Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | GAURAV AGRAWAL | -- | 2237 | 2024-03-07 06:12:13 | | | |
| 2 | Rita Xu | Meta information modification | 2237 | 2024-03-07 06:25:46 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Aitken, J.M.; Aitken, J.E.; Agrawal, G. Mycobacterium avium ssp. paratuberculosis and Crohn’s Disease. Encyclopedia. Available online: https://encyclopedia.pub/entry/55948 (accessed on 06 March 2026).
Aitken JM, Aitken JE, Agrawal G. Mycobacterium avium ssp. paratuberculosis and Crohn’s Disease. Encyclopedia. Available at: https://encyclopedia.pub/entry/55948. Accessed March 06, 2026.
Aitken, John M., Jack E. Aitken, Gaurav Agrawal. "Mycobacterium avium ssp. paratuberculosis and Crohn’s Disease" Encyclopedia, https://encyclopedia.pub/entry/55948 (accessed March 06, 2026).
Aitken, J.M., Aitken, J.E., & Agrawal, G. (2024, March 07). Mycobacterium avium ssp. paratuberculosis and Crohn’s Disease. In Encyclopedia. https://encyclopedia.pub/entry/55948
Aitken, John M., et al. "Mycobacterium avium ssp. paratuberculosis and Crohn’s Disease." Encyclopedia. Web. 07 March, 2024.
Copy Citation
Mycobacterium avium ssp. paratuberculosis (MAP) is the cause of Johne’s disease (JD), which is a chronic infectious gastrointestinal disease of ruminants and is often fatal. In humans, MAP has been associated with Crohn’s disease (CD) for over a century, without conclusive evidence of pathogenicity. Numerous researchers have contributed to the subject, but there is still a need for evidence of the causation of CD by MAP.
Crohn’s disease
Crohn’s dietary treatment
Mycobacterium paratuberculosis
1. Introduction
Johne’s disease (JD) was first described by Johne and Frothingham in 1895 and was thought to be a form of bovine tuberculosis. The association of JD with Mycobacterium avium ssp. paratuberculosis (MAP) was made by Bang in 1906, and MAP was eventually successfully cultured by Fredrick Twort in 1912 on solid media supplemented with an extract of dried Mycobacterium phlei, glycerine–saline and egg [1]. Following quickly after the discovery that MAP was able to be grown, Twort began research on vaccine trials for JD. Reliable new methods for the bacteriological confirmation of MAP in JD-infected animals became available and accelerated veterinary and microbiological research into JD. By 1920 the widespread distribution of MAP globally in ruminants was able to be confirmed.
Ruminant animals with JD are cachectic and malnourished, with MAP infection detrimentally affecting body mass, milk production and reproduction. Diagnostic tests for the laboratory diagnosis of MAP infection in animals include mycobacterial culture, polymerase chain reaction (PCR), and enzyme-linked immunosorbent assay (ELISA). In animals, JD is often fatal if undiagnosed. The time taken for the detection of an infected animal in a herd is between 2–4 years, depending on the first appearance of clinical symptoms and the time taken for the detection of MAP in cultured samples. In ruminant animals, a diagnosis of JD is followed by culling the infected animal to prevent transmission of MAP to other members of the herd.
In humans, the association of MAP with Crohn’s disease has long been conjectured, but methods for the detection, culture and diagnosis of MAP infection have not been available. The primary reason for this absence has been the inability to reliably detect cell-wall-deficient mycobacterial (CWDM) forms of MAP in human blood and tissue samples from patients with CD. The detection of MAP as a CWDM form in patients with CD has been rare and has required extended incubation [2][3].
2. The Pathogenic Potential of MAP
MAP is a member of the Mycobacterium avium complex (MAC), a group of mycobacteria formed around taxonomic and phenotypic traits, with some common features and clear differences. The taxonomy of members of MAC is based on various factors, including opportunistic pathogenicity of the organisms in specific animal hosts, dependence on mycobactin for growth, replication times, temperature ranges for growth in culture, and genome analyses. The inclusion of MAP within MAC has been debated and there are arguments for revising the classification of MAP as a subspecies of Mycobacterium avium. Some authors argue that MAP should be more accurately identified as Mycobacterium paratuberculosis. Factors influencing the taxonomy of MAP include the virulence potential of MAP in ruminant animals, and the increasing occurrence of acid-fast bacillary MAP infections in humans, subsequent to interaction with MAP-infected bison in India [4].
Partly because of the classification of MAP as a member of MAC, it has been proposed that the pathogenic potential of MAP in humans is in line with other members of MAC—that of an environmentally acquired, opportunistic pathogen. Opportunistic mycobacterial pathogens are seldom able to cause significant disease in humans with immunocompetent immune systems. In animals, particularly ruminants, MAP is pathogenic but in humans, the pathogenic range and infectious potential of MAP are largely unknown. However, there is growing global research interest in the role of MAP in association with human and animal diseases [5]. The decision to assign MAP to MAC should be reconsidered given the zoonotic potential of the organism and the dwindling availability of diagnostic tests and therapeutics for CD [6][7][8][9].
