservoirs. Based on the charge type of hydrophobic head, it is commonly divided into four types: cationic, anionic, nonionic, and zwitterionic [65]. Recently, a new type of surfactant has been developed with two heads and two tails called Gemini surfactants [65].
3.1. Wettability Alteration Mechanism by Surfactants
Based on extensive research exploitation on surfactant-assisted EOR, three major phenomena have been commonly identified as wettability alteration mechanisms by surfactants, namely the ion pairing, adsorption, and micellar formation. In general, carbonate reservoirs are mostly composed of calcite, anhydrite, and dolomite, with a positively charged surface
[66]. On the other hand, sandstone reservoirs are composed of quartz and feldspar, which makes their surface negatively charged. The oil contains acidic and basic polar organic compounds
[67]. Therefore, based on the affinity of the reservoir surface charge, the carboxylate group (negatively charged acidic polar organic compound (POC)) or nitrogen-containing aromatic molecules (positively charged basic POC) of the oil become adsorbed to the reservoir surface and become oil-wet.
3.1.1. Ion Pairing Mechanism
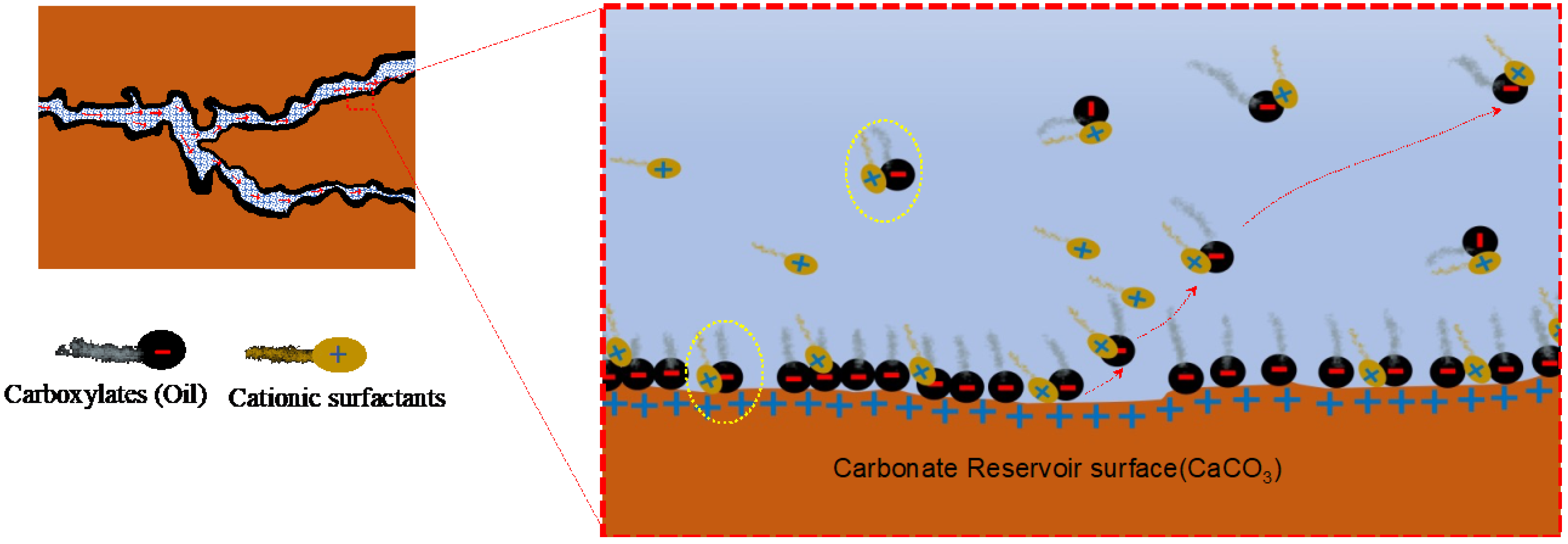
In the ion pairing mechanism, the positively charged surfactant heads (cations) become attached to the negatively charged carboxylates from the crude oil due to electrostatic force and form an ionic pair (
Figure 4). The pair is stabilized by hydrophobic interaction between a surfactant’s tail and oil components, as both tend to aggregate in a water solution. This pair can then easily be imbibed by water from the small pores and becomes stripped off from the rock surface, uncovering the original water-wet layer of rock
[65]. In some literature, this mechanism is called the cleaning or surfactant desorption mechanism
[68].
Figure 4. Illustration of the ion pairing mechanism in a carbonate reservoir
[69].
3.1.2. Surfactant Adsorption Mechanism
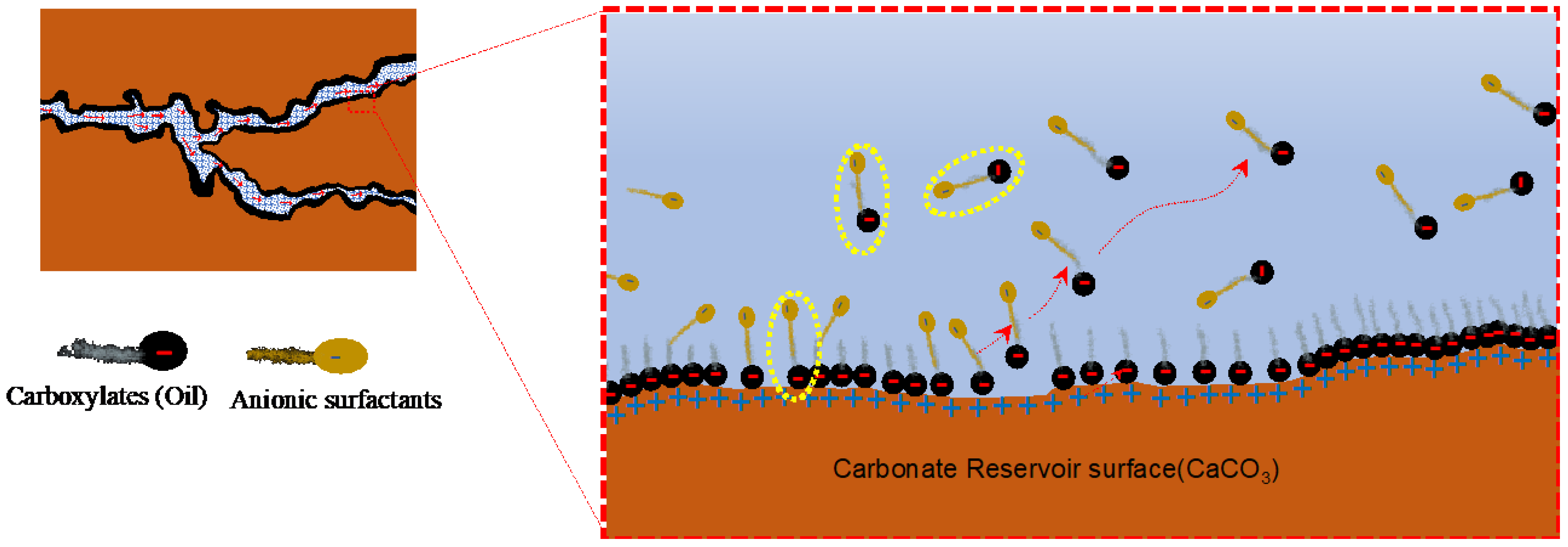
When the negatively charged surfactant heads (anions) are flooded into the reservoir, the surfactants do not form an ionic pair with the carboxylates of oil. Instead, they form a monolayer of surfactants, where the tail of anionic surfactants interacts with the absorbed carboxylates of oil due to van der Waals force (
Figure 5). Meanwhile, the negatively charged heads extend in the opposite direction to the carboxylate layers due to the repulsion force revealing the original water-wet surface of the rock reservoirs. Since the surfactants coat the carboxylate layer, this mechanism is also known as the coating mechanism
[65][68][70].
