Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Tsvetelina Velikova | -- | 1628 | 2024-03-07 10:27:42 | | | |
| 2 | Rita Xu | Meta information modification | 1628 | 2024-03-07 10:39:43 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Velikova, T.; Sekulovski, M.; Peshevska-Sekulovska, M. Biologic Therapy for Inflammatory Bowel Disease Patients. Encyclopedia. Available online: https://encyclopedia.pub/entry/55965 (accessed on 28 February 2026).
Velikova T, Sekulovski M, Peshevska-Sekulovska M. Biologic Therapy for Inflammatory Bowel Disease Patients. Encyclopedia. Available at: https://encyclopedia.pub/entry/55965. Accessed February 28, 2026.
Velikova, Tsvetelina, Metodija Sekulovski, Monika Peshevska-Sekulovska. "Biologic Therapy for Inflammatory Bowel Disease Patients" Encyclopedia, https://encyclopedia.pub/entry/55965 (accessed February 28, 2026).
Velikova, T., Sekulovski, M., & Peshevska-Sekulovska, M. (2024, March 07). Biologic Therapy for Inflammatory Bowel Disease Patients. In Encyclopedia. https://encyclopedia.pub/entry/55965
Velikova, Tsvetelina, et al. "Biologic Therapy for Inflammatory Bowel Disease Patients." Encyclopedia. Web. 07 March, 2024.
Copy Citation
Many patients with inflammatory bowel disease (IBD) experience a loss of effectiveness to biologic therapy (i.e., anti-TNF therapy, etc.). Therefore, in addition to the adverse effects of the treatment, these patients also face failure to achieve and maintain remission. Immunogenicity, the process of production of antibodies to biological agents, is fundamental to the evolution of loss of response to treatment in IBD patients.
inflammatory bowel disease
biologic therapy
antibodies to biologics
1. Introduction—Challenges Related to the Use of Biologic Therapy in IBD Patients
Biologic therapy for treating inflammatory bowel disease (IBD) patients started optimistically 25 years ago [1]. Since then, approvals for clinical use for IBD have been authorized for five anti-TNF drugs (infliximab, adalimumab, golimumab, certolizumab pegol), along with biosimilars [2], and for anti-IL-12/IL-23 (ustekinumab), anti-α4β7-integrin (vedolizumab), and for some countries, for anti-α4-integrins (natalizumab), etc., while other treatments have been implemented for other autoimmune disorders and discussed as having potential efficacy for IBD—etanercept, anti-IL-17 (secukinumab, ixekizumab, brodalumab), etc. Additionally, more recently developed small molecules for the treatment of IBD and other inflammatory diseases have been introduced (i.e., JAK inhibitors (tofacitinib)) [3], although they are not labeled as “biologics”.
However, along with their undeniable effectiveness in enabling IBD patients to achieve and maintain disease remission, biological therapies face some challenges regarding safety, loss of effectiveness, high price, etc. The first and most significant challenge when using biologics as monotherapy in IBD management is that only a maximum of 40% of patients achieve a state of remission at the end of the first year of therapy [4].
Furthermore, patients with poor response to therapy or refractory IBD, including having extraintestinal complications, are often offered dual biologic therapy [3].
Additional issues related to the optimal sequence biological therapy for IBD are still challenging. Many studies for ulcerative colitis (UC), such as GEMINI 1, VARSITY, ULTRA 2, and True North, showed lower clinical remission rates with adalimumab, vedolizumab, and ozanimod after anti-TNF therapy, while other studies, such as OCTAVE 1, 2, U-ACHIEVE/U-ACCOMPLISH, and UNIFI (ustekinumab, tofacitinib, and upadacitinib) did not. At the same time, the EXTEND and GEMINI 1, 2 projects in Crohn’s disease (CD) patients showed that adalimumab and vedolizumab are associated with persistent lower endoscopic remission after anti-TNF therapy, but ustekinumab and risankizumab (from IM-UNIFI, FORTIFY and SEAVUE) do not result in remission. On the other hand, EVOLVE demonstrated that vedolizumab could be used as a first-line biologic because it does not impact further anti-TNF therapy [5]. Therefore, the controversial outcomes regarding the optimal sequence of biologics for IBD emphasize the need to explore this issue more.
