
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Pasquale Longo | -- | 2692 | 2024-03-06 17:16:47 | | | |
| 2 | Catherine Yang | -41 word(s) | 2651 | 2024-03-07 02:00:21 | | |
Video Upload Options
The two Ru(III) and Ru(II) complexes, namely, BOLD-100 and RAPTA-C, are presently being studied in a clinical trial and preclinical studies evaluation, respectively, as anticancer agents. Ruthenium N-heterocyclic carbene (Ru-NHC) complexes show interesting properties in medicinal chemistry, exhibiting multiple biological activities, among them anticancer, antimicrobial, antioxidant, and anti-inflammatory. Among the newly synthesized complexes, RANHC-V and RANHC-VI are the most active against triple-negative human breast cancer cell lines MDA-MB-231. These compounds were selective in vitro inhibitors of the human topoisomerase I activity and triggered cell death by apoptosis. Furthermore, the Ru-NHC complexes’ antimicrobial activity was studied against Gram-positive and -negative bacteria, revealing that all the complexes possessed the best antibacterial activity against the Gram-positive Staphylococcus aureus, at a concentration of 0,025 mg/mL. Finally, the antioxidant effect was assessed by DPPH and ABTS radicals scavenging assays, resulting in a higher ability for inhibiting the ABTS+, with respect to the well-known antioxidant Trolox. Thus, this work provides encouraging insights for further development of novel Ru-NHC complexes as potent chemotherapeutic agents endowed with multiple biological properties.
1. Introduction
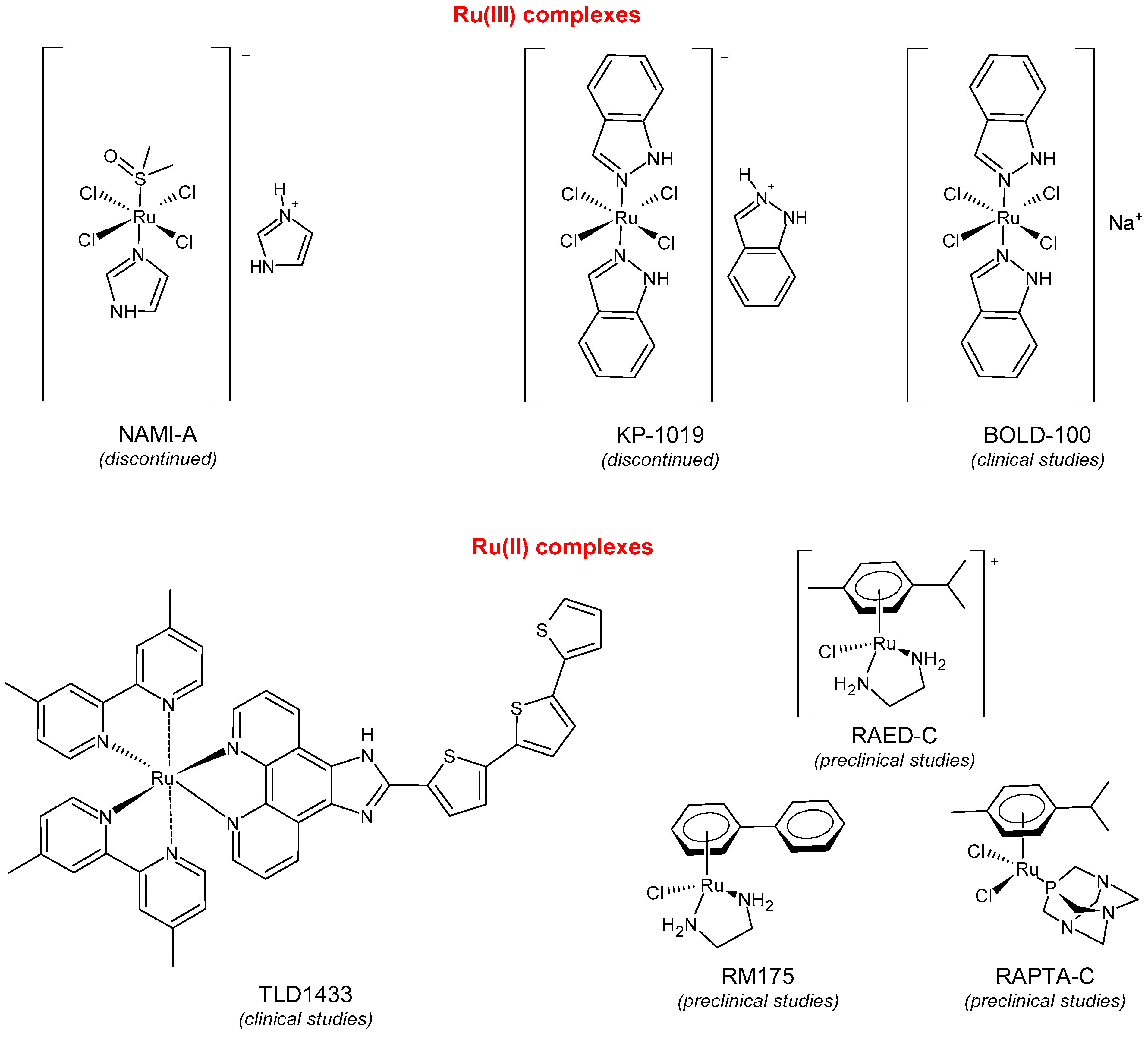
2. Ruthenium(II/III) Complexes in Clinic Trials and Advanced Preclinical Studies as Anticancer Agents
2.1. BOLD-100
2.2. TLD1433
2.3. RAPTA-C
3. Ruthenium Complexes Acting against Viruses
References
- Singh, V.K.; Singh, V.K.; Mishra, A.; Singh, A.A.; Prasad, G.; Singh, A.K. Recent advancements in coordination compounds and their potential clinical application in the management of diseases: An up-to-date review. Polyhedron 2023, 241, 116485.
