Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Philipp E Lambach | -- | 1786 | 2024-03-07 10:29:10 | | | |
| 2 | Peter Tang | Meta information modification | 1786 | 2024-03-08 02:15:00 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Trotter, C.; Giersing, B.; Lindstrand, A.; Bar-Zeev, N.; Cernuschi, T.; Franzel-Sassanpour, L.; Friede, M.; Hombach, J.; Jansen, M.; Hasso-Agopsowicz, M.; et al. Full Value of Vaccine Assessment. Encyclopedia. Available online: https://encyclopedia.pub/entry/55966 (accessed on 07 February 2026).
Trotter C, Giersing B, Lindstrand A, Bar-Zeev N, Cernuschi T, Franzel-Sassanpour L, et al. Full Value of Vaccine Assessment. Encyclopedia. Available at: https://encyclopedia.pub/entry/55966. Accessed February 07, 2026.
Trotter, Caroline, Birgitte Giersing, Ann Lindstrand, Naor Bar-Zeev, Tania Cernuschi, Lauren Franzel-Sassanpour, Martin Friede, Joachim Hombach, Maarten Jansen, Mateusz Hasso-Agopsowicz, et al. "Full Value of Vaccine Assessment" Encyclopedia, https://encyclopedia.pub/entry/55966 (accessed February 07, 2026).
Trotter, C., Giersing, B., Lindstrand, A., Bar-Zeev, N., Cernuschi, T., Franzel-Sassanpour, L., Friede, M., Hombach, J., Jansen, M., Hasso-Agopsowicz, M., Koh, M., Sim, S.Y., Spasenoska, D., Yeung, K.H.T., & Lambach, P. (2024, March 07). Full Value of Vaccine Assessment. In Encyclopedia. https://encyclopedia.pub/entry/55966
Trotter, Caroline, et al. "Full Value of Vaccine Assessment." Encyclopedia. Web. 07 March, 2024.
Copy Citation
A framework to guide the assessment and communication of the value of a vaccine—the Full Value of Vaccine Assessment (FVVA)—has been developed by the WHO. The FVVA framework offers a holistic assessment of the value of vaccines, providing a synthesis of evidence to inform the public health need of a vaccine, describing the supply and demand aspects, its market and its impact from a health, financial and economic perspective.
full value of vaccine assessment
vaccine development
vaccine pipeline
vaccine value
1. Introduction
Vaccination is the most successful public health intervention after clean water. The World Health Organisation (WHO) estimates that over 51 million deaths are expected to be averted due to vaccines against 14 pathogens administered over the period from 2021 to 2030 (on average 5.2 million per year) [1]. There has been an expansion in the number of vaccines in the routine childhood immunisation programme, together with new vaccines being recommended for administration to pregnant women, teenagers and adults [2]. However, there are still barriers in developing and implementing new vaccines to achieve a public health impact [3]. This is particularly the case for diseases with the highest burden in low- and middle-income countries (LMICs). The WHO promotes the development of vaccines where there is the highest unmet public health need and greatest potential for impact. It does so in a variety of ways, including, for example, by publishing technical roadmaps and preferred product characteristics (PPCs, which specify product attributes and their preferential characteristics [4]) for vaccines. It has also been recognised that articulating the wide range of health, social and economic benefits that vaccines offer may help to overcome obstacles such as resource needs in the vaccine pipeline, from clinical development to bottlenecks to use at a public health scale, e.g., the prioritization of interventions by governments and funders [5][6].
A framework to guide the assessment and communication of the value of a vaccine—the Full Value of Vaccine Assessment (FVVA)—has been developed by the WHO [7]. This framework is intended to provide greater consistency than previous vaccine investment cases [8]. In addition, a number of experts have commented on the approach developed, providing views on the theoretical underpinnings and rationale for an FVVA [9].