Another issue related to IBD treatment is the development of new agents. Zurba et al. explored the pipeline of novel therapies for IBD. The authors also discussed the major issues associated with biologics for IBD, namely, primary non-response, secondary loss of response, and adverse effects (short- and long-term) [6]. However, there are novel treatment approaches, such as modulation of host-microbiome interactions, stem cell therapy, fibrosis management, gut-brain axis modulation, and targeted B cell therapy. Still, a definitive therapy or long-term remission for IBD is likely not realistic at this stage of the science [7].
Nonetheless, advances in the medical care of IBD have risen in recent years, boosted by the innovative small molecule and novel biologic medicines discussed here. Although the observed clinical response remains sub-optimal, treatment options for IBD patients are fast evolving to help address the disease burden, morbidity, mortality, and quality of life. In the absence of large-scale trials, physician experience has now led to prospective innovative therapeutic combinations, with most data described in case reports and case series [8].
Despite these advance, the place and future of biologics remain challenging to determine. They and other advanced therapies (i.e., novel small molecules) have made considerable contributions in providing personalized treatment for IBD patients [9]. The problems related to choosing the optimal single-agent, sequential therapy following treatment failure, dual biologic therapy, and small molecules were recently addressed by the British Society of Gastroenterology, which issued consensus guidelines on IBD management in adults [10].
Another unresolved problem associated with biologics is how to discontinue therapy. Miyatani & Kobayashi recommended discontinuation based on the evidence for the risk of relapse and efficacy of re-treatment, where the risk of relapse is higher when ceasing the anti-TNF drugs. However, in the case of withdrawal of immunomodulators combined with biologics (i.e., anti-TNFa), there is a need for therapeutic drug monitoring [11].
Notwithstanding, the determination of the best approach for a new or bio-naïve IBD patient is also an issue. In this case, along with the information from the clinical trials, factors such as comorbidities, genetic background, inflammatory markers, patient preferences, cost of the therapy, etc., should be taken into account [12]. Another debating issue concerns the implementation of biologics early in IBD treatment management. Most evidence supports the early administration of biologics in CD to improve outcomes and prevent complications and disease progression, but not for UC [13]. A meta-analysis by Ben-Horin et al. supported the same observation and recommended early administration of biologics for CD, but not for UC [14].
2. Biologic Therapy—Tailoring the IBD Pathogenetic Mechanisms
The current research focuses on elucidating the immunological mechanisms and the loss of response to various categories of biological agents for treating IBD. However, to better understand these mechanisms, it is essential to underline that biologics are monoclonal antibodies (MAbs), that is, therapeutic immunoglobulins G (IgG) with four polypeptide chains with two heavy and two light chains and two functional regions: the variable (antigen-binding region, Fab) and the constant region (Fc). The nomenclature of the most common mAbs used in IBD therapy are based on their derivation: murine (-omab), chimeric (-ximab), humanized (-zumab), and entirely human (-umab). An understanding of the pathophysiological mechanisms of IBD is critical before discussing immunogenicity [15].
Although the pathogenetic processes in IBD are not entirely comprehended, they undoubtedly influence both the therapeutic effectiveness and adverse effects of biological agents.
In healthy mucosa, the mucus layer and epithelial cells maintain barrier function. The intestinal epithelium, along with Paneth cells, which produce antimicrobial peptides, and M cells, which sample lumenal antigens and IgA dimers, help regulate and separate lumenal bacteria from the mucosal immune system. Dendritic cells (DCs) also sample lumenal contents to maintain immunologic tolerance in the intestine through podocytes across the epithelium. They process and deliver antigens to T and B lymphocytes that reside in the draining lymph nodes to induce immune tolerance [16]. Intestinal dendritic cells can stimulate naïve T and B lymphocytes to express the gut-homing marker α4β7 (Figure 1).