- De, S.; Kazi, S.; Banerjee, S.; Banerjee, S.; Sarkar, N.; Shah, S.K.; Kuo, Y.-C.; Kumar, S.A. Metallotherapeutic complexes with high selective properties for anti-neoplastic therapy. Coord. Chem. Rev. 2024, 498, 215462.
- Gamberi, T.; Hanif, M. Metal-based complexes in cancer treatment. Biomedicines 2022, 10, 2573.
- Paprocka, R.; Wiese-Szadkowska, M.; Janciauskiene, S.; Kosmalski, T.; Kulik, M.; Helmin-Basa, A. Latest developments in metal complexes as anticancer agents. Coord. Chem. Rev. 2022, 452, 214307.
- Ceramella, J.; Mariconda, A.; Sirignano, M.; Iacopetta, D.; Rosano, C.; Catalano, A.; Saturnino, C.; Sinicropi, M.S.; Longo, P. Novel Au carbene complexes as promising multi-target agents in breast cancer treatment. Pharmaceuticals 2022, 15, 507.
- Prathima, T.S.; Choudhury, B.; Ahmad, M.G.; Chanda, K.; Balamurali, M.M. Recent developments on other platinum metal complexes as target-specific anticancer therapeutics. Coord. Chem. Rev. 2023, 490, 215231.
- Bruijnincx, P.C.; Sadler, P.J. New trends for metal complexes with anticancer activity. Curr. Opin. Chem. Biol. 2008, 12, 197–206.
- Todorov, L.; Kostova, I. Recent Trends in the development of novel metal-based antineoplastic drugs. Molecules 2023, 28, 1959.
- Esquezaro, P.G.; Manzano, C.M.; Nakahata, D.H.; ISantos, I.A.; Ruiz, U.E.; Santiago, M.B.; Silva, N.B.; Martins, C.H.; Pereira, D.H.; Bergamini, F.R.G.; et al. Synthesis, spectroscopic characterization and in vitro antibacterial and antiviral activities of novel silver(I) complexes with mafenide and ethyl-mafenide. J. Mol. Struct. 2021, 1246, 131261.
- El-Lateef, H.M.A.; El-Dabea, T.; Khalaf, M.M.; Abu-Dief, A.M. Recent overview of potent antioxidant activity of coordination compounds. Antioxidants 2023, 12, 213.
- Abate, C.; Carnamucio, F.; Giuffrè, O.; Foti, C. Metal-Based Compounds in Antiviral Therapy. Biomolecules 2022, 12, 933.
- Singh, A.K.; Kumar, A.; Singh, H.; Sonawane, P.; Pathak, P.; Grishina, M.; Yadav, J.P.; Verma, A.; Kumar, P. Metal Complexes in cancer treatment: Journey so far. Chem. Biodivers. 2023, 20, e202300061.