The FVVA framework offers a holistic assessment of the value of vaccines, providing a synthesis of evidence to inform the public health need of a vaccine, describing the supply and demand aspects, its market and its impact from a health, financial and economic perspective. The ultimate goals of an FVVA are to accelerate the development of vaccines that meet a country’s needs and preferences, supporting the evaluation of vaccines and ultimately sustained introduction in countries. Lead by an independent technical agency (usually the WHO), the process of developing an FVVA brings together relevant national, regional and global experts in a working group, establishing lines of communication and alignment among these to gather, evaluate and synthesize evidence on the value of vaccines from a range of perspectives. Key stakeholders and audiences for the FVVA include the vaccine research and development (R&D) community; funders of research and vaccine implementation; vaccine market experts; global policy makers; regulatory authorities, national policy makers and programme managers; immunisation partner organisations; and civil society organisations.
An FVVA can be viewed as a compendium of different elements that define the full value of a vaccine from a range of different perspectives. These elements are illustrated in Figure 1. Some elements will be informed by literature reviews and stakeholder consultation, whereas other elements will require specific research studies to be commissioned. An FVVA will therefore be supported by an extensive body of literature and evidence.
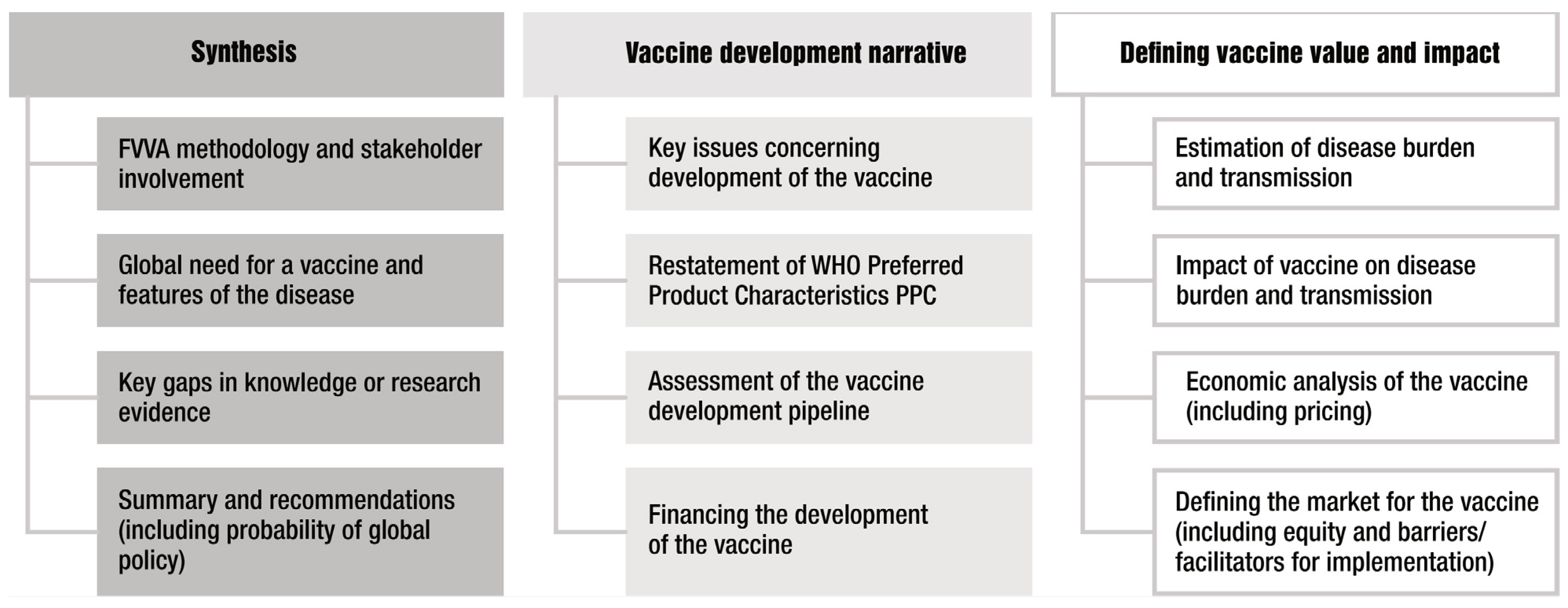
Figure 1. Key elements of a Full Value of Vaccine Assessment (FVVA). The columns broadly define the purpose of each element, with the third column representing areas where most new research is likely needed to inform the FVVA.
