The ever-increasing world population and environmental stress are leading to surging demand for nutrient-rich food products with cleaner labeling and improved sustainability. Plant proteins, accordingly, are gaining enormous popularity compared with counterpart animal proteins in the food industry. While conventional plant protein sources, such as wheat and soy, cause concerns about their allergenicity, peas, beans, chickpeas, lentils, and other pulses are becoming important staples owing to their agronomic and nutritional benefits. However, the utilization of pulse proteins is still limited due to unclear pulse protein characteristics and the challenges of characterizing them from extensively diverse varieties within pulse crops.
1. Introduction
By 2050, the world’s population will exponentially increase to over 10 billion from the current 7.9 billion, according to the World Health Organization (UNFPA, 2020). A major challenge of food scarcity will arise from climate change, the rapid growth of the global population, and imbalance in food production, which may inevitably lead to severe human malnutrition. Protein-energy malnutrition (PEM) is responsible for six million deaths worldwide annually
[1]. The main source of dietary protein is highly reliant on animal-derived products, such as muscles, eggs, dairy, and their processed products, although livestock farming generates more pollution including sewage and greenhouse gas than crop production
[2][3][4].
Pulse crops have drawn increasing attention in the food industry due to their low production cost, non-GMO status, and high yield of nutritious proteins
[5]. According to the Food and Agriculture Organization (FAO) of the United Nations in 2023, pulses, the seeds of leguminous plants, serve 36 food and feed purposes and offer benefits to both food security and environmental sustainability. In terms of nutritional composition, pulse seeds contain more than 30% protein, carbohydrates including digestible and resistant starch, and dietary fibers, as well as essential vitamins and minerals and bioactive phytochemicals
[6]. For these reasons, pulses have been suggested as wholesome alternatives to animal proteins. With their advantages of hypoallergenicity, broad acceptance, and better bioaccessibility, pulse proteins have gained popularity in the supply chain
[7]. However, pulse crops, unlike common staple crops (e.g., soybeans), originate from a vast array of sources and showcase a remarkable diversity of species
[8][9]. As a result, pulse proteins derived from different crops are distinguished from each other in structure, composition, and especially in functional properties. To achieve the full potential of pulse proteins in food applications, it is important to gain a comprehensive understanding of primary pulse crop varieties, their geographic distribution, their production quantities, and, most notably, the specific differentiations in protein attributes.
2. Origins and Compositions of Pulse Crops
The term “pulse” is defined as the nutritional-dense edible legume crops that are harvested solely for dry seeds, e.g., dry peas (
Pisum sativum), pigeon peas (
Cajanus cajan), chickpeas (
Cicer arietinum), cowpeas (
Vigna unguiculata), lentils (
Lens culinaris), common beans (
Phaseolus vulgaris), faba beans (
Vicia faba), bambara beans (
Vigna subterranea), mung beans (
Phaseolus aureus), black gram (
Phaseolus mungo), moth beans (
Phaseolus aconitifolius), and velvet beans (
Stizolobium spp.)
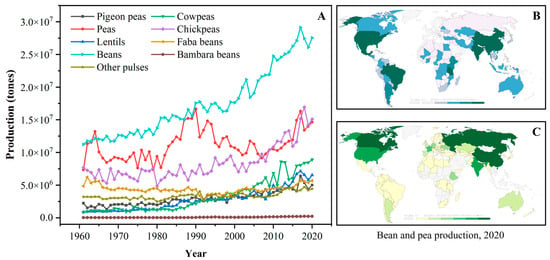
[5][9]. According to the U.N. Food and Agriculture Organization (FAO), the annual worldwide production quantities (from 1961 to 2020) of some pulses are depicted in
Figure 1A. Over the past four decades, the production of pulse crops experienced obvious upward trends; meanwhile, pulses have also become the second most consumed crops, after cereals, for human diets around the world
[8][9]. Dry beans, peas, and chickpeas are the most popular strains among all pulses, and their annual production is approximately three times higher than those in 1961. Owing to the reduced moisture contents, pulses exhibit a relatively long storage life compared to fresh legumes and thus are widely cultivated all over the world
[9]. Geographically, pulse crops are grown in India, North America, China, and Europe, which exhibit excellent soil and climate tolerance
[8][10]. For example, beans are primarily produced in South America, North America, Asia, and Africa (
Figure 1B), while most peas are grown in Asia, Europe, and North America (
Figure 1C).