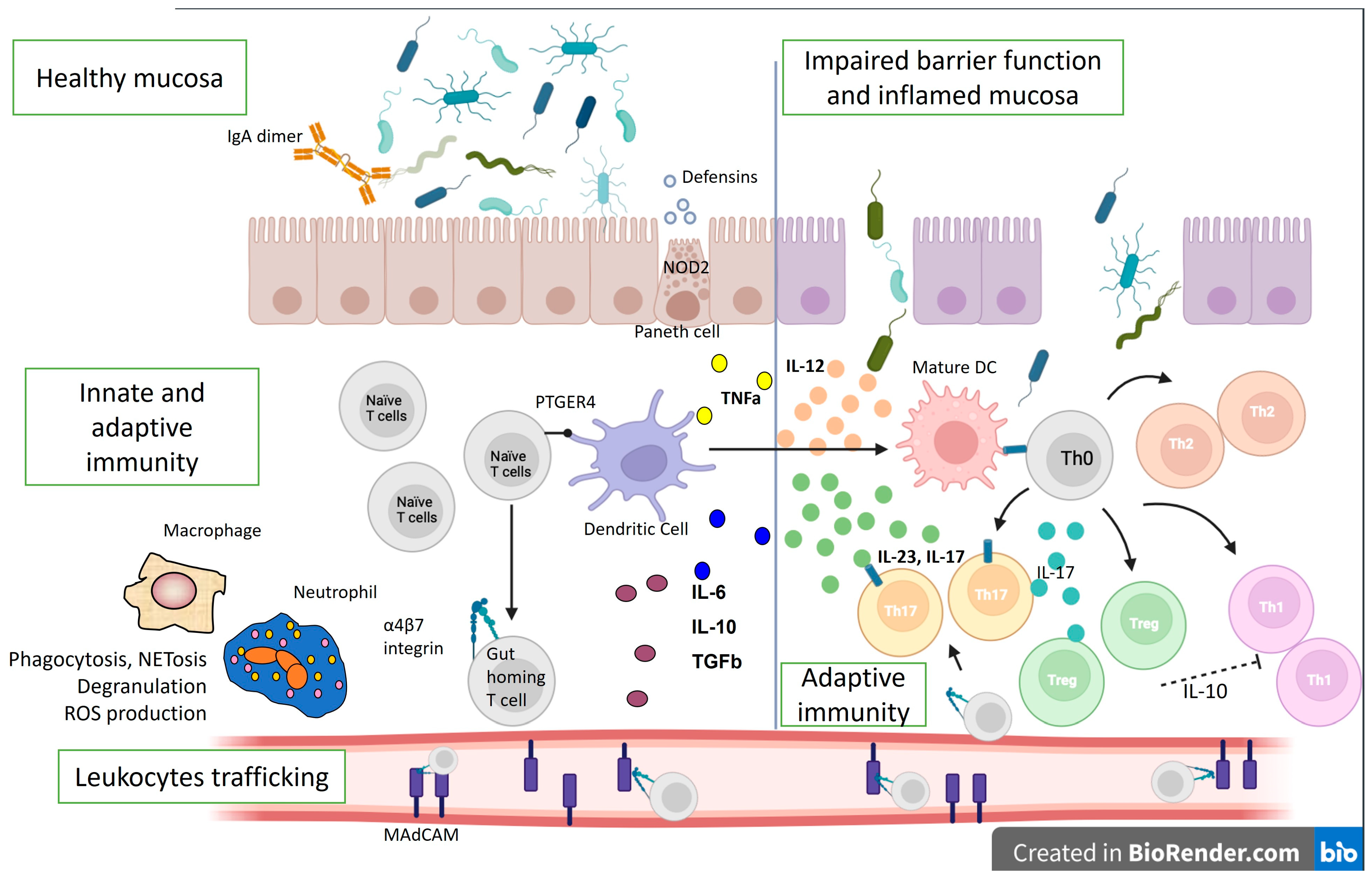
Figure 1. Immunological mechanisms in IBD pathogenesis. The lines represent differentiation, and the dotted line—suppression. IL—interleukin; ROS—reactive oxygen species; Th—T helper cell; DC—dendritic cell; TGFb—transforming growth factor beta; PTGER4—Prostaglandin EP4 receptor; NOD2—Nucleotide Binding Oligomerization Domain Containing 2; TNFa—tumor necrosis factor-alpha; MAdCAM—Mucosal vascular addressin cell adhesion molecule 1.
Intestinal lymphocytes imprinted with α4β7 interact with locally generated MAdCAM to re-enter the intestinal lamina propria and avoid circulation. Additionally, the intestinal lamina propria contains Th1, Th17, and Treg cells. The gut mucosa maintains balance through coordinated innate and adaptive immune cells, where Treg cells regulate Th1 and Th17 cells, reducing inflammation [17].