Figure 5. Illustration of the surfactant adsorption mechanism in a carbonate reservoir
[69].
Ion pairing and surfactant adsorption mechanisms were initially hypothesized by Austad et al.
[65][70]. In their study of improving the spontaneous imbibition process, alkyl-propoxythoxy sulfate (anionic) and dodecyltrimethylammonium bromide (cationic) surfactants were used for oil recovery from cores with different wetting orders. These hypotheses were simply based on the oil recovery results from the flooding experiment. Later, one of the first mechanistic studies on these hypotheses was conducted
[71]. Using synthetic polyethylene cores, the reservoir condition, like wetting order and preventing unwanted adsorbed compounds in the flooding experiment, was controlled. Then, to measure the performance of the surfactants, the core was flooded and aged with anionic (STEOL CS-330) and cationic (C12TAB) surfactants. Based on the adsorption isotherm results, cationic surfactants perform better than anionic surfactants, as the ionic interaction is much stronger. Consequent core-based experimental studies found similar results
[72][73]. Many parameters were discovered during these core flooding experiments that provide insight into the effectiveness of these mechanisms in different conditions like salinity, pH, and concentration of surfactants or polymers. The discussion on these parameters is not in the scope of this entry, but researchers have mentioned some comprehensive reviews in the literature for interested readers.
Even though core flooding experiments have served greatly in terms of enhancing researchers' understanding of the wettability alteration mechanisms, the microscopic analysis with real-time quantitative measures was still a challenge with these experimental setups because the hypothesized wettability alteration mechanisms were still proven based on indirect measurements like adsorption isotherm on post-experiment core samples. The implementation of microfluidic techniques in flooding experiments has removed these challenges. For example, in the microfluidic experiment that measured the contact angle of the flooding liquid (surfactant) in a real-time experiment to assess the performance of different surfactants, it was found that anionic surfactant (SDS) was able to detach adsorbed octadecylamine from the pore surface by an ion pair mechanism (
Figure 6)
[56]. Additionally, the nonionic surfactant nonylphenol ethoxylated decyl ether (NP-10) showed comparatively less effectiveness for wettability alteration. These results were aligned with the first core flooding-based mechanistic study.
Figure 6. (
a) A pore network model of a reservoir. (
b) A magnified view of the pore network. (
c–
f) The surfactant flooding at different mass flow rates shows different contact numbers (Ca): (
c) v = 0.26 ft/day (Ca = 1.4 × 10
−6); (
d) v = 1.3 ft/day (Ca = 7.0 × 10
−6); (
e) v = 2.6 ft/day (Ca = 1.4 × 10
−5); (
f) v = 13 ft/day (Ca = 7.0 × 10
−5)
[48].
Similarly, the visualization of polymer adsorption on the Polydimethylsiloxane (PDMS) surface was shown in the experimental study
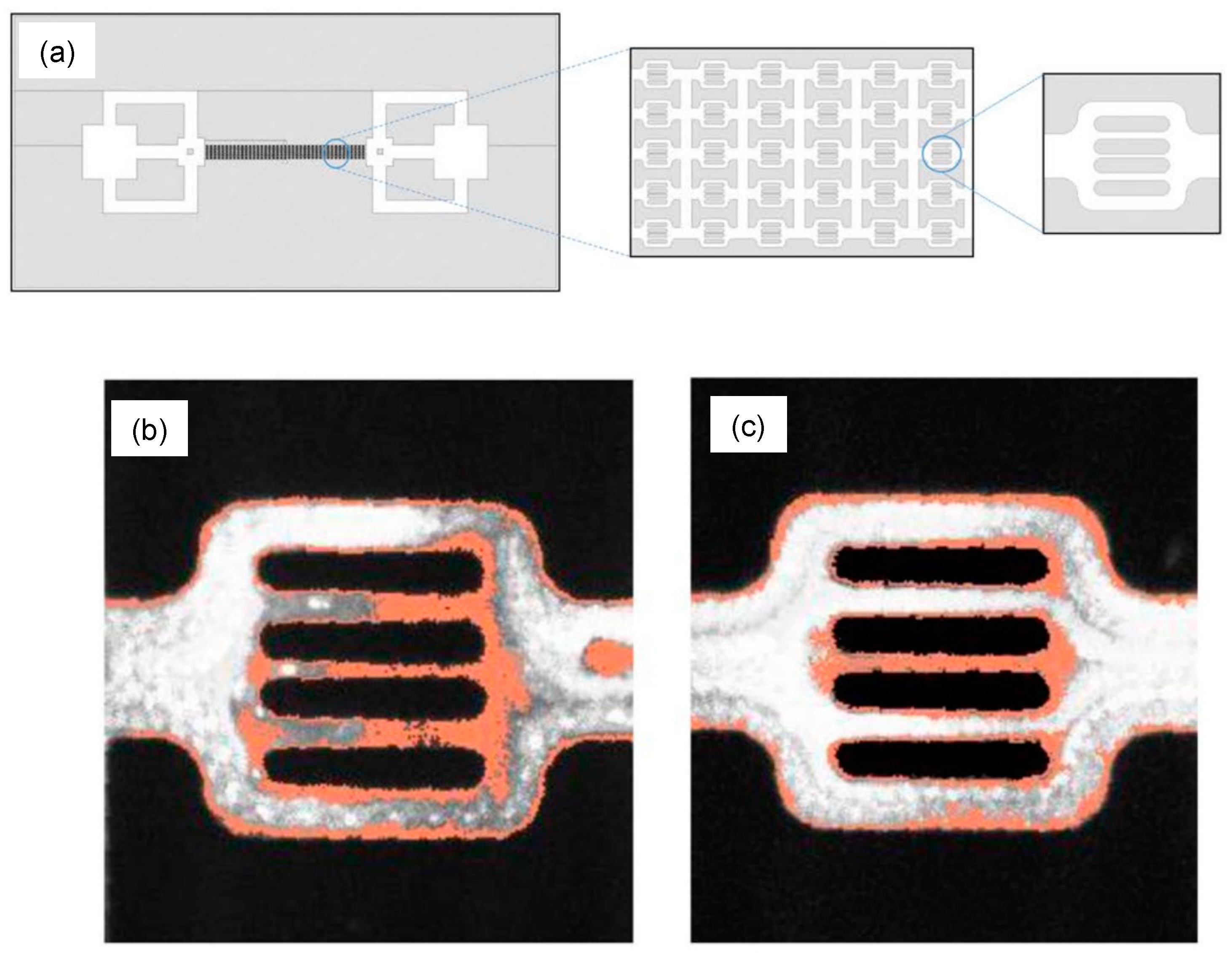
[74]. A fluorescently labeled polymer poly(fluorescein isothiocyanate allylamine hydrochloride) solution was pumped into the PDMS microchannels, and the real-time video was recorded with the help of a fluorescence microscope (
Figure 7). This study shows the instantaneous adsorption with a non-homogeneous adsorbed thickness of polymer on the PDMS surface, providing insights into polymer retention with static and flow-induced adsorption. Researchers believe these studies have opened a new gateway for a more thorough analysis of molecule- and pore-scaled flooding mechanisms in EOR. It opens the scope of using closely mimicking reservoir surfaces in micromodels to analyze the adsorption behavior of different polymers and flooding fluids.
Figure 7. (
a) Schematic of a microfluidic model with a magnified pore network. The total size of the channel is 1 cm × 2 cm with 2 to 10 µm pore channels. (
b,
c) Two random flow units where polymer adsorption can be seen on the surface of the channel (in orange) with the fluid flow (in grey)
[70].