- Anthony, E.A.; Bolitho, E.M.; Bridgewater, R.J.; Carter, O.W.L.; Donnelly, J.M.; Imberti, C.; Lant, E.C.; Lermyte, F.; Needham, R.J.; Palau, M.; et al. Metallodrugs are unique: Opportunities and challenges of discovery and development. Chem. Sci. 2020, 11, 12888–12917.
- Iacopetta, D.; Ceramella, J.; Catalano, A.; Saturnino, C.; Pellegrino, M.; Mariconda, A.; Longo, P.; Sinicropi, M.S.; Aquaro, S. COVID-19 at a glance: An up-to-date overview on variants, drug design and therapies. Viruses 2022, 14, 573.
- Pal, M.; Musib, D.; Roy, M. Transition metal complexes as potential tools against SARS-CoV-2: An in silico approach. New J. Chem. 2021, 45, 1924.
- Cirri, D.; Pratesi, A.; Marzo, T.; Messori, L. Metallo therapeutics for COVID-19. Exploiting metal-based compounds for the discovery of new antiviral drugs. Expert Opin. Drug Discov. 2021, 16, 39–46.
- Karges, J.; Cohen, S.M. Metal complexes as antiviral agents for SARS-CoV-2. ChemBioChem 2021, 22, 2600–2607.
- Gopal, J.; Muthu, M.; Sivanesan, I. A Comprehensive survey on the expediated anti-COVID-19 options enabled by metal complexes—Tasks and trials. Molecules 2023, 28, 3354.
- Allardyce, C.S.; Dyson, P.J. Ruthenium in medicine: Current clinical uses and future prospects. Platin. Met. Rev. 2001, 45, 62–69.
- D’Amato, A.; Mariconda, A.; Longo, P. New insights into the catalytic activity of second generation Hoveyda–Grubbs complexes having phenyl substituents on the backbone. Inorganics 2023, 11, 244.
- Rajabi, S.; Rüttger, F.; Lücken, J.; Dechert, S.; John, M.; Meyer, F. Ruthenium Complexes of Rigid, Dianionic, Tetradentate N-Donor Ligands and their Potential as Catalysts for Water Oxidation. Eur. J. Inorg. Chem. 2023, 26, e202200597.
- Yang, F.; Zhou, P.; Huang, Z.; Liao, J.; Huang, G.; Liang, T.; Zhang, Z. Ruthenium(II)-catalyzed remote C–H sulfonylation of 2-pyridones. Org. Lett. 2023, 25, 5779–5783.
- Gobbo, A.; Ma, X.; Ciancaleoni, G.; Zacchini, S.; Biancalana, L.; Guelfi, M.; Pampaloni, G.; Nolan, S.P.; Marchetti, F. Ruthenium(II) tris-pyrazolylmethane complexes in transfer hydrogenation reactions. Eur. J. Inorg. Chem. 2023, 26, e202300078.
- Hafeez, J.; Bilal, M.; Rasool, N.; Hafeez, U.; Adnan Ali Shah, S.; Imran, S.; Amiruddin Zakaria, Z. Synthesis of ruthenium complexes and their catalytic applications: A review. Arab. J. Chem. 2022, 15, 104165.
- Donnici, C.L.; Araujo, M.H.; Stoianoff, M.A.R. Ruthenium complexes as antifungal agents. In Ruthenium Complexes; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; pp. 293–318.
- Munteanu, A.C.; Uivarosi, V. Ruthenium complexes in the fight against pathogenic microorganisms. An extensive review. Pharmaceutics 2021, 13, 874.
- Kostova, I. Ruthenium complexes as anticancer agents. Curr. Med. Chem. 2006, 13, 1085–1107.
- Shutkov, I.A.; Okulova, Y.N.; Mazur, D.M.; Melnichuk, N.A.; Babkov, D.A.; Sokolova, E.V.; Spasov, A.A.; Milaeva, E.R.; Nazarov, A.A. New organometallic Ru(II) compounds with lonidamine motif as antitumor agents. Pharmaceutics 2023, 15, 1366.
- Pete, S.; Roy, N.; Kar, B.; Paira, P. Construction of homo and heteronuclear Ru(II), Ir(III) and Re(I) complexes for target specific cancer therapy. Coord. Chem. Rev. 2022, 460, 214462.
- Ribeiro, G.H.; Costa, A.R.; de Souza, A.R.; da Silva, F.V.; Martins, F.T.; Plutin, A.M.; Batista, A.A. An overview on the anticancer activity of Ru(II)/acylthiourea complexes. Coord. Chem. Rev. 2023, 488, 215161.