Figure 1. (
A): The annual production quantity of pulse crops worldwide from 1961 to 2020 (FAO, 2020); (
B): The distribution of bean production worldwide in 2020; (
C): The distribution of pea production worldwide in 2020 (data obtained from Our World in Data,
https://ourworldindata.org/, accessed on 17 July 2023).
From the nutritional perspective, pulse crops contain carbohydrates, fibers, minerals, vitamins, and other significant bioactive substances, with >30% protein as the most noticeable attribute
[9]. While carbohydrate is typically the highest content among nutrients, protein ranks second in these pulse products. Due to their high protein content and being non-demanding in farming conditions, pulses have been staple crops in regions where meat protein is scarce. Except for chickpeas and lupins, all pulses are low in fat content (<5%,
w/
w)
[11]. Even so, a high unsaturated fatty acid profile in pulses has been reported
[12]. Combined with the high content of dietary fibers, pulses including peas have been proven to protect against cardiovascular disease and obesity
[13].
3. Composition and Structure of Pulse Protein Isolates
3.1. Amino Acid Composition
The nutritional properties and functional characteristics of pulse protein isolates are determined by their amino acid compositions and sequences (primary structure) as well as the derived higher-level structures, i.e., secondary, tertiary, and quaternary, during their folding and complexation. On this basis, the additional advanced structures are assembled through dynamic bonding such as hydrogen bonds, hydrophobic contacts, electrostatic interaction, and disulfide bonds
[14]. The amino acid (AA) composition of several selected pulse proteins is given in
Table 1 [6][8][11][15][16][17][18][19]. It is notable that the contents of essential amino acids, including lysine, leucine, aspartic acid, glutamic acid, and arginine, are relatively high in pulse proteins. Particularly, lysine, a well-known limiting essential AA in cereals, is abundant in pulse proteins; for example, the lysine content is about 7.7 g per 100 g in chickpea and pea proteins
[16][17]. However, according to the sequences, pulse proteins are deficient in two essential AAs, methionine and tryptophan. Therefore, it is viable to complement pulse with tryptophan-rich proteins such as canola protein to offer a complementary essential AA composition
[8][19]. Variations in AA profiles of different pulse proteins are caused by their species, growth environments, and differences in measurement methods
[9][11][16].
Table 1. Amino acid composition of pulse proteins (g/100 g dry matter)
[6][8][11][15][16][17][18][19].
| Amino Acid |
Pea |
Chickpea |
Lentil |
Mung Bean |
Lupin |
Cowpea |
Faba Bean |
Pigeon Pea |
| Essential AA |
|
|
|
|
|
|
|
|
| Isoleucine (Ile, I) |
0.4–4.9 |
0.4–4.1 |
0.5–5.0 |
1.0–4.7 |