In a microfluidic and core-based experiment to analyze the effect of salinity on a novel green surfactant (S-2-Dodecanamido-Aminobutanedioic acid), the flow pattern with different salinity was visualized. The experiment shows wettability alteration of 24.98% higher than salinity flooding due to the surfactant adsorption mechanism
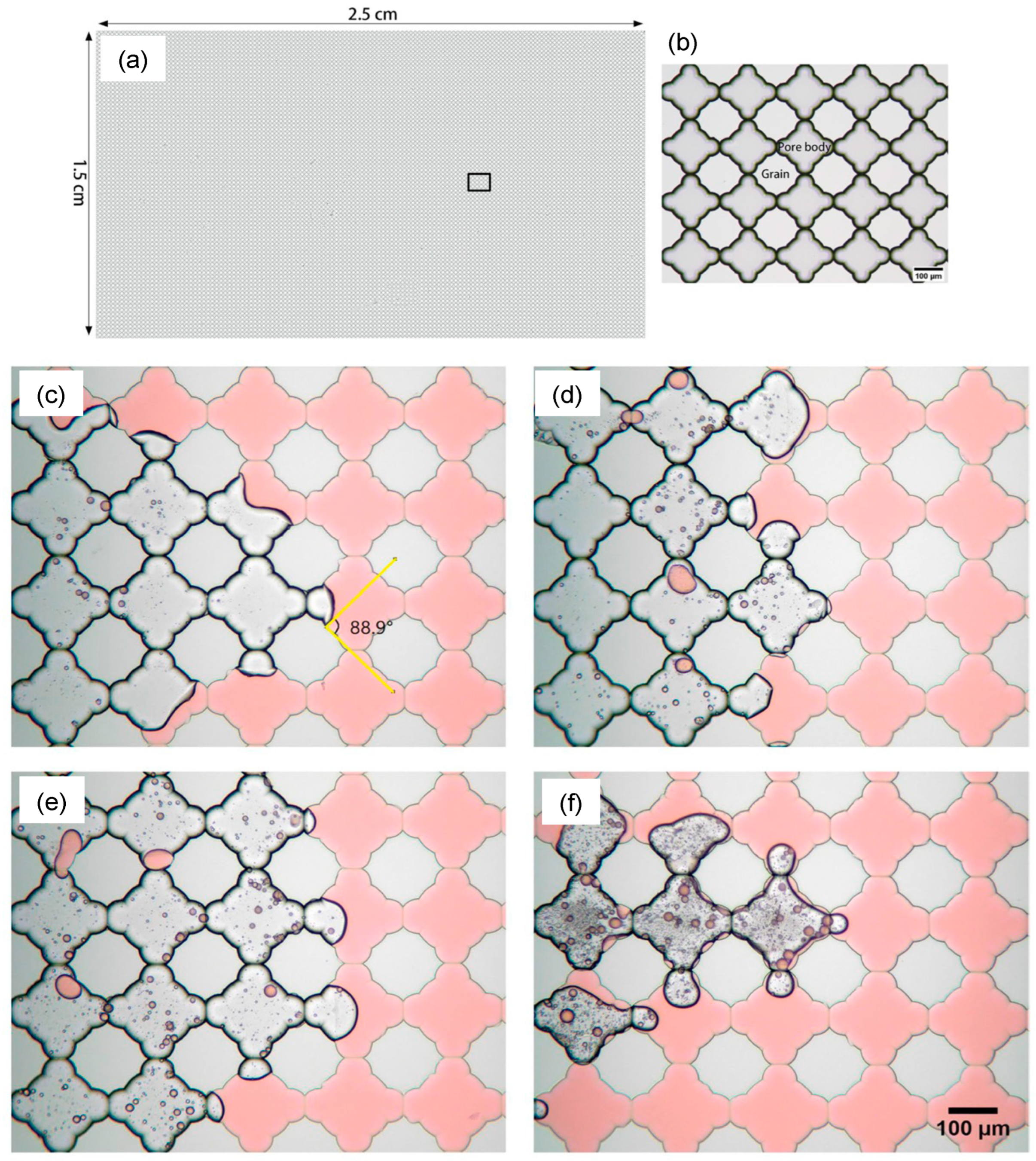
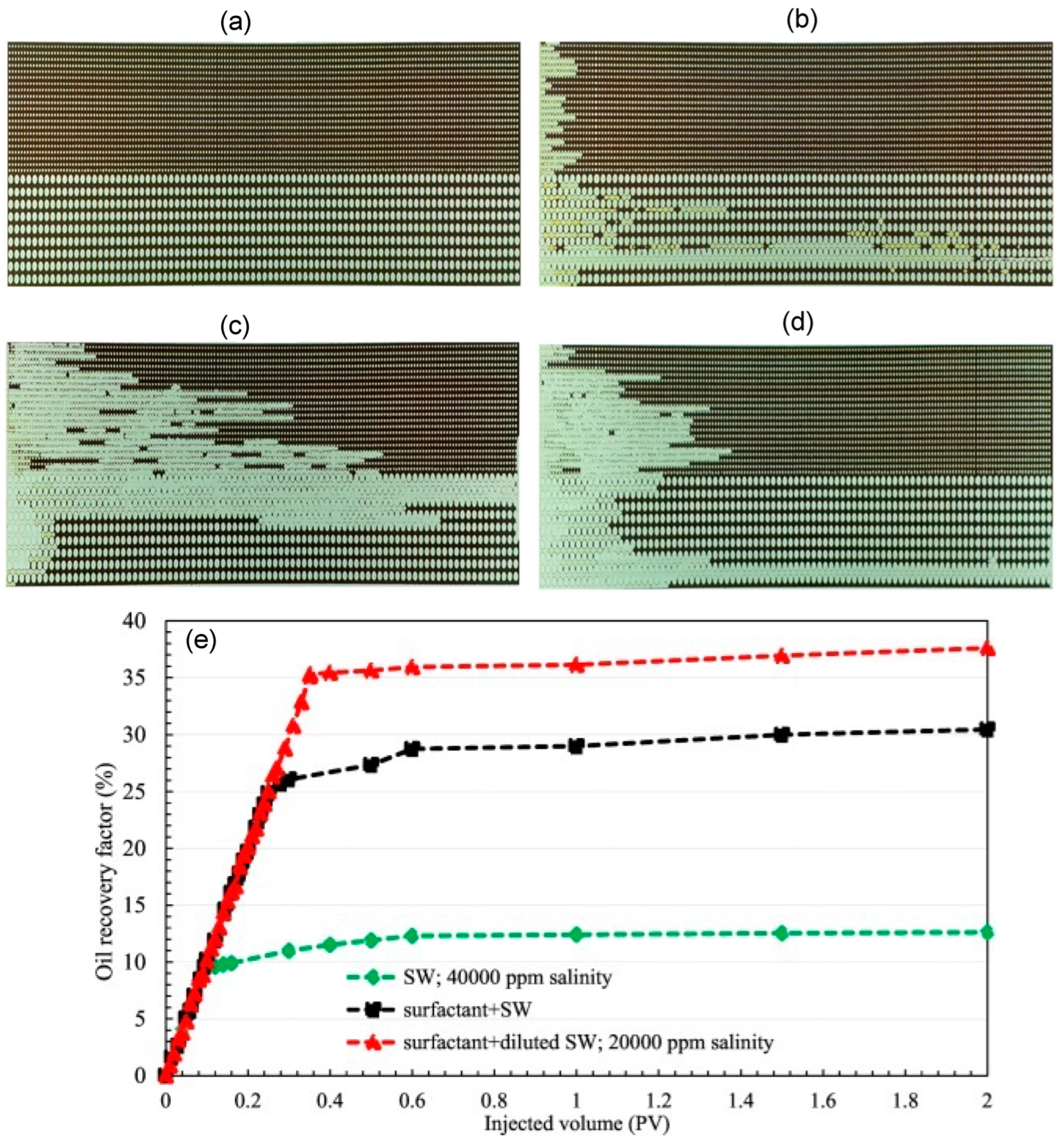
[75]. These results were further verified by the sandstone core flooding experiment (
Figure 8).