- Rafols, L.; Josa, D.; Aguila, D.; Barrios, L.A.; Roubeau, O.; Cirera, J.; Soto-Cerrato, V.; Pérez-Tomás, R.; Martinez, M.; Grabulosa, A. Piano-stool ruthenium(II) complexes with delayed cytotoxic activity: Origin of the lag time. Inorg. Chem. 2021, 60, 7974–7990.
- Wang, Z.F.; Huang, X.Q.; Wu, R.C.; Xiao, Y.; Zhang, S.H. Antitumor studies evaluation of triphenylphosphine ruthenium complexes with 5, 7-dihalo-substituted-8-quinolinoline targeting mitophagy pathways. J. Inorg. Biochem. 2023, 248, 112361.
- Florio, D.; La Manna, S.; Annunziata, A.; Iacobucci, I.; Monaco, V.; Di Natale, C.; Mollo, V.; Ruffo, F.; Monti, M.; Marasco, D. Ruthenium complexes bearing glucosyl ligands are able to inhibit the amyloid aggregation of short histidine-peptides. Dalton Trans. 2023, 52, 8549.
- Honorato, J.; Oliveira, K.M.; Leite, C.M.; Colina-Vegas, L.; Nóbrega, J.A.; Castellano, E.E.; Ellena, J.; Correa, R.S.; Batista, A.A. “Half-sandwich”/Ru II anticancer complexes containing triphenylphosphine and p-substituted benzoic acids. J. Brazil. Chem. Soc. 2020, 31, 2237–2249.
- Srivastava, P.; Shukla, M.; Kaul, G.; Chopra, S.; Patra, A.K. Rationally designed curcumin based Ruthenium(II) antimicrobials effective against drug-resistant: Staphylococcus aureus. Dalton Trans. 2019, 48, 11822–11828.
- Catalano, A.; Mariconda, A.; Sinicropi, M.S.; Ceramella, J.; Iacopetta, D.; Saturnino, C.; Longo, P. Biological activities of ruthenium NHC complexes: An update. Antibiotics 2023, 12, 365.
- Hu, H.; Zhang, H.; Zhong, R.; Yang, Y.; Huang, C.; Chen, J.; Liang, L.; Chen, Y.; Liu, Y. Synthesis, RNA-sequence and evaluation of anticancer efficacy of ruthenium(II) polypyridyl complexes toward HepG2 cells. J. Inorg. Biochem. 2023, 244, 112230.
- Huang, C.; Zhang, H.; Yang, Y.; Liu, H.; Chen, J.; Wang, Y.; Liang, L.; Hu, H.; Liu, Y. Synthesis, characterization, molecular docking, RNA-sequence and anticancer efficacy evaluation in vitro of ruthenium(II) complexes on B16 cells. J. Inorg. Biochem. 2023, 247, 112329.
- Khan, R.A.; Alterary, S.S.; BinSharfan, I.I.; Alsaeedi, H.; AlFawaz, A.; Khan, M.S.; Jaafar, M.H.; Shi, Y.; Arman, H.D.; Alsalme, A. Piano-stool type (η6-p-cymene) ruthenium(II) thiazole-derived motifs complexes: Synthesis, crystal structures, DFT studies, molecular docking and in-vitro binding studies with HSA and cytotoxicity. Inorg. Chim. Acta 2022, 537, 120925.
- Iacopetta, D.; Ceramella, J.; Catalano, A.; Mariconda, A.; Giuzio, F.; Saturnino, C.; Longo, P.; Sinicropi, M.S. Metal Complexes with Schiff Bases as Antimicrobials and Catalysts. Inorganics 2023, 11, 320.
- Sinicropi, M.S.; Ceramella, J.; Iacopetta, D.; Catalano, A.; Mariconda, A.; Rosano, C.; Saturnino, C.; El-Kashef, H.; Longo, P. Metal complexes with Schiff bases: Data collection and recent studies on biological activities. Int. J. Mol. Sci. 2022, 23, 14840.
- Parveen, S. Recent advances in anticancer ruthenium Schiff base complexes. Appl. Organometal. Chem. 2020, 34, e5687.