1.2–3.2 |
4.3–4.4 |
1.1–4.3 |
4.8 |
| Leucine (Leu, L) |
1.3–8.4 |
0.5–7.0 |
0.8–7.9 |
1.8–8.4 |
2.0–7.4 |
7.1–7.5 |
2.0–8.2 |
7.5 |
| Lysine (Lys, K) |
1.4–7.7 |
0.9–7.7 |
0.5–7.2 |
1.7–4.2 |
1.2–7.6 |
3.9–6.6 |
1.9 |
4.4 |
| Methionine (Met, M) |
0.2–3.3 |
0.1–1.9 |
0.1–2.9 |
0.3–1.9 |
0.2–0.3 |
1.2–1.3 |
0.2–0.8 |
1.2 |
| Phenylalanine (Phe, F) |
0.2–8.1 |
0.4–5.9 |
0.6–7.8 |
1.1–5.7 |
1.0–3.3 |
4.0–5.6 |
1.2 |
3.9 |
| Threonine (Thr, T) |
0.9–4.5 |
0.1–3.6 |
0.6–3.8 |
0.8–3.2 |
1.0–4.3 |
2.5–3.7 |
1.0–13.0 |
2.8 |
| Tryptophan (Trp, W) |
0.2–1.0 |
0.2–1.1 |
0.7–0.8 |
0.3–1.0 |
0.2–0.3 |
0.3–1.1 |
0.2–1.1 |
NR |
| Valine (Val, V) |
0.4–5.2 |
0.4–3.8 |
0.7–5.3 |
1.2–5.2 |
1.1–3.5 |
4.6–4.9 |
1.2 |
4.7 |
| Arginine (Arg, R) |
1.2–8.7 |
0.5–10.3 |
0.9–7.8 |
1.7–6.3 |
2.8–10.9 |
7.3 |
2.6–10.3 |
NR |
| Histidine (His, H) |
0.5–2.8 |
0.2–3.4 |
0.4–3.4 |
0.7–3.6 |
0.7–3.1 |
2.8–3.5 |
0.9–2.7 |
4.0 |
| Non-essential AA |
|
|
|
|
|
|
|
|
| Alanine (Ala, A) |
0.8–4.8 |
0.3–4.8 |
2.0–4.2 |
3.5–4.4 |
0.9–2.8 |
3.7–4.3 |
1.2–4.2 |
4.5 |
| Aspartic acid (Asp, D) |
2.1–11.9 |
0.6–11.4 |
1.1–11.3 |
8.4–13.5 |
2.8–8.4 |
7.8–11.9 |
3.1 |
8.2 |
| Cystine (Cys, C) |
0.4–1.6 |
1.3–2.3 |
0.0–1.0 |
0.8–1.8 |
0.3–0.6 |
1.0–1.8 |
0.4–1.9 |
2.2 |
| Glutamic acid (Glu, E) |
2.9–18.5 |
1.7–17.3 |
2.4–15.1 |
6.1–21.7 |
6.2–26.1 |
6.0–18.5 |
4.6–13.0 |
6.2 |
| Glycine (Gly, G) |
0.8–4.8 |
0.3–4.1 |
1.0–4.8 |
4.1–4.26 |
1.0–3.7 |
4.1–4.2 |
1.2–4.2 |
4.6 |
| Proline (Pro, P) |
0.8–4.6 |
0.2–4.6 |
0.9–3.8 |
2.8–4.2 |
1.1–4.3 |
2.8–3.6 |
1.2–3.9 |
3.0 |
| Serine (Ser, S) |
0.8–5.7 |
0.1–4.9 |
1.1–4.9 |
2.5–5.0 |
1.3–6.0 |
2.6–5.6 |
1.3 |
2.7 |
| Tyrosine (Tyr, Y) |
0.6–3.8 |
0.2–3.7 |
0.5–3.2 |
3.3–3.4 |
1.0–4.3 |
3.2–5.0 |
0.9 |
3.2 |
3.2. Protein Fractions and Structures
Based on solubility in water, saline, dilute acid or alkali, and alcohol, pulse proteins are empirically divided into four primary fractions known as albumin, globulin, glutelin, and prolamin, respectively
[20][21]. The reported range of primary protein compositions is given in
Table 2 [15][18][22][23][24][25][26]. Pulse protein isolates are predominantly constituted by globulin and albumin at approximately 50–80% and 10–20% of total storage proteins, respectively, where glutelin (10%) and prolamin (less than 5%) are minor constituents. These subunit compositions vary considerably in structures and functions
[27][28].
Table 2. Osborne protein composition of pulse proteins (g/100 g dry matter).
| |
Albumin |
Globulin |
Glutelins |
Prolamins |
| Pea [21][24] |
18–25 |
55–65 |
3–4 |
4–5 |
| Chickpea [23] |
8–12 |
53–60 |
19–25 |
3–7 |
| Lentil [18][25] |
16–17 |
51–70 |
11 |
3–4 |
| Mung bean [18][22] |
16.3 |
62 |
13.3 |
0.9 |
| Faba bean [26] |
18.4–21.9 |
61.6–68 |
10.2–12.2 |
3.4–4.3 |
| Cowpea [15] |
4–12 |
58–80 |
10–15 |
1–3 |
| Lupin [15] |
9–22 |
44–60 |
6–23 a |