
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jianan Xian | -- | 2170 | 2024-03-07 01:16:03 | | | |
| 2 | Wendy Huang | Meta information modification | 2170 | 2024-03-07 09:00:40 | | |
Video Upload Options
Potassium diformate (KDF) is an organic acid salt. It is a dimer formed through hydrogen bonding between one molecule of formic acid and one molecule of potassium formate. The chemical formula of KDF is HCOOH·HCOOK, with a molecular weight of 130.14. It is a white or slightly yellow crystalline powder with no discernible pungent odor. KDF dissolves in water and exhibits a pronounced hygroscopic nature. Its aqueous solution is acidic and remains stable under acidic conditions, while it decomposes into formate and formic acid under neutral or slightly alkaline conditions. Compared with formic acid, KDF overcomes the irritability, corrosiveness, and instability of formic acid. Therefore, KDF is a more suitable additive in feed, providing a safer and more stable solution in maintaining the balance of microbial communities in aquatic animals.
1. Introduction
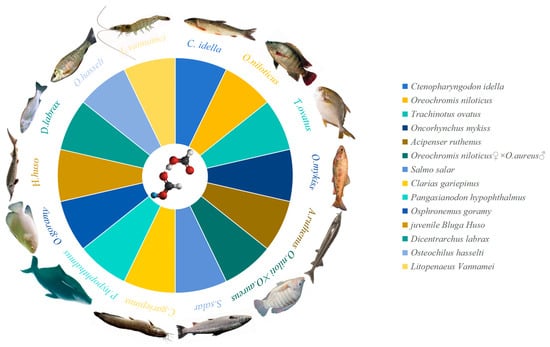
2. Enhancing Growth Performance and Feed Efficiency
3. Improving Disease Resistance
4. Modulation of the Gut Microbiota
5. Reducing Intestinal pH Levels
References
- Gatlin, D.M., III; Yamamoto, F.Y. Nutritional supplements and fish health. In Fish Nutrition; Elsevier: Amsterdam, The Netherlands, 2022; pp. 745–773.
- Nugraha, A.A.; Yustiati, A.; Bangkit, I.; Andriani, Y. Growth performance and survival rate of giant gourami fingerlings (Osphronemus goramy Lacepede, 1801) with potassium diformate addition. World Sci. News 2020, 143, 103–114.
- Shah, S.Z.H.; Afzal, M.; Khan, S.Y.; Hussain, S.M.; Habib, R.Z. Prospects of using citric acid as fish feed supplement. Int. J. Agric. Biol. 2015, 17, 1–8.
- Sayah, A.B.; Mohammadian, T.; Mesbah, M.; Jalali, S.M.; Tabandeh, M.R. The effects of different levels of potassium diformate and calcium diformate on growth, digestion, antioxidant capacity, intestinal flora, stress markers, and some serum biochemical analytes in juvenile Bluga Huso huso. Res. Sq. 2023. preprint.
- Safari, O.; Sarkheil, M.; Shahsavani, D.; Paolucci, M. Effects of single or combined administration of dietary synbiotic and sodium propionate on humoral immunity and oxidative defense, digestive enzymes and growth performances of African cichlid (Labidochromis lividus) challenged with Aeromonas hydrophila. Fishes 2021, 6, 63.
- Castillo, S.; Rosales, M.; Pohlenz, C.; Gatlin, D.M. Effects of organic acids on growth performance and digestive enzyme activities of juvenile red drum Sciaenops ocellatus. Aquaculture 2014, 433, 6–12.
- Demas, G.E.; Zysling, D.A.; Beechler, B.R.; Muehlenbein, M.P.; French, S.S. Beyond phytohaemagglutinin: Assessing vertebrate immune function across ecological contexts. J. Anim. Ecol. 2011, 80, 710–730.
- Monalisa, S.S.; Rozik, M.; Pratasik, S.B. Effectivity of Arcangelisia flava as immunostimulant to prevent streptococcosis on Nile tilapia, Oreochromis niloticus. Aquac. Aquar. Conserv. Legis. 2018, 11, 1834–1843.
- Neumann, N.F.; Stafford, J.L.; Barreda, D.; Ainsworth, A.; Belosevic, M. Antimicrobial mechanisms of fish phagocytes and their role in host defense. Dev. Comp. Immunol. 2001, 25, 807–825.
- Vadstein, O. The use of immunostimulation in marine larviculture: Possibilities and challenges. Aquaculture 1997, 155, 401–417.
- Suryadi, I.B.B.; Ulfa, D.N.; Yustiati, A.; Rosidah, R. The Effect of Potassium Diformate as Feed Additive on Immune Performances of Nilem (Osteochilus hasselti Valenciennes, 1842) Under Infection of Aeromonas hydrophila. Omni-Akuatika 2020, 16, 11–23.
- Wassef, E.A.; Saleh, N.E.; Abdel-Latif, H.M. Beneficial effects of some selected feed additives for European seabass (Dicentrarchus labrax L.): A review. Int. Aquat. Res. 2023, 15, 271–288.
- Lantu, S. Osmoregulasi pada hewan akuatik. J. Perikan. Dan Kelaut. Trop. 2010, 6, 46–50.
- Awan, F.; Dong, Y.; Wang, N.; Liu, J.; Ma, K.; Liu, Y. The fight for invincibility: Environmental stress response mechanisms and Aeromonas hydrophila. Microb. Pathog. 2018, 116, 135–145.
- Soliman, M.K.; Easa, M.E.S.; Faisal, M.; Abou-Elazm, I.M.; Hetrick, F.M. Motile aeromonas infection of striped (grey) mullet Mugil cephalus. Antonie Van Leeuwenhoek 1989, 56, 323–335.