- Međedović, M.; Mijatović, A.; Baošić, R.; Lazić, D.; Milanović, Ž.; Marković, Z.; Milovanović, J.; Arsenijević, D.; Stojanović, B.; Arsenijević, M. Synthesis, characterization, biomolecular interactions, molecular docking, and in vitro and in vivo anticancer activities of novel ruthenium(III) Schiff base complexes. J. Inorg. Biochem. 2023, 248, 112363.
- Sumithaa, C.; Ganeshpandian, M. Half-sandwich ruthenium arene complexes bearing clinically approved drugs as ligands: The importance of metal–drug synergism in metallodrug design. Mol. Pharm. 2023, 20, 1453–1479.
- Mahmud, K.M.; Niloy, M.S.; Shakil, M.S.; Islam, M.A. Ruthenium complexes: An alternative to platinum drugs in colorectal cancer treatment. Pharmaceutics 2021, 13, 1295.
- Popolin, C.P.; Cominetti, M.R. A review of ruthenium complexes activities on breast cancer cells. Mini-Rev. Med. Chem. 2017, 17, 1435–1441.
- Sun, Q.; Li, Y.; Shi, H.; Wang, Y.; Zhang, Q. Ruthenium complexes as promising candidates against lung cancer. Molecules 2021, 26, 4389.
- Paulus, L.; Gallardo-Villagrán, M.; Carrion, C.; Ouk, C.; Martin, F.; Therrien, B.; Léger, D.Y.; Liagre, B. The effect of photosensitizer metalation incorporated into arene–ruthenium assemblies on prostate cancer. Int. J. Mol. Sci. 2023, 24, 13614.
- Thota, S.; Rodrigues, D.A.; Crans, D.C.; Barreiro, E.J. Ru(II) compounds: Next-generation anticancer metallotherapeutics? J. Med. Chem. 2018, 61, 5805–5821.
- Dyson, P.J.; Sava, G. Metal-Based Antitumour Drugs in the Post Genomic Era. Dalton Trans. 2006, 16, 1929–1933.
- Hong, W.X.; Huang, F.; Huan, T.; Xu, X.; Han, Q.; Wang, G.; Xu, H.; Duan, S.; Duan, Y.; Long, X.; et al. Comparative studies on DNA-binding and in vitro antitumor activity of enantiomeric ruthenium(II) complexes. J. Inorg. Biochem. 2018, 180, 54–60.
- Sonkar, C.; Sarkar, S.; Mukhopadhyay, S. Ruthenium (II)–arene complexes as anti-metastatic agents, and related techniques. RSC Med. Chem. 2022, 13, 22–38.
- Abid, M.; Shamsi, F.; Azam, A. Ruthenium complexes: An emerging ground to the development of metallopharmaceuticals for cancer therapy. Mini Rev. Med. Chem. 2016, 16, 772–786.
- Kanaoujiya, R.; Singh, M.; Singh, J.; Srivastava, S. Ruthenium based anticancer compounds and their importance. J. Sci. Res. 2020, 64, 264–268.
- Silva, M.J.S.A.; Vinck, R.; Wang, Y.; Saubaméa, B.; Tharaud, M.; Dominguez-Jurado, E.; Karges, J.; Gois, P.M.P.; Gasser, G. Towards selective delivery of a ruthenium(II) polypyridyl complex-containing bombesin conjugate into cancer cells. ChemBioChem 2023, 24, e202200647.
- Kundu, B.K.; Mukhopadhyay, S. Target based chemotherapeutic advancement of ruthenium complexes. Coord. Chem. Rev. 2021, 448, 214169.
- Yang, G.G.; Su, X.X.; Liang, B.B.; Pan, Z.Y.; Cao, Q.; Mao, Z.W. A platinum–ruthenium hybrid prodrug with multi-enzymatic activities for chemo-catalytic therapy of hypoxic tumors. Chem. Sci. 2022, 13, 11360–11367.
- Juszczak, M.; Kluska, M.; Kosińska, A.; Rudolf, B.; Woźniak, K. Antioxidant activity of ruthenium cyclopentadienyl complexes bearing succinimidato and phthalimidato ligands. Molecules 2022, 27, 2803.
- Małecka, M.; Skoczyńska, A.; Goodman, D.M.; Hartinger, C.G.; Budzisz, E. Biological properties of ruthenium (II)/(III) complexes with flavonoids as ligands. Coord. Chem. Rev. 2021, 436, 213849.