2. Synthesis
The first column in Figure 1 represents elements that provide the rationale for a vaccine (i.e., high-level global public health need), methodology of the FVVA and importantly, synthesis of both the overall findings of the FVVA and key evidence and research gaps. The latter can be used to inform the ongoing research agenda, and it highlights that an FVVA can best be considered a living document that can be updated periodically rather than presenting a snapshot at only one point in time. The synthesis of the overall findings is essential for effective communication and, in particular, making the FVVA accessible to a wider range of actors. This will also facilitate a common understanding and help in stakeholder alignment, leading to action.
3. Vaccine Development Narrative
The second column of Figure 1 shows the elements that describe the vaccine characteristics and status of vaccine development. This will include a narrative assessment of the key challenges in, and potential barriers to, vaccine development as well as an up-to-date evaluation of the vaccine pipeline. These elements most closely align with the remit of the Product Development for Vaccines Advisory Committee (PDVAC [10]). To fulfil the purpose of the FVVA as a compendium, some elements, such as Preferred Product Characteristics (PPCs, which describe the WHO’s preferences for vaccine parameters—principally, indications for vaccination, target groups, immunization strategies and the clinical data required for the assessment of safety and efficacy), will be restated here. Usually, the PPCs will inform other elements of the FVVA—for example, as input parameters in the modelling of a vaccine’s impact where future vaccine characteristics are as yet unknown. For TB vaccines, the FVVA actually assessed two different PPCs—one for adults and adolescents and one for infants. It is also in this section where the size of the required investment to develop a vaccine would be estimated and discussed. Additional considerations could include the cost of goods, price of a vaccine, or potential financing mechanisms such as through Gavi, the PAHO’s Revolving Fund or other means. An important barrier to vaccine development from the perspective of the manufacturer is uncertainty around their return on investment. To be inclusive of vaccine developer stakeholders, it is desirable to also include financial and global demand analysis, as was the case for the GBS FVVA.
4. Defining Vaccine Impact and Value
The third column of Figure 1 includes the main elements where most new research is likely required to inform the FVVA and therefore represents the main novel substance of the compendium. These elements most closely align with the remit of the Immunization and Vaccine-Related Implementation Research Advisory Committee (IVIR-AC) [11]. Ultimately an FVVA serves to inform global and national policy-making bodies such as the Strategic Advisory Group of Experts on Immunization (SAGE) or NITAGs [12]. For these downstream uses, the FVVA may need to be updated from the original if there is a considerable lag between FVVA publication and phase 3 results.
For these elements, there is some flexibility in the range of analyses presented, and their emphasis may vary according to the properties of the vaccines and epidemiology of disease. For example, considering the transmission dynamics of some infections is crucially important for understanding vaccine impact, whereas this is less the case for other infections, such as GBS, where the vaccination of pregnant women is unlikely to influence the dynamics of GBS, an organism that is widely carried in the population. Likewise, assessing the impact of vaccination on antimicrobial resistance (AMR) may be a critical factor for some infections where there are high levels of resistance or antibiotic use but less so where antibiotics are not used or where pathogens remain susceptible. The modelling of disease burden and vaccine impact in terms of DALYs, cases, deaths averted and cost-effectiveness is now well appreciated as a methodology to inform vaccine decision making. Such analyses are an essential component of the FVVA framework and would be expected to conform to high standards, demonstrated, for example, through the use of internationally recognised guidelines and checklists, such as GATHER [13], CHEERS [14] and the ISPOR Modelling Good Research Practices [15]. In particular, the uncertainty of outcomes should be appropriately represented.
There is clearly opportunity for both choice and innovation in assessing the value of a vaccine based on information needs identified by stakeholders. The FVVA includes evidence-based assessment methods driven by policy questions and decision contexts, including, among others, vaccine impact modelling; the cost of disease burden, development and delivery; investment impact; and cost-effectiveness [7]. More practically, within an FVVA, the choice of analysis should be well justified, particularly where methods that would be less familiar than a standard cost-effectiveness analysis are used. In the case of the FVVA for GBS vaccines [16], a cost-effectiveness study was performed from the perspective of the health-care provider, with results presented in terms of net monetary benefit. Sensitivity analyses were presented on some of the normative assumptions that may vary between different decision makers, such as the QALY loss assigned to a stillbirth [17]. For GAS vaccines, the global societal gains from prospective vaccines was estimated through a value-per-statistical-life approach [18]. In the case of TB vaccines, a wider range of economic analyses were performed, including analyses of the potential impact of novel vaccines on both economic growth in LMICs [19] and on health equity and financial protection in LMICs [20]. Such analyses are well justified given the epidemiology and burden of TB, a common disease affecting adults of working age. TB is also interesting in this regard because of the assessment of two different vaccine approaches, which reshaped the TB field to focus on vaccines for adults and adolescents rather than infants because of the greater potential for impact. For shigella vaccines, the association between shigella and linear growth faltering has suggested that the impact of a vaccine on child development and future productivity should be included [21]. One could also consider analyses that are pertinent to a group of pathogens—for example, those with high levels of antimicrobial resistance or antibiotic use [22]. Health security may be another dimension that requires consideration for some diseases/vaccines.