3. Isolation of MAP in Humans
MAP has the longest replication time of any opportunistically pathogenic Mycobacterium species (>24 h) resulting in extended incubation times for detection of growth.
Diagnostic medical laboratories using automated mycobacterial detection systems to culture opportunistic mycobacteria from human samples will only rarely isolate MAP from human samples. Such systems are optimised for the detection of MTBC but the short incubation times, from 6 to 8 weeks, that are suitable for isolation of MTBC, are unsuitable for some members of MAC—including MAP. These require extended incubation at varied temperatures and specific media supplementation.
Reports of the successful isolation of MAP in the bacillary form from human samples indicate that 12 to 18 months incubation is needed for detection.
In lieu of the culture detection of MAP in human samples, researchers have opted for polymerase chain reaction (PCR) to detect unique MAP sequences. The most common DNA sequences used for detection of MAP in samples are the insertion sequence IS900 and the F57 sequence element. Using PCR for IS900, carriage of MAP has been detected in both healthy controls and patients diagnosed with CD. PCR detection of MAP (IS900) in healthy patients has been widely reported, but only occasionally explained. One theory holds that viable MAP are widely distributed in the environment and various foodstuffs, (milk, water, and meat) and therefore transient intestinal colonisation is possible. Another possible explanation is the phenomenon of “environmental vaccination”.
This term was first proposed by Abrahams in 1970, as the theory of “original mycobacterial sin” in relation to primary mycobacterial contact and carriage [10].
This theory explores how initial contact by the host with a Mycobacterium species influences subsequent antigenic responses elicited by secondary exposure of the same host to other Mycobacterium species later in life. The theory suggests that viable mycobacteria may be retained in the bloodstream to augment “immune memory”.
Studies of healthy individuals who had been vaccinated with Bacillus Calmette-Guerin vaccine (BCG) at birth have demonstrated that BCG was retained in a cell-wall-deficient state by the vaccinated subjects, and could be reisolated decades later [11][12]. The long-term carriage of viable BCG suggests the possibility that primary exposure to MAP in early childhood, through environmental sources, may similarly explain the high levels of MAP carriage found in healthy adults.
CWDM forms of MAP present additional technical problems for diagnostic medical laboratories using methods that are optimised for the detection of MTBC. To successfully detect MAP in clinical samples, modifications to existing methods, culture media, and stains are required.
Partly for the reasons outlined above, the cultivation of MAP in the bacillary form in diagnostic medical laboratories is uncommon, unless MAP is specifically cultured for. In 1984, Chiodini et al. reported the cultivation of uncharacterised cell-wall-deficient mycobacteria from patients presenting with Crohn’s disease. The isolates reverted to bacillary forms of MAP after 18 months incubation [13]. There have been reports of the spread of MAP in the bacillary form among animal attendants tending MAP-infected bison in India [8]. Sechi et al., have reported a high level of culture-positive MAP infections in Sardinian patients with CD and some controls [14].
In 2002 Richter et al. reported the isolation of a bacillary form of MAP from a patient with HIV infection [15]. Samples collected from the patient included intestinal biopsy tissues, bone marrow and liver punch biopsies; all showed the presence of numerous acid- fast bacilli. The authors described the difficulties in culturing the acid-fast bacilli in samples collected from the patient. Despite laboratory visualisation of the acid-fast bacilli, cultures using commercial mycobacterial culture media and in-house media were inconclusive. After two years, the authors considered the possibility of MAP infection. Mycobactin J was thereafter added to culture media, and MAP in the bacillary form was grown after four weeks. The identity of the isolates as M. avium ssp. paratuberculosis was confirmed by PCR. This case highlighted the difficulties faced by diagnostic microbiology laboratories in the culture of MAP, even when the identity of the acid-fast bacillary isolate is suspected.
In 2023, Estevinho et al., succeeded in isolating bacillary MAP after 18 months, using a commercial culture system [16].
4. The Importance of the Diagnostic Laboratory
Parts of the role of a diagnostic microbiology department include the detection and identification of potentially pathogenic organisms, carrying out isolation and susceptibility tests, and providing guidance to clinicians regarding therapies. In addition, the 21st century ‘One Health’ concept includes “front line” monitoring for emerging zoonotic health threats by diagnostic microbiology laboratories. These tasks are counterbalanced by constrained health resources that limit these surveillance activities [17].