Figure 8. The micromodel was 21 cm × 5 cm with the variable pore network. (
a) Image of the microchannel saturated with oil. (
b) After the flooding of seawater (SW) with 40,000 ppm salinity. (
c) After the flooding of surfactant with SW (20,000 ppm salinity). (
d) After injecting surfactant + SW. (
e) Oil recovery with different solutions
[71].
Apart from the direct visualization of fluid flows into the pore network, the microchannel also enables the spectroscopic analysis of the chemical condition of the surface without compromising the reservoir model. Spectroscopic analyses like Fourier-transform infrared spectroscopy (FTIR) and Raman spectroscopy can provide new insights into the chemical interactions of flooding solutions with the surface and a compound change in the surface after the experiment. For example, in a study for the surfactant-based brine solution on oil recovery, the low surface energy surfactant, iC
18S (FO-180) based brine solution, increased the OOIP from 25% to 58%
[76]. The Raman spectroscopy revealed that the improvement in oil recovery was caused by reduced asphaltene aggregation from 1.4 to 0.7 nm because the surfactant changed the asphaltene interaction with the surface during the adsorption. With this method, the absorption effectiveness of different surfactants and polymers could be analyzed, which is useful for selecting the best surfactant/polymers in the chemical EOR process.
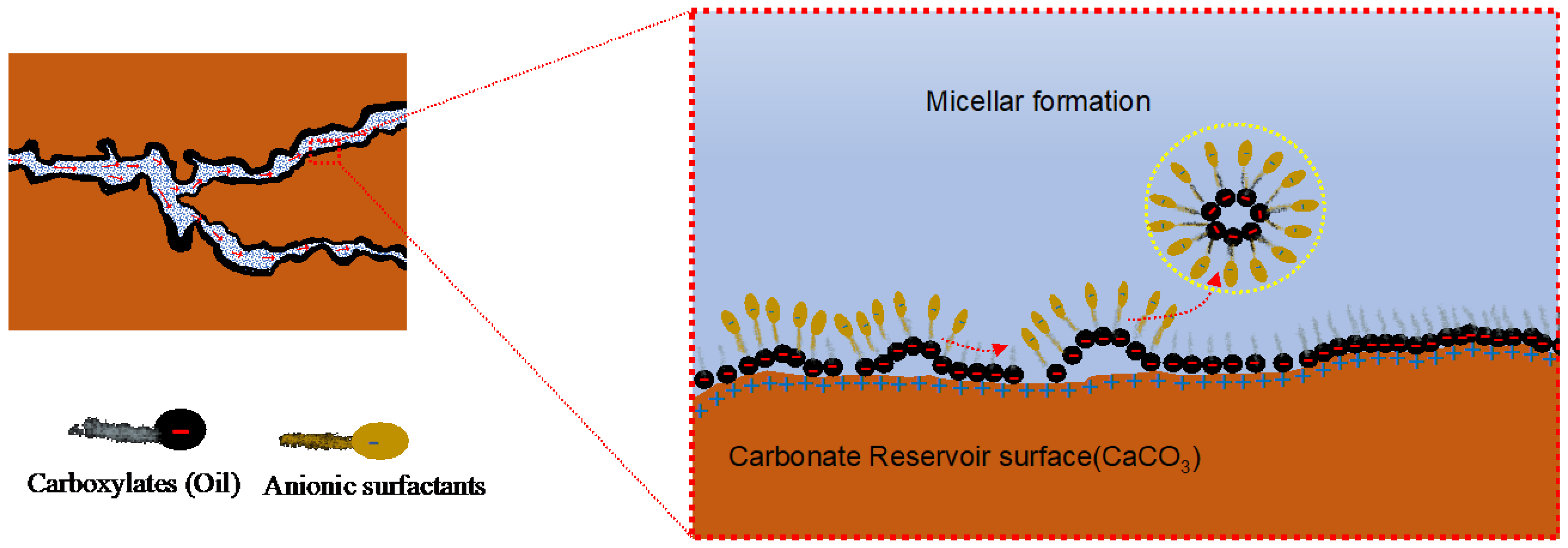
3.1.3. Micelle Formation Mechanism (Micelle Layer Solubilization)
The micelle is a self-assembled colloidal structure made from surfactants, polymers, or ions (
Figure 9). It forms when enough ions become concentrated together
[77]. The minimum value of these concentrations to form the micelles is called critical micelle concentration (CMC). In the micelle layer solubilization, the layer of surfactants integrates with hydrocarbon molecules via van der Waal forces, becomes concentrated, forms micelle and is diffused into the flooding flow with the hydrocarbon droplets, exposing the reservoir rock surface, which is initially water-wet
[78].
Figure 9. Illustration of the micelle formation mechanism in a carbonate reservoir
[73].
Oil recovery by the micelle formation mechanism was introduced in 1988, where the study explained the oil removal mechanisms from the solid surface in the presence of anionic micelle solutions
[79]. The study showed that the contact line of oil and the solid surface was suppressed by the surfactant solution, and then surfactant molecules diffused along with the oil droplets into the stream. These phenomena were explained as the combination of rolling up and diffusion mechanisms. Many other studies that support the micelle formation mechanisms for enhanced oil recovery followed this pioneering work. About 20 years later, the crude oil interaction with the mineral plates mimicking the reservoir surface in the presence of anionic and cationic surfactants was examined
[80]. Based on the force adhesion study, it was found that anionic surfactants were more efficient in achieving wettability alteration compared to cationic surfcasters. This is because micelle solubilization of adsorbed anionic oil molecules leads to wettability alteration. This observation was further supported by the recent study showing the spontaneous imbibition experiment for the wettability alteration
[81]. Most researchers generally believed that micelle formation requires more surfactant to achieve ultra-low IFT. However, some reviews show that even reduced surfactants can produce a higher rate of oil through EOR via micellar formation. A microfluidic experiment was conducted to investigate the process further. This research revealed the role of micellar solubilization in wettability alteration
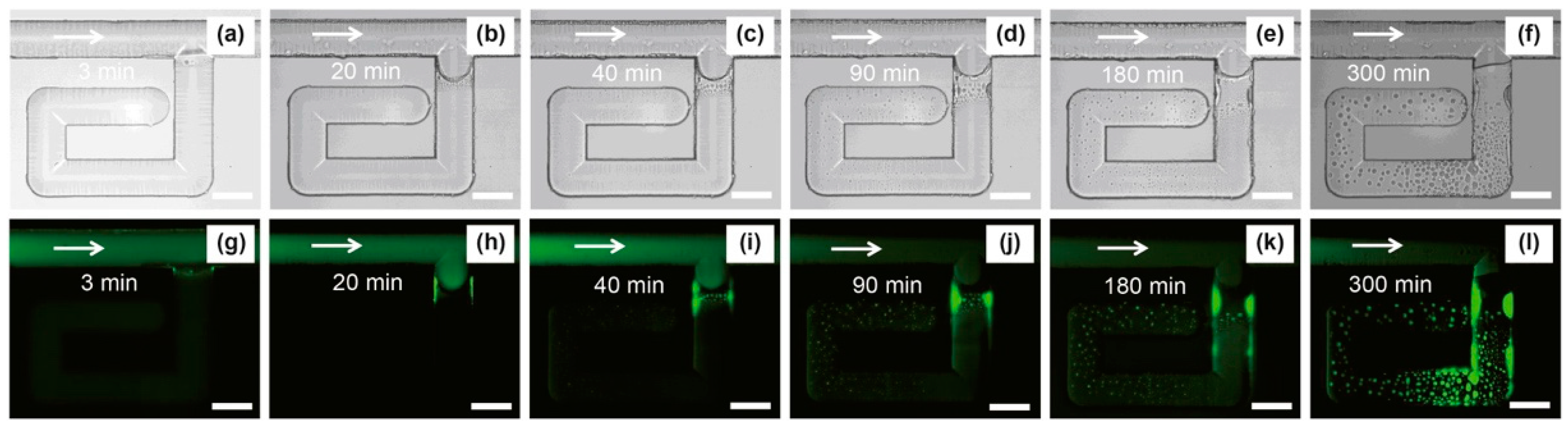
[82]. It was found that the oil solubility was increased up to 2 times with the higher micellar formation, even with the 0.2%wt concentration of naphthenic arylsulfonate (NAS). In the dead end of the microchannel, the oil solubilization can be visually seen with fluorescence microscopy in
Figure 10. Based on the visualization analysis, new phenomena were discovered that the formed emulsion could plug the pore throat to divert the driving fluid from going into the more permeable region, subsequently optimizing the sweep efficiency. This emulsification-entrainment effect can significantly reduce the residual oil saturation by 46% for the naphthenic arylsulfonate (NAS)/alkanes system.