Both innate and adaptive immune mechanisms are also involved in IBD pathogenesis. Allelic variations in NOD2 have faulty intracellular bacteria sensing and reduced defensin synthesis by Paneth cells in the base of the intestinal crypts in the mucosa of CD patients. The result is an increased adaptive immune response to compensate for ineffective innate immunity. The interleukin (IL)-12/IL-23 pathway can also disrupt adaptive immunity, shifting the helper T-cell response to the Th17 spectrum. Ustekinumab, which blocks the p40 component of IL-23 and IL-12, was shown to be effective in CD.
Additionally, Th1 and Th17-associated inflammation outweighs Treg regulation by making Tregs ineffective and suppressed [18], complicating the pathophysiological picture. However, many other immune cells and cytokines are involved in the pathogenesis of IBD [19]. For example, in IBD patients, DCs and macrophages are activated during inflammation and secrete large amounts of mucosal TNF and other mediators. This pleiotropic cytokine has several pro-inflammatory effects, and anti-TNF antibodies (i.e., infliximab, adalimumab, certolizumab, golimumab) are among the biologics that are used in the treatment of CD and UC. Furthermore, when exposed to MHC class II antigens and a co-stimulatory signal, macrophages and DCs activate T lymphocytes, involving adaptive immune mechanisms.
Additionally, the receptor variant PTGER4 can cause intestinal mucosa barrier defects that promote microbial and antigenic penetration and immune activation [20]. Mucosal immune response amplification requires leukocyte movement. α4-integrins (α4β1 and α4β7) bind to ICAM-1 in inflamed tissues and MAdCAM-1, which is particular to the intestinal endothelium. In line with this, homing inhibitors, such as vedolizumab, natalizumab, and etrolizumab, disrupt inflammation by blocking inflammatory cell adhesion and recruitment [21]. Moreover, a breach in the epithelial mucosal barrier permits lumenal bacteria to cause uncontrollable inflammatory responses in UC. Th9 inflammatory cells further promote enterocyte death and hinder mucosal repair, and NKT cells generate IL-13, which damages epithelial cells. Cytokines secreted by the innate lymphoid cells (ILCs) also contribute to inflammation. Therefore, ILCs are significant mediators of chronic intestinal inflammation and drivers of disease pathogenesis, making them targets for prospective novel therapeutics, such as the JAK pathway inhibitor, tofacitinib [22].