There are few papers describing the isolation of MAP from human samples in commercial mycobacteria culture media [15]. Culture systems, such as the BD BACTEC™ MGIT™ automated mycobacterial detection system (MGIT), are optimised for the isolation of MTBC in the acid-fast bacillary (AFB) phase of the mycobacterial life cycle. However, MAP requires additional supplementation with mycobactin J, which is not readily available in many human diagnostic laboratories. In addition, the extended incubation times required for MAP growth are outside the recommended incubation times of most commercial automated systems [18]. The isolation of CWDM forms of MAP is extremely difficult and as well as mycobactin J supplementation, complex, high-nutrient media and specific conditions for growth are required [19]. Chiodini et al., Timms et al., and more recently Estevinho et al., took approximately 18 months to isolate MAP from human samples [2][3][16].
Using alternative staining techniques and culture media, researchers are able to detect the presence of CWDM in human blood and tissue samples within two months, if the subject is in an acute phase of the illness [20]. The variant of the Ziehl Neelsen (ZN) stain used by many laboratories is optimised for the detection of MTBC. Researchers have found that different ZN methods are more reliable for NTM and CWDM. Decolourising solutions using ethanol should be avoided. Ehrich’s modification of the ZN decolouriser (1883) is suitable for the detection of CWDM in cultures [21]. The CWDM isolates found in association with CD are acid fast, but are not acid–alcohol fast, particularly if ethanol is used in the decolouriser.
MAP detection is an example of the difficulties faced by diagnostic medical laboratories presented with the reality of emerging bacterial pathogens. A similar circumstance applies to Mycobacterium xenopi which has a slow growth rate and an optimal temperature for growth at 42 °C, so cannot easily be detected in automated systems [22].
5. Cell-Wall-Deficient Mycobacteria in Crohn’s Disease
In humans, the CWDM form of MAP remains elusive to bacteriologists. In vivo, CWDM are induced through enzymatic action which removes the outer membrane and the cell wall from the bacillary form. However, the cytoplasm remains intact and is encased by the remaining inner membrane. The inner membrane is semi-permeable and surrounds the cytoplasm, the region of the cell where mycolic acid is produced [23]. The presence of mycolic acid in proximity to the inner membrane explains why ZN staining of CWDM, with relevant changes in the decolouriser, shows acid-fastness in CWDM. Mycolic acids are highly antigenic and stimulate the production of tumour necrosis factor alpha (TNFα) in the host [24]. Mycolic lipid deposition in vitro is evident in the ‘donut’ morphology of the CWDM in cultures, where there is an accretion of