Figure 10. Water and oil emulsification process in the microchannel. (
a–
l) shows in normal light and fluorescence microscopic images at different time steps, respectively. The green color shows displacing fluid. The scale bar shows a 100 μm length
[82].
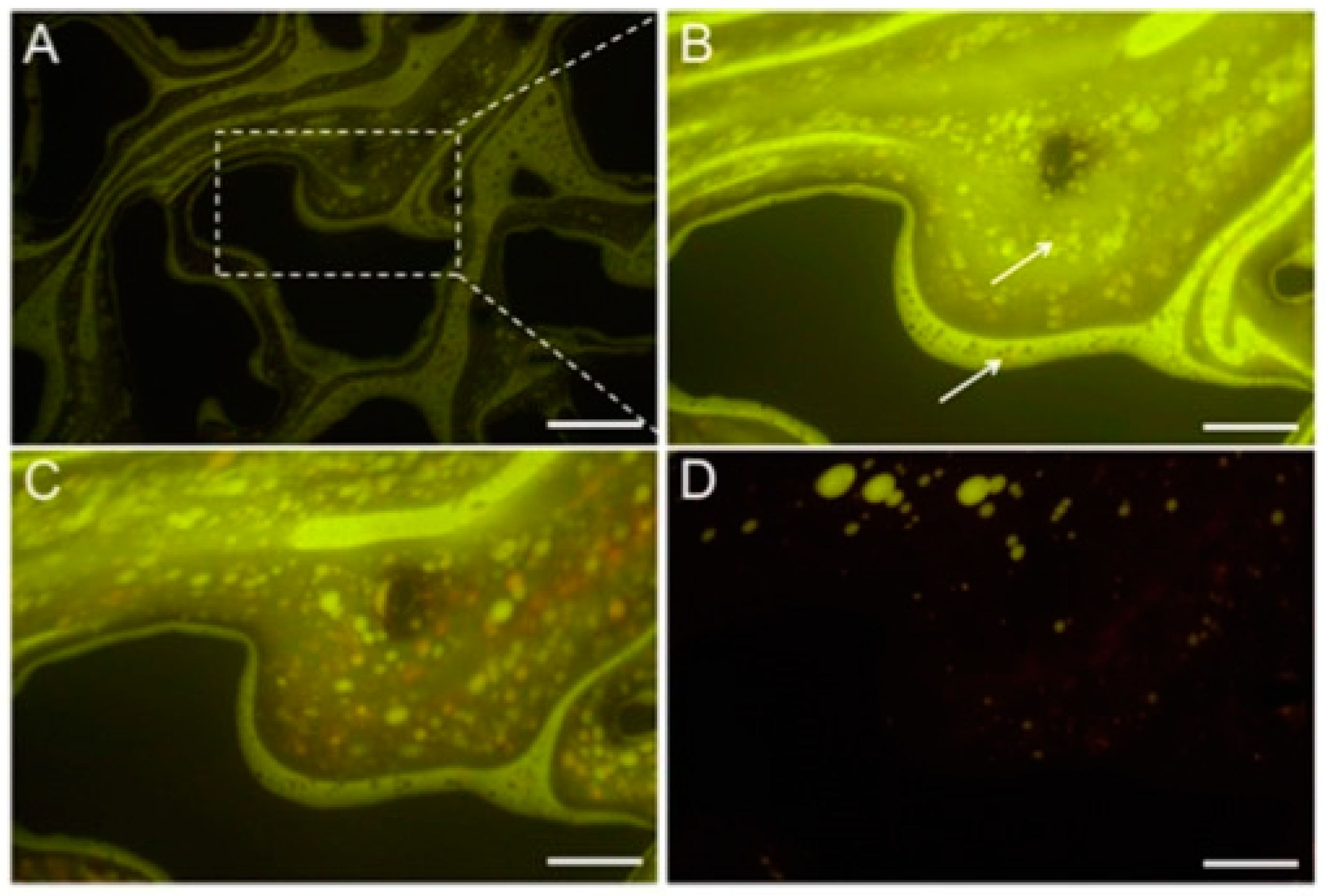
Similarly, sodium 4-dodecyl benzenesulfonate (SDBS) and alcohol propoxy sulfate (APS) surfactants were employed in the micellar solubilization with the microfluidic experiment
[83]. The micro-emulsification process was visualized by in situ fluorescent labeling and ex situ cryo-transmission electron microscopy (cryo-TEM) and small angle X-ray scattering (
Figure 11). The results showed that low mobility microemulsion was initially formed due to micellar solubilization when surfactant solution was in contact with the trapped oil. Subsequently, it diluted into the new driving fluid, inducing ultra-low-IFT in trapped oil. With the core flooding experiment, only the ultimate results would be noticed; however, due to the real-time visualization, this synergic effect of micellar solubilization and trapped oil mobilization was first time observed in the micromodel experiment. Researchers believe that more extensive micromodel reservoir studies can reveal new insights into the micelle solubilization mechanism for EOR and can be useful to gain a complete understanding of this EOR mechanism.
Figure 11. (
A) Fluorescent image of the microemulsion formation process in the microchannel (The scale bar shows 500 μm length). (
B) Five min of flooding; (
C) 30 min after flooding; (
D) 60 min after the flooding. Around the time, microemulsion of oil forms, and eventually, as shown in image d, the oil is mainly mobilized. (The scale bar shows 200 μm length in (
B–
D) images)
[78].