Flexibility in determining analyses presented in the FVVA presents opportunities for estimating the broader benefits of vaccination. However, there is also a need for standardisation to ensure consistency across FVVAs (or other analyses to be used by stakeholders) and enable fair comparisons to be made with other vaccines or health interventions. This will also mitigate the loss of objectivity. As Hutubessy et al. emphasise, “the remit of an FVVA should always be aligned with the standard reference cases so as to avoid the appearance of ‘special pleading’ for particular vaccines and to avoid explicit or hidden donor-driven agendas that are not aligned with country needs” [9]. This challenge could be addressed and balanced against the desire for innovation by having, for example, at least an economic analysis from the health provider perspective with other analyses, such as those assessing broader societal value—presented separately, as was the case for TB.
References
- Carter, A.; Msemburi, W.; Sim, S.Y.; Gaythorpe, K.A.; Lambach, P.; Lindstrand, A.; Hutubessy, R. Modeling the impact of vaccination for the immunization Agenda 2030: Deaths averted due to vaccination against 14 pathogens in 194 countries from 2021 to 2030. Vaccine, 2023; in press.
- WHO. A Brief History of Vaccination. Available online: https://www.who.int/news-room/spotlight/history-of-vaccination/a-brief-history-of-vaccination (accessed on 30 January 2024).
- Piot, P.; Larson, H.J.; O’Brien, K.L.; N’kengasong, J.; Ng, E.; Sow, S.; Kampmann, B. Immunization: Vital progress, unfinished agenda. Nature 2019, 575, 119–129.
- Vekemans, J.; Moorthy, V.; Friede, M.; Alderson, M.R.; Meulen, A.S.-T.; Baker, C.J.; Heath, P.T.; Madhi, S.A.; Doare, K.M.-L.; Saha, S.K.; et al. Maternal immunization against Group B streptococcus: World Health Organization research and development technological roadmap and preferred product characteristics. Vaccine 2019, 37, 7391–7393.
- Bärnighausen, T.; Bloom, D.E.; Canning, D.; Friedman, A.; Levine, O.S.; O’brien, J.; Privor-Dumm, L.; Walker, D. Rethinking the benefits and costs of childhood vaccination: The example of the Haemophilus influenzae type b vaccine. Vaccine 2011, 29, 2371–2380.
- Gessner, B.D.; Kaslow, D.; Louis, J.; Neuzil, K.; O’Brien, K.L.; Picot, V.; Pang, T.; Parashar, U.D.; Saadatian-Elahi, M.; Nelson, C.B. Estimating the full public health value of vaccination. Vaccine 2017, 35, 6255–6263.
- WHO. Meeting of the Immunization and Vaccine-related Implementation Research Advisory Committee (IVIR-AC), septembre 2022. Wkly. Epidemiol. Rec. 2022, 97, 599–612.
- Sim, S.Y.; Jit, M.; Constenla, D.; Peters, D.H.; Hutubessy, R.C.W. A Scoping Review of Investment Cases for Vaccines and Immunization Programs. Value Health 2019, 22, 942–952.
- Hutubessy, R.; Lauer, J.A.; Giersing, B.; Sim, S.Y.; Jit, M.; Kaslow, D.; Botwright, S. The Full Value of Vaccine Assessments (FVVA): A framework for assessing and communicating the value of vaccines for investment and introduction decision-making. BMC Med. 2023, 21, 229.