mycolic lipids on the inner membrane. (Figure 1). This aggregation is a consequence of the inability of the CWDM to transport mycolic lipids from the inner membrane to the absent outer membrane. The precipitation of mycolic acid is a consequence of the elimination of the mmpL3 transporter [25][26] and the loss of the outer membrane.
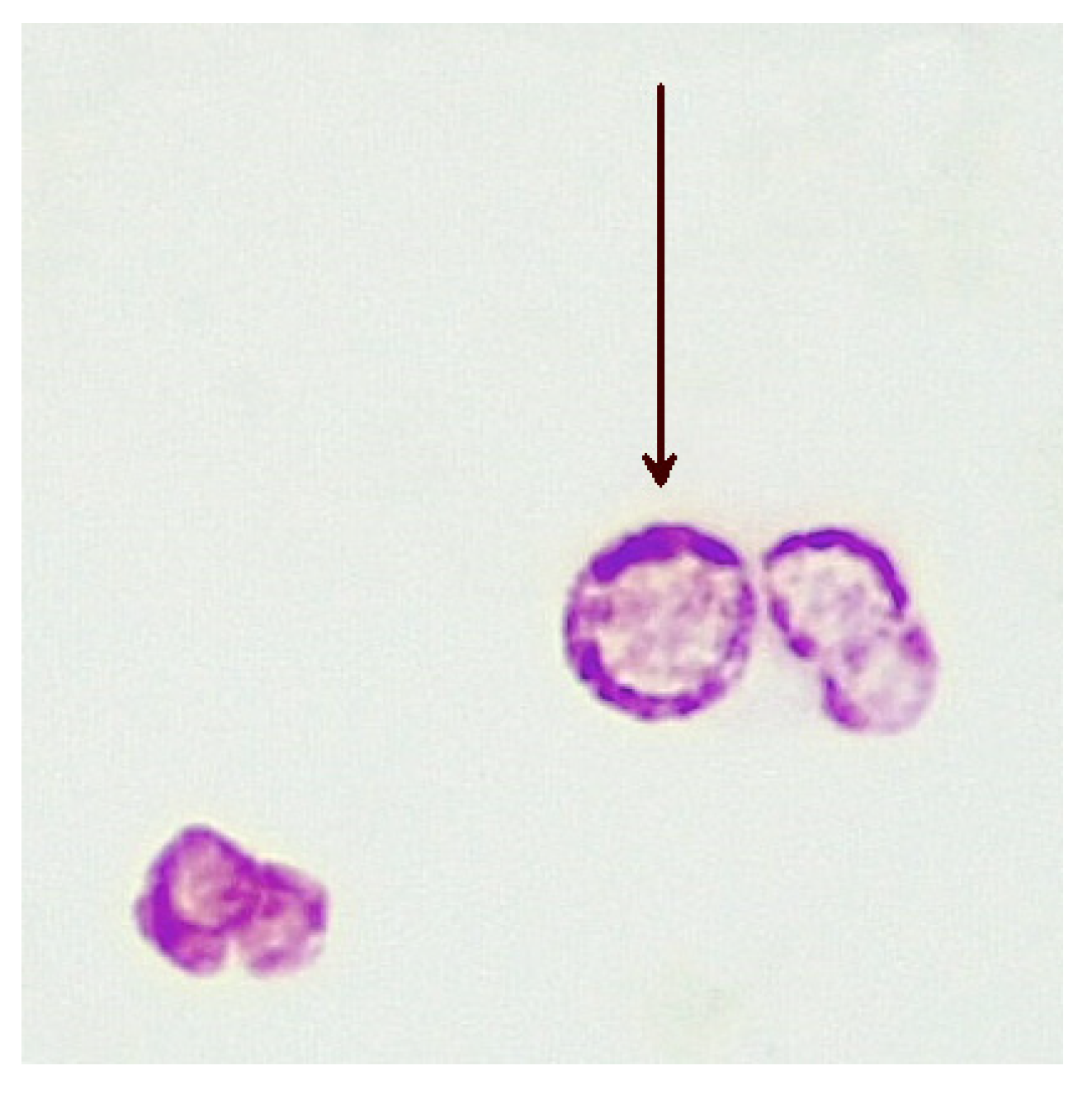
Figure 1. Deposition of mycolic lipids in the wall of the inner membranes of CWDMs (arrow) isolated from a patient with CD (×1000 magnification oil immersion ZN stain). Samples were positive for IS900.
Some antibiotics may penetrate the inner membrane of CWDM forms and enter the cytoplasm. However, the inner membranes of naturally occurring CWDM isolates are often enveloped in extracellular biofilm (Figure 2). In combination with biofilm, the deposition of mycolic lipids on the inner membrane wall presents challenges for the uptake of antimicrobial compounds. Despite these obstructions, antibiotics may infuse into the biofilm and thus have (limited) access to the inner membrane. Biofilm may also act as a ‘surrogate cell wall’ and retain the organism in the non-replicating persister (NRP) state. This biofilm matrix is also known to contain enzymes, such as nucleases [27]. The permeability of the inner membrane is bidirectional, so mycolic acid may also be present in the extracellular biofilm. The presence of NRP mycobacteria in the host subsequent to