4. Wettability Alteration by Nanofluids
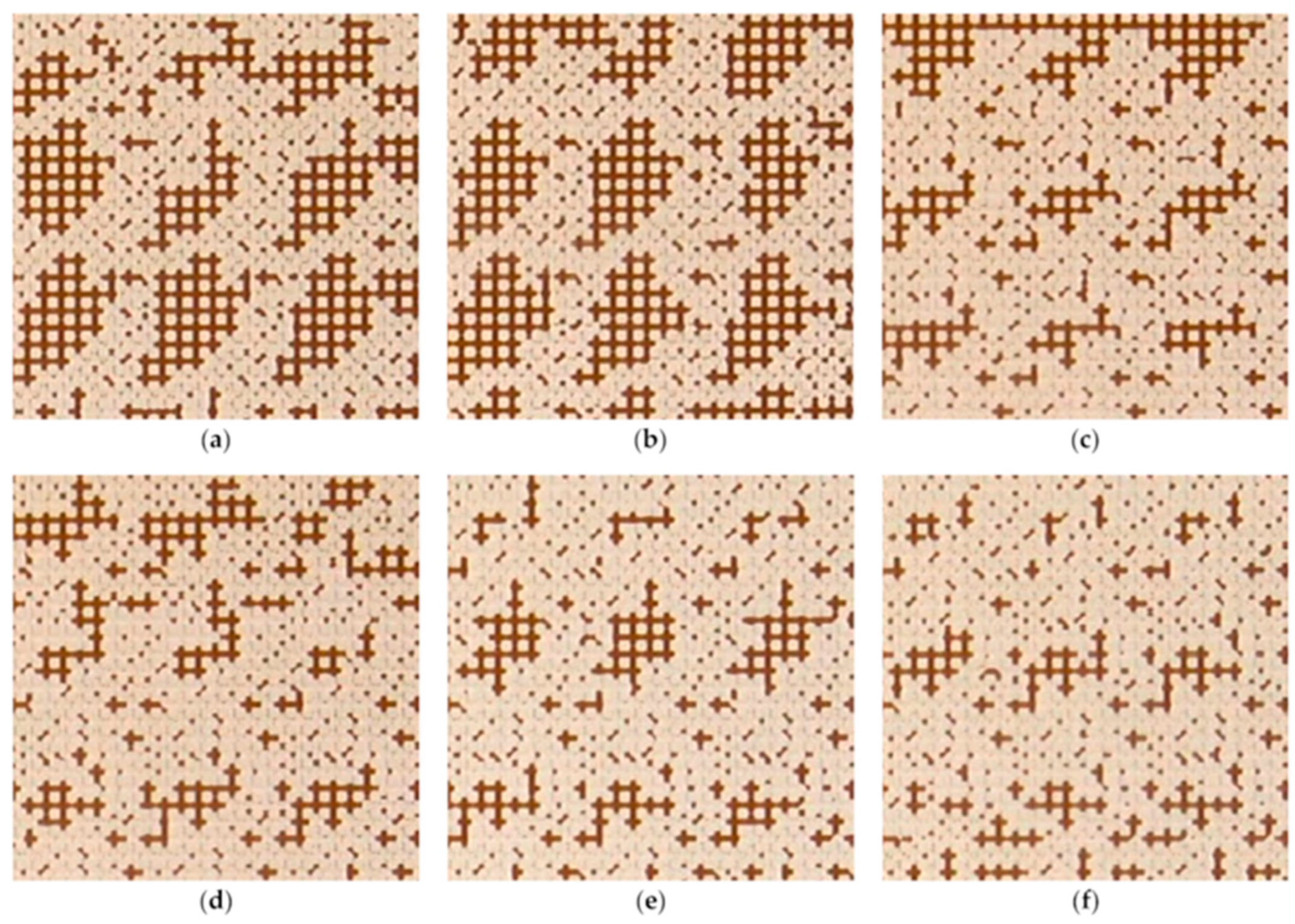
Although surfactants have been favored in enhanced oil recovery (EOR) for many years due to their excellent wettability alteration and interfacial tension reduction abilities, their performance is substantially reduced in harsh reservoir conditions like high salinity and high temperature. Nanofluid has gained much attention from researchers because of its potential for EOR in harsh reservoir conditions. Many core-flooding experimental results have shown improved oil recovery with different types of nanoparticles, along with various parametric studies in high salinity and temperature conditions. With the micromodel experiments, the effect of nanofluid on the improvement in oil recovery was revealed with visualization of the flow patterns. For example, a pore network experiment showed that in a water flooding experiment, droplets remained in a large quantity in washed-up areas of pore networks, retained by capillary forces near the small pores, while the amount of trapped oil was significantly less in the case of nanofluid flooding (
Figure 12)
[84].
Figure 12. The effect of nanoparticle concentration on the residual oil in the microchannel (6 × 6 mm). (
a) water; (
b) 0.1
w/
v% SiO
2; (
c) 0.25
w/
v% SiO
2; (
d) 0.5
w/
v% SiO
2; (
e) 1
w/
v% SiO
2; (
f)
w/
v% SiO
2 [85].
These clear visual proofs of oil recovery improvement have been shown in plenty of experimental studies. However, relatively few studies have been conducted to understand its mechanism and performance-affecting parameters for EOR. In the literature, various mechanisms like wettability alteration, reduction in interfacial tension, increased viscosity of flooding solution, viscosity reduction in reservoir oil, and log jamming are found to be responsible for nanofluid-assisted EOR. Among these proposed mechanisms, wettability alteration was found to be the most accepted mechanism for EOR by nanofluid, while other mechanisms have inconsistent results and have not been fully proven
[86][87].
4.1. Mechanisms for Wettability Alteration by Nanofluid
Nanofluids effectively transform the oil-wet reservoir into more water-wet. The experimental studies acknowledged that disjoining pressure is an underlying phenomenon for the alteration of wettability by nanofluids
[86]. Disjoining pressure can be explained as the required pressure gradient to remove the fluid adhered to the reservoir surface due to the adhesion force between the fluid and the solid surface. During a disjoining process, nanoparticles in the fluid create a wedge film structure at the three-phase contact area, as shown in
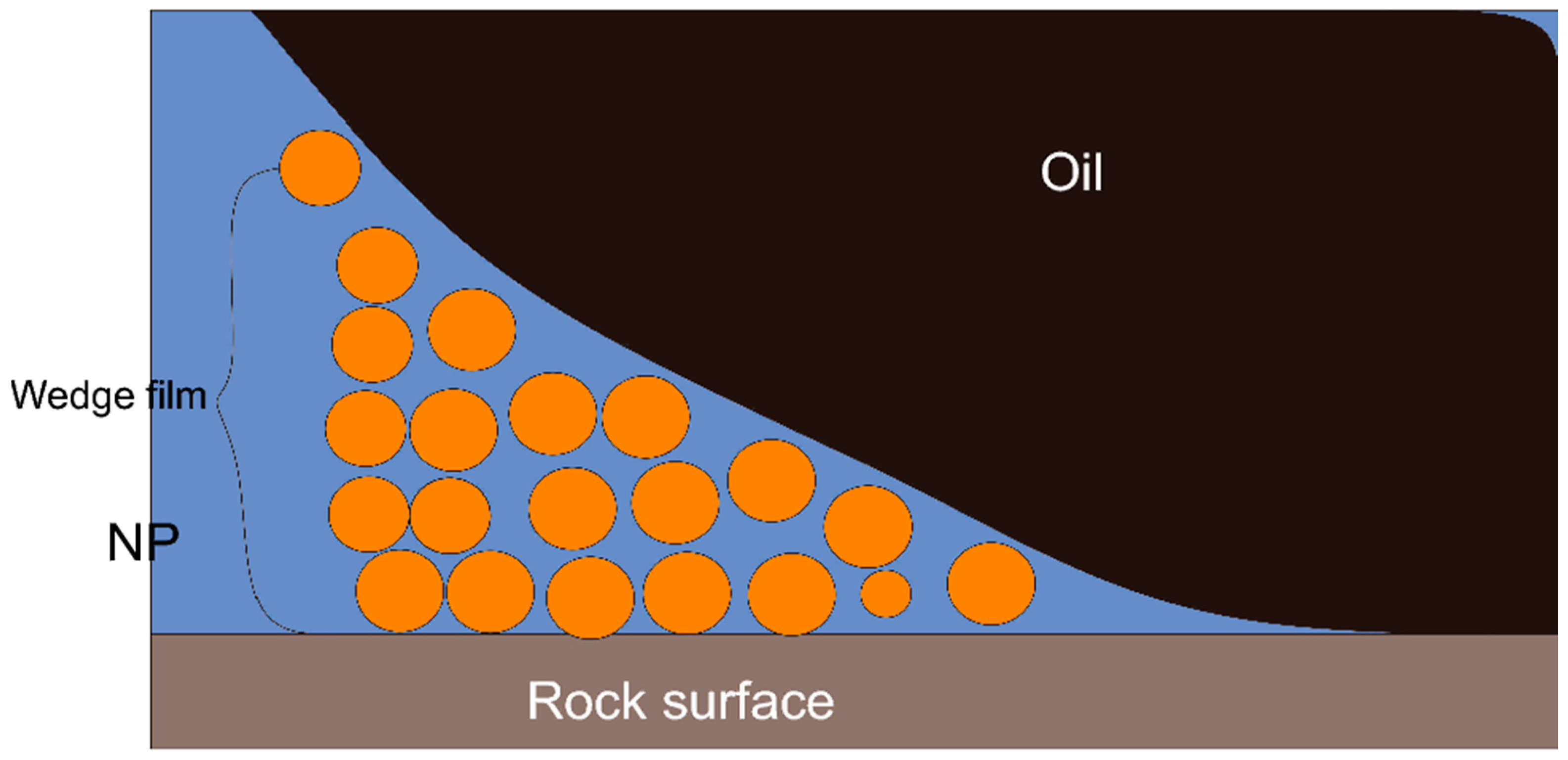
Figure 13. This wedge film spreads further on the rock surface and changes its wettability. Many experimental studies have demonstrated different responsible forces for creating the wedge film and removing the adhered fluid to the rock surface.