- WHO. Product Development for Vaccines Advisory Committee (PDVAC). Available online: https://www.who.int/groups/product-development-for-vaccines-advisory-committee (accessed on 30 January 2024).
- WHO. Immunization and Vaccines Related Implementation and Research Advisory Committee (IVIR-AC). Available online: https://www.who.int/groups/immunization-and-vaccines-related-implementation-research-advisory-committee (accessed on 30 January 2024).
- WHO. Strategic Advisoty Group of Experts on Immunization (SAGE). Available online: https://www.who.int/groups/strategic-advisory-group-of-experts-on-immunization (accessed on 30 January 2024).
- Stevens, G.A.; Alkema, L.; Black, R.E.; Boerma, J.T.; Collins, G.S.; Ezzati, M.; Grove, J.T.; Hogan, D.R.; Hogan, M.C.; Horton, R.; et al. Guidelines for Accurate and Transparent Health Estimates Reporting: The GATHER statement. Lancet 2016, 388, e19–e23.
- Husereau, D.; Drummond, M.; Augustovski, F.; de Bekker-Grob, E.; Briggs, A.H.; Carswell, C.; Caulley, L.; Chaiyakunapruk, N.; Greenberg, D.; Loder, E.; et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: Updated reporting guidance for health economic evaluations. BMC Med. 2022, 20, 23.
- Pitman, R.; Fisman, D.; Zaric, G.S.; Postma, M.; Kretzschmar, M.; Edmunds, J.; Brisson, M. Dynamic transmission modeling: A report of the ISPOR-SMDM Modeling Good Research Practices Task Force—5. Value Health 2012, 15, 828–834.
- WHO. Group B Streptococcus Vaccine: Full Value of Vaccine Assessment. Available online: https://iris.who.int/bitstream/handle/10665/347595/9789240037526-eng.pdf?sequence=1 (accessed on 30 January 2024).
- Procter, S.R.; Gonçalves, B.P.; Paul, P.; Chandna, J.; Seedat, F.; Koukounari, A.; Hutubessy, R.; Trotter, C.; Lawn, J.E.; Jit, M. Maternal immunisation against Group B Streptococcus: A global analysis of health impact and cost-effectiveness. PLoS Med. 2023, 20, e1004068.
- Cadarette, D.; Ferranna, M.; Cannon, J.W.; Abbas, K.; Giannini, F.; Zucker, L.; Bloom, D.E. The full health, economic, and social benefits of prospective Strep A vaccination. NPJ Vaccines 2023, 8, 166.
- Portnoy, A.; Arcand, J.-L.; Clark, R.A.; Weerasuriya, C.K.; Mukandavire, C.; Bakker, R.; Patouillard, E.; Gebreselassie, N.; Zignol, M.; Jit, M.; et al. The potential impact of novel tuberculosis vaccine introduction on economic growth in low- and middle-income countries: A modeling study. PLoS Med. 2023, 20, e1004252.
- Portnoy, A.; Clark, R.A.; Weerasuriya, C.K.; Mukandavire, C.; Quaife, M.; Bakker, R.; Baena, I.G.; Gebreselassie, N.; Zignol, M.; Jit, M.; et al. The potential impact of novel tuberculosis vaccines on health equity and financial protection in low-income and middle-income countries. BMJ Glob. Health 2023, 8, e012466.
- Puett, C.; Anderson, J.D.; Bagamian, K.H.; Muhib, F.; Scheele, S.; Hausdorff, W.P.; Pecenka, C. Projecting the long-term economic benefits of reducing Shigella-attributable linear growth faltering with a potential vaccine: A modelling study. Lancet Glob. Health 2023, 11, e892–e902.
- Vekemans, J.; Hasso-Agopsowicz, M.; Kang, G.; Hausdorff, W.P.; Fiore, A.; Tayler, E.; Klemm, E.J.; Laxminarayan, R.; Srikantiah, P.; Friede, M.; et al. Leveraging Vaccines to Reduce Antibiotic Use and Prevent Antimicrobial Resistance: A World Health Organization Action Framework. Clin. Infect. Dis. 2021, 73, e1011–e1017.
More
Information
Subjects:
Health Policy & Services
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
498
Revisions:
2 times
(View History)
Update Date:
08 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