antimicrobial therapy, adverse environmental conditions or lack of sufficient nutrients and oxygen, may result in reduced effectiveness of antimicrobials [28] and require an extended period of effective therapy, because of the slow growth and metabolic activity of the organism.
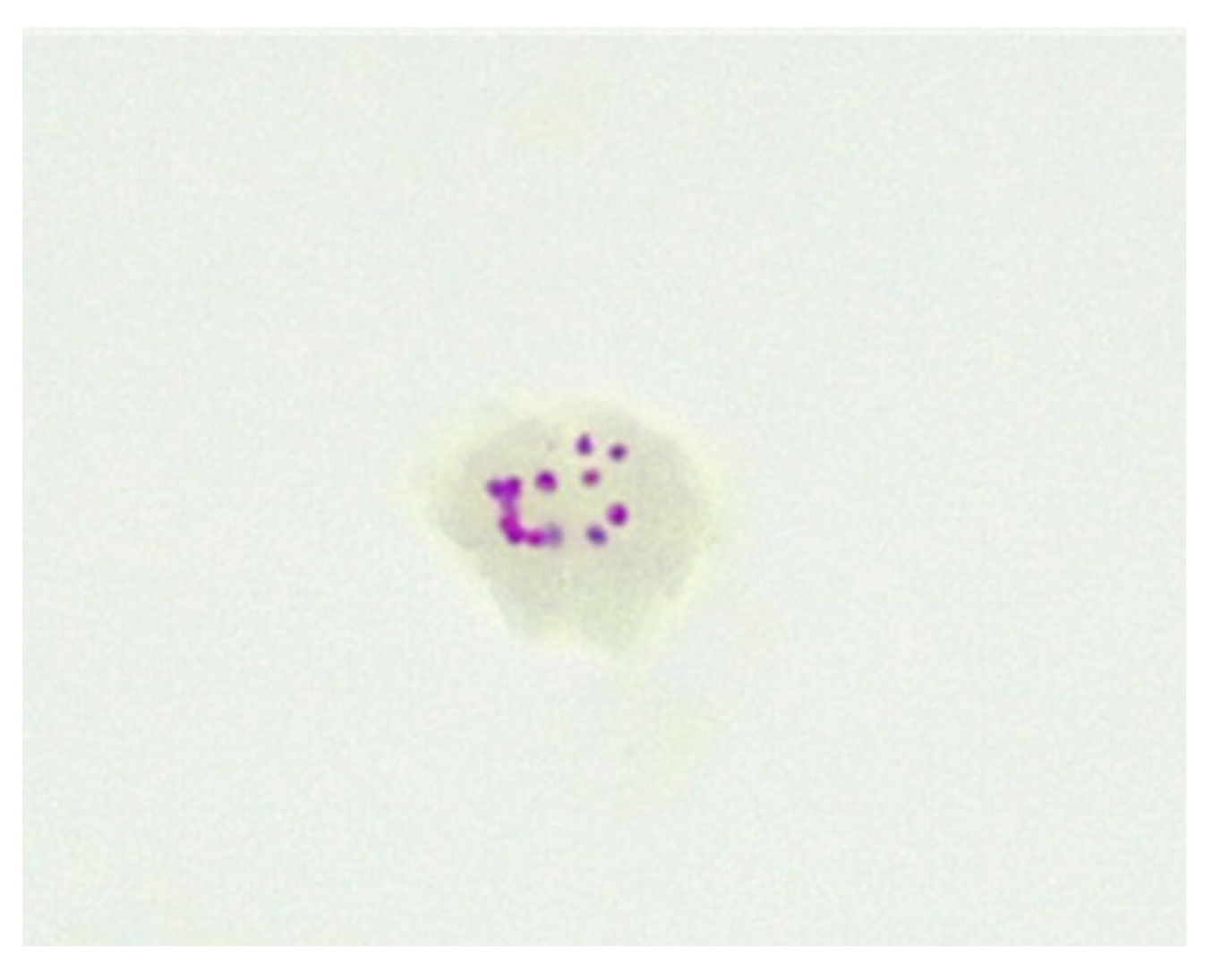
Figure 2. CWDM encased in biofilm from a patient with CD (×1000 magnification oil immersion ZN stain). Samples were positive for IS900.
The precise methods of replication of MAP CWDM are unclear, but there is evidence of a form of bilateral division (Figure 3). Microscopic examination of CWDM in cultures that suggest division are in contrast with the report that the FtsZ gene may be deficient in some intracellular MTBC [29]. There is also some evidence of blebs forming on the inner membranes [30], suggesting an alternative method of replication (Figure 4). Microscopy can demonstrate the presence of both bilateral division and “budding” in actively growing in cultures, though bilateral division is seen more commonly in culture.
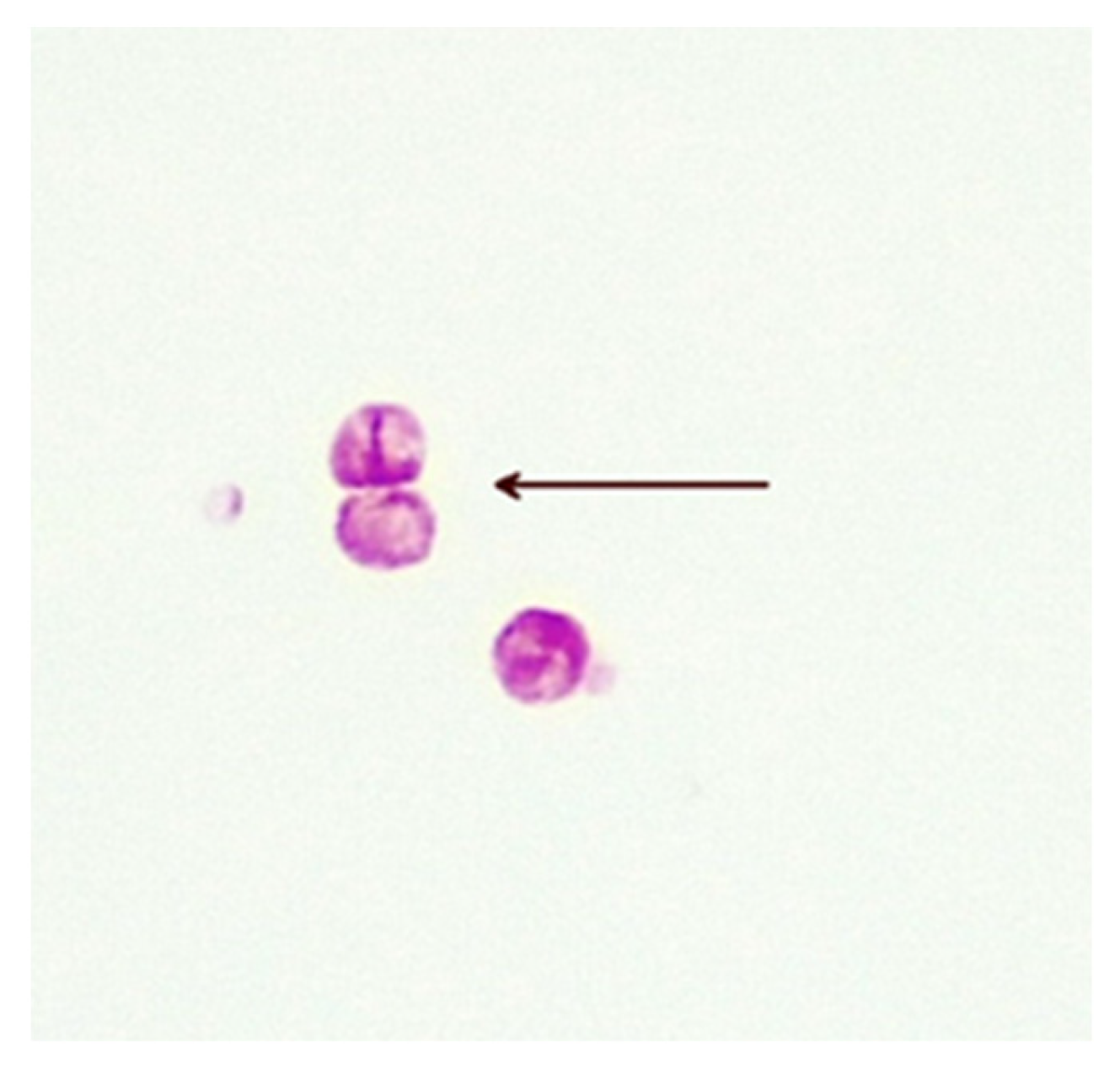
Figure 3. Bilateral division of CWDM forms (arrow) from a patient with CD (×1000 magnification oil immersion ZN stain). Samples were positive for IS900.
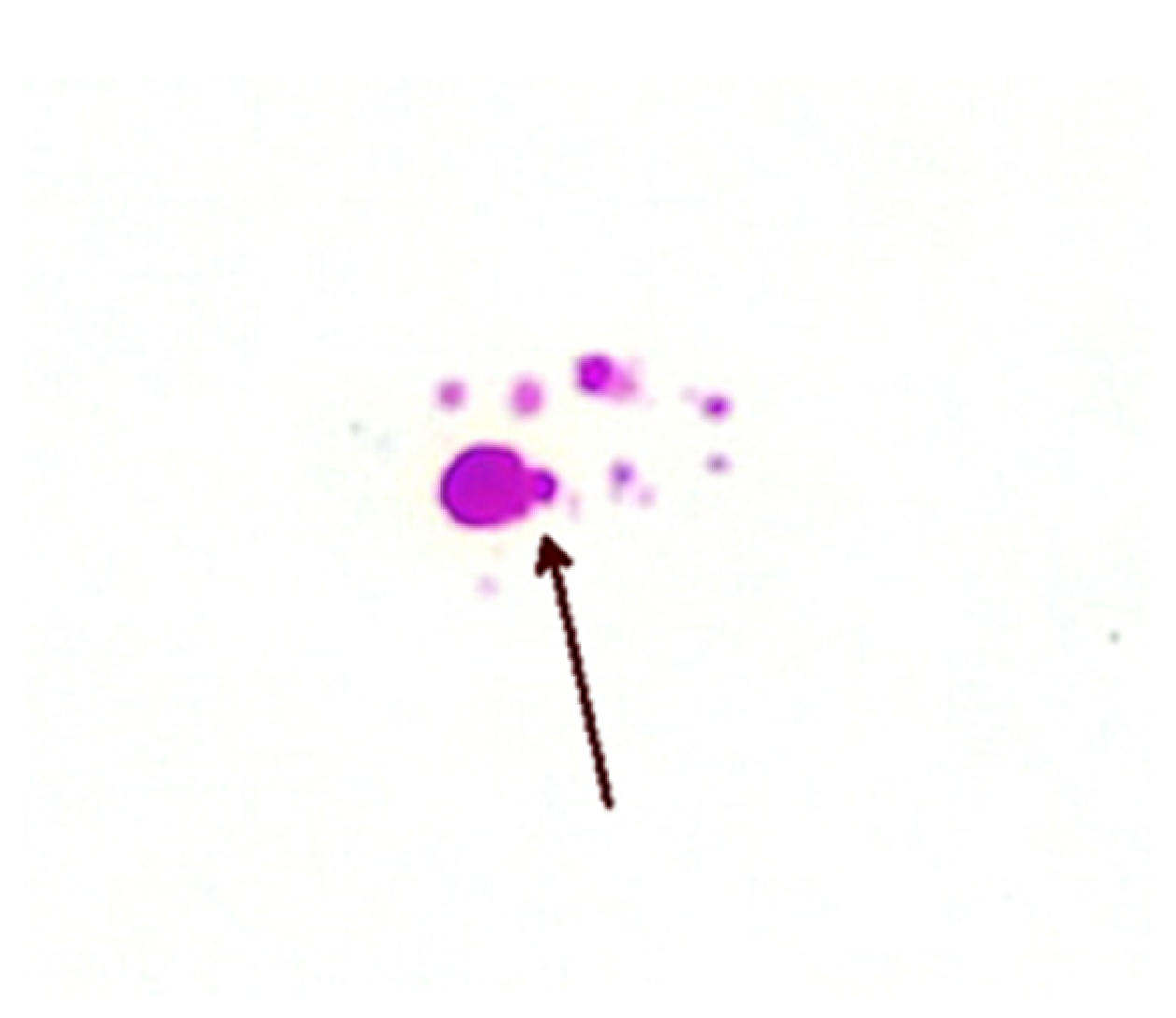
Figure 4. Bleb formation (arrow) on inner membrane from a patient with CD (×1000 magnification oil immersion ZN stain). Samples were positive for IS900.