Figure 13. Illustrative image of a disjoining pressure mechanism
[15].
A van der Waal’s force seems responsible for the spreading of the wedge film, but there is another argument that van der Waal’s and electrostatic forces are responsible in combination for the wedge film spread
[25][88]. The other concept has been suggested to explain the wedge film creating and spreading—the excess pressure on the film edge is caused by the layering and structuring arrangement of nanoparticles
[89]. Another experiment confirmed that the detachment of oil was well correlated by structural disjoining pressure with layer formation
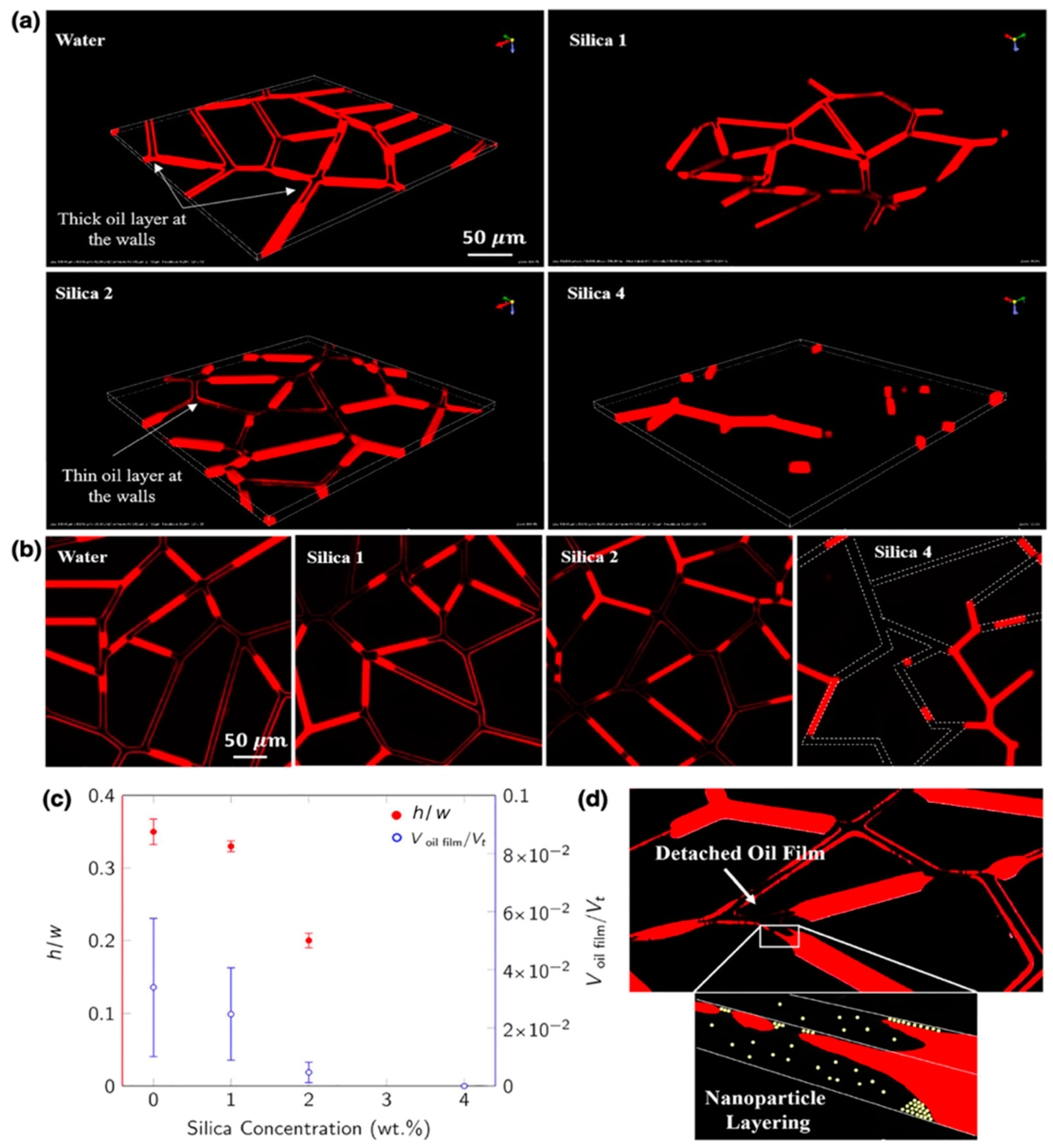
[90]. Lastly, PDMS-based micro-reservoir patterns were used in one study, where with the help of three-dimensional confocal imaging, it was revealed that silica nanoparticles were thinning the wetting fluid with the wedge formation advancing between the surface and wetting phase (
Figure 14)
[91]. This microfluidic study showed one of the first visual proofs of the disjoining pressure mechanism during nanofluid-assisted EOR. Visual access to nanofluid performance during the oil recovery in this study weighs high importance as it could provide the easy optimization of concentration and size of nanoparticles.
Figure 14. (
a,
b) Two-dimensional and Three-dimensional images of the micromodel showing the detachment mechanism of the wetting film (in red) during nanofluid flooding, respectively. (
c) h/w represents a dimensionless thickness of the channel (height/width), and the right axis shows the ratio of oil volume to the total volume. (
d) Schematics of the wedge shape formation of nanoparticles
[92].
Researchers found that most literature acknowledges the disjoining pressure mechanism as the prominent mechanism for nanofluid-assisted EOR. Researchers also found that interfacial tension (IFT) reduction has been receiving attention from many researchers, especially in fractured reservoirs, where the removal of trapped oil requires a reduction in the capillary pressure. The IFT reduction promotes lower capillary pressure to ease the spontaneous imbibition process
[15]. There is a review article that comprehensively discusses the effect of nanoparticles on IFT with various proposed IFT reduction mechanisms for some interested readers
[93].