On the other hand, dysbiosis also causes mucosal damage and inflammation. Due to a better understanding of the gut immune system, the development of novel therapeutic targets has expanded. TNFα antagonists, integrin inhibitors, anti-IL-12/23 inhibitors, and JAK inhibitors are now in clinical use, and others are in early to advanced phases of development (Figure 2).
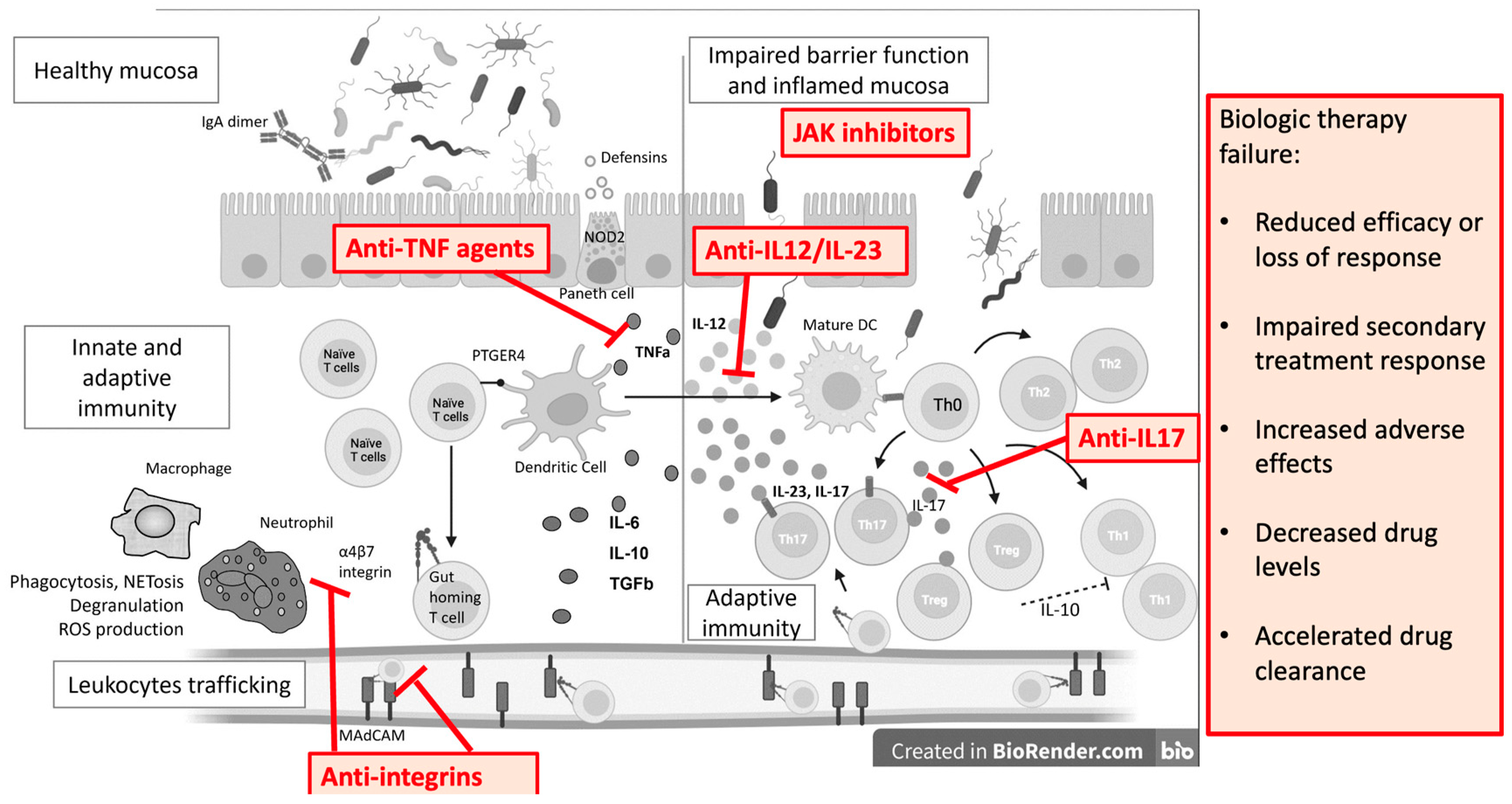
Figure 2. Monoclonal antibodies and small molecules in IBD treatment and causes of treatment failure. IL—interleukin; ROS—reactive oxygen species; Th—T helper cell; DC—dendritic cell; TGFb—transforming growth factor beta; PTGER4—Prostaglandin EP4 receptor; NOD2—Nucleotide Binding Oligomerization Domain Containing 2; TNFa—tumor necrosis factor alpha; MAdCAM—Mucosal vascular addressin cell adhesion molecule 1.
References
- Kornbluth, A. Infliximab Approved for Use in Crohn’s Disease: A Report on the FDA GI Advisory Committee Conference. Inflamm. Bowel Dis. 1998, 4, 328–329.
- Leone, G.M.; Mangano, K.; Petralia, M.C.; Nicoletti, F.; Fagone, P. Past, Present and (Foreseeable) Future of Biological Anti-TNF Alpha Therapy. J. Clin. Med. 2023, 12, 1630.
- Gold, S.L.; Steinlauf, A.F. Efficacy and Safety of Dual Biologic Therapy in Patients with Inflammatory Bowel Disease: A Review of the Literature. Gastroenterol. Hepatol. 2021, 17, 406–414.
- Hirten, R.P.; Iacucci, M.; Shah, S.; Ghosh, S.; Colombel, J.-F. Combining Biologics in Inflammatory Bowel Disease and Other Immune Mediated Inflammatory Disorders. Clin. Gastroenterol. Hepatol. 2018, 16, 1374–1384.
- Bressler, B. Is there an optimal sequence of biologic therapies for inflammatory bowel disease? Ther. Adv. Gastroenterol. 2023, 16, 17562848231159452.
- Zurba, Y.; Gros, B.; Shehab, M. Exploring the Pipeline of Novel Therapies for Inflammatory Bowel Disease; State of the Art Review. Biomedicines 2023, 11, 747.