References
- Twort, F.W.; Ingram, G.L.Y.; Hill, L.E. A method for isolating and cultivating the mycobacterium enteritidis chronicæ pseudotuberculosæ bovis, Jöhne, and some experiments on the preparation of a diagnostic vaccine for pseudo-tuberculous enteritis of bovines. Proc. R. Soc. Lond. Ser. B Contain. Pap. A Biol. Character 1997, 84, 517–542.
- Chiodini, R.J.; Van Kruiningen, H.J.; Thayer, W.R.; Coutu, J.A. Spheroplastic phase of mycobacteria isolated from patients with Crohn’s disease. J. Clin. Microbiol. 1986, 24, 357–363.
- Timms, V.J.; Daskalopoulos, G.; Mitchell, H.M.; Neilan, B.A. The Association of Mycobacterium avium subsp. paratuberculosis with Inflammatory Bowel Disease. PLoS ONE 2016, 11, e0148731.
- Singh, A.V.; Chauhan, D.S.; Singh, S.V.; Kumar, V.; Singh, A.; Yadav, A.; Yadav, V.S. Current status of Mycobacterium avium subspecies paratuberculosis infection in animals & humans in India: What needs to be done? Indian J. Med. Res. 2016, 144, 661–671.
- Ekundayo, T.C.; Okoh, A.I. Systematic Assessment of Mycobacterium avium Subspecies paratuberculosis Infections from 1911–2019: A Growth Analysis of Association with Human Autoimmune Diseases. Microorganisms 2020, 8, 1212.
- Mintz, M.J.; Lukin, D.J. Mycobacterium avium subspecies paratuberculosis (MAP) and Crohn’s disease: The debate continues. Transl. Gastroenterol. Hepatol. 2023, 8, 28.
- Magro, F.; Moreira, P.L.; Catalano, G.; Alves, C.; Roseira, J.; Estevinho, M.M.; Silva, I.; Dignass, A.; Peyrin-Biroulet, L.; Danese, S.; et al. Has the therapeutical ceiling been reached in Crohn’s disease randomized controlled trials? A systematic review and meta-analysis. United Eur. Gastroenterol. J. 2023, 11, 202–217.
- Singh, A.V.; Singh, S.V.; Singh, P.K.; Sohal, J.S.; Singh, M.K. High prevalence of Mycobacterium avium subspecies paratuberculosis (‘Indian bison type’) in animal attendants suffering from gastrointestinal complaints who work with goat herds endemic for Johne’s disease in India. Int. J. Infect. Dis. 2011, 15, e677–e683.
- Ananthakrishnan, A.N.; Adler, J.; Chachu, K.A.; Nguyen, N.H.; Siddique, S.M.; Weiss, J.M.; Sultan, S.; Velayos, F.S.; Cohen, B.L.; Singh, S. AGA Clinical Practice Guideline on the Role of Biomarkers for the Management of Crohn’s Disease. Gastroenterology 2023, 165, 1367–1399.
- Abrahams, E.W. Original mycobacterial sin. Tubercle 1970, 51, 316–321.
- Markova, N.; Slavchev, G.; Michailova, L. Presence of mycobacterial L-forms in human blood: Challenge of BCG vaccination. Human. Vaccines Immunother. 2015, 11, 1192–1200.
- Dimova, T.; Terzieva, A.; Djerov, L.; Dimitrova, V.; Nikolov, A.; Grozdanov, P.; Markova, N. Mother-to-newborn transmission of mycobacterial L-forms and Vδ2 T-cell response in placentobiome of BCG-vaccinated pregnant women. Sci. Rep. 2017, 7, 17366.
- Chiodini, R.J.; Van Kruiningen, H.J.; Merkal, R.S.; Thayer, W.R.; Coutu, J.A. Characteristics of an unclassified Mycobacterium species isolated from patients with Crohn’s disease. J. Clin. Microbiol. 1984, 20, 966–971.
- Sechi, L.A.; Scanu, A.M.; Molicotti, P.; Cannas, S.; Mura, M.; Dettori, G.; Fadda, G.; Zanetti, S. Detection and Isolation of Mycobacterium avium subspecies paratuberculosis from intestinal mucosal biopsies of patients with and without Crohn’s disease in Sardinia. Am. J. Gastroenterol. 2005, 100, 1529–1536.