- Kamphues, J.; Visscher, C.; Mößeler, A.; Häbich, A.; Wolf, P. 16.1 EFFORTS TO REDUCE THE AMOUNTS OF ANTIBIOTICS USED IN LIVESTOCK, FOCUSED ON YOUNG FOOD PRODUCING ANIMALS (PIGS/POULTRY). In Production Diseases in Farm Animals; Zentrum GmbH: Leipzig, Germany, 2007.
- Thomson, J.R.; Friendship, R.M. Digestive system. In Diseases of Swine; Wiley Online Library: Hoboken, NJ, USA, 2019; pp. 234–263.
- Mangunwardoyo, W.; Ratih, I.; Etty, R. Pathogenicity and virulency of Aeromonas hydrophila stainer on nila fish (Oreochromis niloticus Lin.) using koch postulate. J. Ris. Akuakultur 2010, 5, 245–255.
- Pandey, A. Screening, Detection and Characterization of Bacterial Fish Pathogens in Coastal Region of Goa. Doctoral Dissertation, Goa University, Panaji, India, 2011.
- Kumar, R.; Pande, V.; Singh, L.; Sharma, L.; Saxena, N.; Thakuria, D.; Singh, A.K.; Sahoo, P.K. Pathological findings of experimental Aeromonas hydrophila infection in golden mahseer (Tor putitora). Fish. Aquac. J. 2016, 7, 2150–3508.
- El Deen, A.N.; Dorgham-Sohad, M.; Hassan-Azza, H.M.; Hakim, A.S. Studies on Aeromonas hydrophila in cultured Oreochromis niloticus at Kafr El Sheikh Governorate, Egypt with reference to histopathological alterations in some vital organs. World J. Fish Mar. Sci. 2014, 6, 233–240.
- Maroccolo, S. Inclusion of a Species-Specific Probiotic or Calcium Diformate in Young Calves Diets: Effects on Gut Microbial Balance, Health Status and Growth Performance. Ph.D. Thesis, University of Milan, Milano, Italy, 2013.
- da Silva, B.C.; do Nascimento Vieira, F.; Mouriño, J.L.P.; Ferreira, G.S.; Seiffert, W.Q. Salts of organic acids selection by multiple characteristics for marine shrimp nutrition. Aquaculture 2013, 384, 104–110.
- Ajingi, Y.S.; Ruengvisesh, S.; Khunrae, P.; Rattanarojpong, T.; Jongruja, N. The combined effect of formic acid and Nisin on potato spoilage. Biocatal. Agric. Biotechnol. 2020, 24, 101523.
- Ariño, J.; Ramos, J.; Sychrova, H. Monovalent cation transporters at the plasma membrane in yeasts. Yeast 2018, 36, 177–193.
- Mulcahy, M.F. Serum protein changes associated with ulcerative dermal necrosis (UDN) in the trout Salmo trutta L. J. Fish Biol. 1971, 3, 199–201.
- da Silva, B.C.; Vieira, F.D.N.; Mouriño, J.L.P.; Bolivar, N.; Seiffert, W.Q. Butyrate and propionate improve the growth performance of Litopenaeus vannamei. Aquac. Res. 2016, 47, 612–623.
- Adams, D.; Boopathy, R. Use of formic acid to control vibriosis in shrimp aquaculture. Biologia 2013, 68, 1017–1021.
- Elala, N.M.A.; Ragaa, N.M. Eubiotic effect of a dietary acidifier (potassium diformate) on the health status of cultured Oreochromis niloticus. J. Adv. Res. 2015, 6, 621–629.
- Zhou, Z.; Liu, Y.; He, S.; Shi, P.; Gao, X.; Yao, B.; Ringø, E. Effects of dietary potassium diformate (KDF) on growth performance, feed conversion and intestinal bacterial community of hybrid tilapia (Oreochromis niloticus♀× O. aureus♂). Aquaculture 2009, 291, 89–94.
- Sivakumar, M.; Amirtharaj, K.V.; Chrisolite, B.; Sivasankar, P.; Subash, P. Dietary organic acids on growth, immune response, hepatopancreatic histopathology and disease resistance in Pacific white shrimp, Penaeus vannamei against Vibrio harveyi. Res. Sq. 2022. preprint.
- Ng, W.K.; Koh, C.B.; Sudesh, K.; Siti-Zahrah, A. Effects of dietary organic acids on growth, nutrient digestibility and gut microflora of red hybrid tilapia, Oreochromis sp., and subsequent survival during a challenge test with Streptococcus agalactiae. Aquac. Res. 2009, 40, 1490–1500.
- Yustiati, A.; Aminah, S.; Lili, W.; Andriani, Y.; Bioshina, I.B. Effect of using potassium diformate as a feed additive to growth rate and feed efficiency of Nirwana tilapia (Oreochromis niloticus). J. GSD 2019, 7, 738–750.
- Siqwepu, O.; Salie, K.; Goosen, N. Evaluation of potassium diformate and potassium chloride in the diet of the African catfish, Clarias gariepinus in a recirculating aquaculture system. Aquaculture 2020, 526, 735414.
- Sun, Y.; Yu, P.; Cheng, Y.; Liu, J.; Chen, X.; Zhang, T.; Gao, T.; Zhou, R.; Li, L. The feed additive potassium diformate prevents Salmonella enterica Serovar Pullorum infection and affects intestinal flora in chickens. Antibiotics 2022, 11, 1265.
- Lückstädt, C. Use of organic acids as feed additives—Sustainable aquaculture production the non-antibiotic way. Int Aquafeed 2006, 9, 21–26.
- Lim, C.; Lückstädt, C.; Webster, C.D.; Kesius, P. Organic acids and their salts. In Dietary Nutrients, Additives, and Fish Health; Wiley Online Library: Hoboken, NJ, USA, 2015; pp. 305–319.