Apart from disjoining pressure and IFT reduction, there is another phenomenon called adsorption/desorption responsible for the wettability alteration. Like the surfactant adsorption mechanism, nanoparticles become attracted to the reservoir surface while removing the wetting fluid particles (oil), which is known as the adsorption of nanoparticles. When the nanofluid is injected into a porous medium, van der Waals, the repulsion force of the electric double layer, acid-base interaction, and hydrodynamic forces govern the interaction between nanoparticles and reservoir surfaces
[94][95]. The resultant negative force leads to the attraction between nanoparticles and reservoir surfaces, leading to the adsorption of nanoparticles. Conversely, when the resultant force is positive, desorption of nanoparticles from the reservoir surface will occur. In an experimental study, the hydrophilic nanoparticles were able to alter the wettability of the sandstone reservoir surface by a reduction of 10 degrees more the contact angle between oil and brine on the glass surface than hydrophobic nanoparticles
[96]. The higher reduction in contact angle was achieved because of the adsorption of hydrophilic nanoparticles, where the hydrophobic nanoparticles experienced desorption
[96]. Similar results, the adsorption of silica nanoparticles onto the glass substrate, were shown by some researchers (
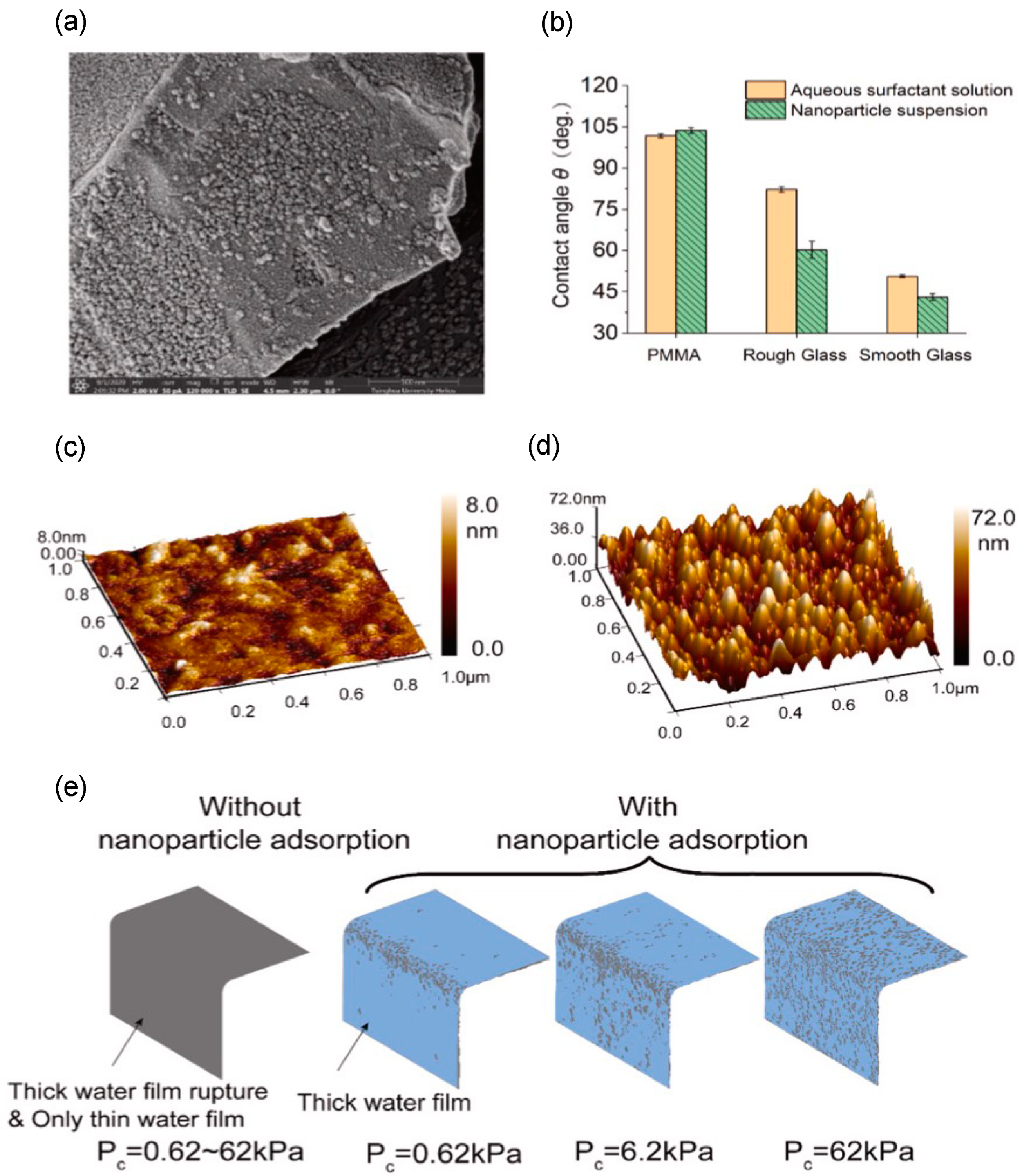
Figure 15)
[97]. The surface roughness was found to be increased after the nanoparticle adsorption, which helps to detach the wetting fluid by altering the wettability.
Figure 15. (
a) A scanning electron microscopic image of the glass surface with nanoparticle adsorption. (
b) The comparison of the contact angle of surfactant solution and nanofluid in different surfaces with the same IFT and the presence of decane. (
c) Surface roughness of the acid-etched glass. (
d) Surface roughness of the glass after nanoparticle adsorption. (
e) Water film thinness (in blue) onto the 100 nm convex glass surface at different capillary pressures (P
c) with and without nanoparticle adsorption
[93].
5. Discussion and Summary
Wettability is among the most significant attributes of the reservoir system for EOR
[64][68][98]. Significant research has been performed within the decade to obtain insights into wettability alteration. However, an absolute understanding of the overall effect of all the underlying mechanisms is still required to achieve the optimum EOR method. With advanced technology, the pace of research has increased, especially after the incorporation of microfluidic technology. With access to real-time microscopic analysis, low cost, and higher accuracy experimental conditions, many challenges can be addressed, like parametric studies on the effect of various reservoir conditions on chemical flooding, especially with the new surfactants and polymers, which was expensive and time-consuming in the core flooding experiments. The tailored wetting order and mimicking of different geochemical conditions in microfluidic channels have been demonstrated. However, a combined approach of these fabrications to achieve as close as the real reservoir core-like condition has not been achieved. In the future, these types of microchips, with the available advanced microscopic and spectroscopic analysis, could provide the complete analysis of underlying mechanisms to achieve optimum EOR methods. When it comes to surfactant-based EOR methods, the adsorption of the surfactants onto the reservoir surface is considered the foremost challenge
[99][100]. Because of the adsorption of the surfactants, a higher concentration of surfactant solution is required to maintain the high EOR efficiency, which makes the overall process expensive. To overcome this drawback, new methods must be developed where the adsorbed surfactants can be recovered for reuse after the oil recovery. Along with surfactant-based EOR, nanofluid-based EOR has shown great potential. The nanofluid-assisted EOR method has yet to be explored as it is relatively new
[101]. Many literature reviews state the need to understand the mechanisms associated with the nanofluidic-based EOR
[15][102][103]. In addition to the underlying mechanism of nanofluid EOR, preparing nanofluid and its operating conditions in different reservoir conditions requires extensive research. Especially, many parameters like size, concentration, and stability, with their different dependency on external parameters like temperature and salinity, make it challenging to find the optimized performance of the nanoparticles in EOR processes. There is a strong need for research required in that aspect. Lastly, to obtain full advantage of the chemical EOR process, the synergic effect of brine surfactant/polymers and nanoparticles needs to be extensively evaluated as there is a lack of research compared to their individual cases.