- Richter, E.; Wessling, J.; Lügering, N.; Domschke, W.; Rüsch-Gerdes, S. Mycobacterium avium subsp. paratuberculosis Infection in a Patient with HIV, Germany. Emerg. Infect. Dis. 2002, 8, 729–731.
- Estevinho, M.M.; Cabeda, J.; Santiago, M.; Machado, E.; Silva, R.; Duro, M.; Pita, I.; Morais, R.; Macedo, G.; Bull, T.J.; et al. Viable Mycobacterium avium subsp. paratuberculosis Colonizes Peripheral Blood of Inflammatory Bowel Disease Patients. Microorganisms 2023, 11, 1520.
- Reller, L.B.; Weinstein, M.P.; Peterson, L.R.; Hamilton, J.D.; Baron, E.J.; Tompkins, L.S.; Miller, J.M.; Wilfert, C.M.; Tenover, F.C.; Thomson, R.B., Jr. Role of Clinical Microbiology Laboratories in the Management and Control of Infectious Diseases and the Delivery of Health Care. Clin. Infect. Dis. 2001, 32, 605–610.
- Mahomed, S.; Dlamini-Mvelase, N.R.; Dlamini, M.; Mlisana, K. Failure of BACTECTM MGIT 960TM to detect Mycobacterium tuberculosis complex within a 42-day incubation period. Afr. J. Lab. Med. 2017, 6, 537.
- Dane, H.; Stewart, L.D.; Grant, I.R. Culture of Mycobacterium avium subsp. paratuberculosis: Challenges, limitations and future prospects. J. Appl. Microbiol. 2023, 134, lxac017.
- Aitken, J.M.; Phan, K.; Bodman, S.E.; Sharma, S.; Watt, A.; George, P.M.; Agrawal, G.; Tie, A.B.M. A Mycobacterium species for Crohn’s disease? Pathology 2021, 53, 818–823.
- Bishop, P.J.; Neumann, G. The history of the Ziehl-Neelsen stain. Tubercle 1970, 51, 196–206.
- Piersimoni, C.; Nista, D.; Bornigia, S.; Gherardi, G. Unreliable Detection of Mycobacterium xenopi by the Nonradiometric Bactec MGIT 960 Culture System. J. Clin. Microbiol. 2009, 47, 804–806.
- Bansal-Mutalik, R.; Nikaido, H. Mycobacterial outer membrane is a lipid bilayer and the inner membrane is unusually rich in diacyl phosphatidylinositol dimannosides. Proc. Natl. Acad. Sci. USA 2014, 111, 4958–4963.
- Sueoka, E.; Nishiwaki, S.; Okabe, S.; Iida, N.; Suganuma, M.; Yano, I.; Aoki, K.; Fujiki, H. Activation of protein kinase C by mycobacterial cord factor, trehalose 6-monomycolate, resulting in tumor necrosis factor-alpha release in mouse lung tissues. Jpn. J. Cancer Res. 1995, 86, 749–755.
- Thouvenel, L.; Rech, J.; Guilhot, C.; Bouet, J.-Y.; Chalut, C. In vivo imaging of MmpL transporters reveals distinct subcellular locations for export of mycolic acids and non-essential trehalose polyphleates in the mycobacterial outer membrane. Sci. Rep. 2023, 13, 7045.
- Xu, Z.; Meshcheryakov, V.A.; Poce, G.; Chng, S.-S. MmpL3 is the flippase for mycolic acids in mycobacteria. Proc. Natl. Acad. Sci. USA 2017, 114, 7993–7998.
- Zang, X.; Dang, G.; Cai, Z.; Shao, M.; Tang, Y.; Cao, J.; Cui, Z.; Liu, S. Extracellular DNase MAP3916c attacks the neutrophil extracellular traps and is needed for Mycobacterium avium subsp. paratuberculosis virulence. Vet. Microbiol. 2022, 273, 109529.
- Quigley, J.; Lewis, K. Noise in a Metabolic Pathway Leads to Persister Formation in Mycobacterium tuberculosis. Microbiol. Spectr. 2022, 10, e0294822.
- Chauhan, A.; Madiraju, M.V.V.S.; Fol, M.; Lofton, H.; Maloney, E.; Reynolds, R.; Rajagopalan, M. Mycobacterium tuberculosis cells growing in macrophages are filamentous and deficient in FtsZ rings. J. Bacteriol. 2006, 188, 1856–1865.
- Lazenby, J.J.; Li, E.S.; Whitchurch, C.B. Cell wall deficiency—An alternate bacterial lifestyle? Microbiology 2022, 168, 001218.
More
Information
Subjects:
Gastroenterology & Hepatology; Infectious Diseases
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
583
Revisions:
2 times
(View History)
Update Date:
07 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