- Higashiyama, M.; Hokaria, R. New and Emerging Treatments for Inflammatory Bowel Disease. Digestion 2023, 104, 74–81, Erratum in: Digestion 2023, 104, 164.
- Haider, M.; Lashner, B. Dual Targeted Therapy for the Management of Inflammatory Bowel Disease. J. Clin. Gastroenterol. 2021, 55, 661–666.
- Juillerat, P.; Grueber, M.M.; Ruetsch, R.; Santi, G.; Vuillèmoz, M.; Michetti, P. Positioning biologics in the treatment of IBD: A practical guide—Which mechanism of action for whom? Curr. Res. Pharmacol. Drug Discov. 2022, 3, 100104.
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.; Lomer, M.C.E.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019, 68 (Suppl. 3), s1–s106.
- Miyatani, Y.; Kobayashi, T. Evidence-Based Approach to the Discontinuation of Immunomodulators or Biologics in Inflammatory Bowel Disease. Digestion 2023, 104, 66–73.
- Laredo, V.; Gargallo-Puyuelo, C.J.; Gomollón, F. How to Choose the Biologic Therapy in a Bio-naïve Patient with Inflammatory Bowel Disease. J. Clin. Med. 2022, 11, 829.
- Berg, D.R.; Colombel, J.-F.; Ungaro, R. The Role of Early Biologic Therapy in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2019, 25, 1896–1905.
- Ben-Horin, S.; Novack, L.; Mao, R.; Guo, J.; Zhao, Y.; Sergienko, R.; Zhang, J.; Kobayashi, T.; Hibi, T.; Chowers, Y.; et al. Efficacy of Biologic Drugs in Short-Duration Versus Long-Duration Inflammatory Bowel Disease: A Systematic Review and an Individual-Patient Data Meta-Analysis of Randomized Controlled Trials. Gastroenterology 2022, 162, 482–494.
- Moreno, L.O.; Fernández-Tomé, S.; Abalo, R. Biological Treatments in Inflammatory Bowel Disease: A Complex Mix of Mechanisms and Actions. Biologics 2021, 1, 189–210.
- Kofla-Dłubacz, A.; Pytrus, T.; Akutko, K.; Sputa-Grzegrzółka, P.; Piotrowska, A.; Dzięgiel, P. Etiology of IBD—Is It Still a Mystery? Int. J. Mol. Sci. 2022, 23, 12445.
- Perez-Jeldres, T.; Tyler, C.J.; Boyer, J.D.; Karuppuchamy, T.; Bamias, G.; Dulai, P.S.; Boland, B.S.; Sandborn, W.J.; Patel, D.R.; Rivera-Nieves, J. Cell Trafficking Interference in Inflammatory Bowel Disease: Therapeutic Interventions Based on Basic Pathogenesis Concepts. Inflamm. Bowel Dis. 2019, 25, 270–282.
- Pan, X.; Zhu, Q.; Pan, L.-L.; Sun, J.; Dong, X.; Li, J.; Liu, H.; Ren, Z.; Li, B. Macrophage immunometabolism in inflammatory bowel diseases: From pathogenesis to therapy. Pharmacol. Ther. 2022, 238, 108176.
- Velikova, T.; Kyurkchiev, D.; Ivanova-Todorova, E.; Spassova, Z.; Stanilova, S.; Altankova, I. Cytokines in Inflamed Mucosa of IBD Patients; InTech: London, UK, 2016.
- Illig, D.; Kotlarz, D. Dysregulated inflammasome activity in intestinal inflammation—Insights from patients with very early onset IBD. Front. Immunol. 2022, 13, 1027289.
- Zundler, S.; Becker, E.; Weidinger, C.; Siegmund, B. Anti-Adhesion Therapies in Inflammatory Bowel Disease—Molecular and Clinical Aspects. Front. Immunol. 2017, 8, 891.
- Dai, C.; Huang, Y.-H.; Jiang, M. Combination therapy in inflammatory bowel disease: Current evidence and perspectives. Int. Immunopharmacol. 2023, 114, 109545.
More
Information
Subjects:
Gastroenterology & Hepatology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
483
Revisions:
2 times
(View History)
Update Date:
07 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