- Allardyce, C.S.; Dyson, P.J.; Ellis, D.J.; Salter, P.A.; Scopelliti, R. Synthesis and characterisation of some water soluble ruthenium(II)–arene complexes and an investigation of their antibiotic and antiviral properties. J. Organomet. Chem. 2003, 668, 35–42.
- de Oliveira, D.M.; Santos, I.D.A.; Martins, D.O.S.; Gonçalves, Y.G.; Cardoso-Sousa, L.; Sabino-Silva, R.; Von Poelhsitz, G.; Franca, E.D.F.; Nicolau-Junior, N.; Pacca, C.C.; et al. Organometallic complex strongly impairs Chikungunya virus entry to the host cells. Front. Microbiol. 2020, 11, 608924.
- Wu, C.Y.; Chen, H.J.; Wu, Y.C.; Tsai, S.W.; Liu, Y.H.; Bhattacharya, U.; Lin, D.; Tai, H.C.; Kong, K.V. Highly efficient singlet oxygen generation by BODIPY–ruthenium(II) complexes for promoting neurite outgrowth and suppressing Tau Protein aggregation. Inorg. Chem. 2023, 62, 1102–1112.
- Yawson, G.K.; Will, M.F.; Huffman, S.E.; Strandquist, E.T.; Bothwell, P.J.; Oliver, E.B.; Apuzzo, C.F.; Platt, D.C.; Weitzel, C.S.; Jones, M.A.; et al. A dual-pronged approach: A ruthenium(III) complex that modulates amyloid-β aggregation and disrupts its formed aggregates. Inorg. Chem. 2022, 61, 2733–2744.
- Guo, L.; Li, P.; Li, J.; Gong, Y.; Li, X.; Liu, Y.; Yu, K.; Liu, Z. Half-sandwich iridium(III), rhodium(III), and ruthenium(II) complexes chelating hybrid sp2-N/sp3-N donor ligands to achieve improved anticancer selectivity. Inorg. Chem. 2023, 62, 15118–15137.
- Sadique, S.; Baqer, A.A.; Salman, A.W.; Iqbal, M.A.; Kadim, M.M.; Jamil, F.; Majeed, A.; Manahil, S.; Altaf, A. Ruthenium complexes for breast cancer therapy. Rev. Inorg. Chem. 2023, in press.
- Skoczynska, A.; Lewinski, A.; Pokora, M.; Paneth, P.; Budzisz, E. An overview of the potential medicinal and pharmaceutical properties of Ru (II)/(III) complexes. Int. J. Mol. Sci. 2023, 24, 9512.
- Li, W.; Li, S.; Xu, G.; Man, X.; Yang, T.; Zhang, Z.; Liang, H.; Yang, F. Developing a ruthenium(III) complex to trigger gasdermin E-mediated pyroptosis and an immune response based on decitabine and liposomes: Targeting inhibition of gastric tumor growth and metastasis. J. Med. Chem. 2023, 66, 13072–13085.
- Lee, S.Y.; Kim, C.Y.; Nam, T.G. Ruthenium complexes as anticancer agents: A brief history and perspectives. Drug. Des. Dev. Ther. 2020, 14, 5375–5392.
- Kenny, R.G.; Marmion, C.J. Toward multi-targeted platinum and ruthenium drugs—A new paradigm in cancer drug treatment regimens? Chem. Rev. 2019, 119, 1058–1137.
- Swaminathan, S.; Deepak, R.J.; Karvembu, R. Interweaving catalysis and cancer using Ru-and Os-arene complexes to alter cellular redox state: A structure-activity relationship (SAR) review. Coord. Chem. Rev. 2023, 491, 215230.
- Borutzki, Y.; Skos, L.; Gerner, C.; Meier-Menches, S.M. Exploring the potential of metal-based candidate drugs as modulators of the cytoskeleton. ChemBioChem 2023, 24, e202300178.
- Toupin, N.; Herroon, M.K.; Thummel, R.P.; Turro, C.; Podgorski, I.; Gibson, H.; Kodanko, J.J. Metalloimmunotherapy with rhodium and ruthenium complexes: Targeting tumor-associated macrophages. Chem. Eur. J. 2022, 28, e202104430.
- Kanaoujiya, R.; Srivastava, S.; Singh, R.; Mustafa, G. Recent advances and application of ruthenium complexes in tumor malignancy. Mater. Today Proc. 2023, 72, 2822–2827.
- Bijelic, A.; Theiner, S.; Keppler, B.K.; Rompel, A. X-ray structure analysis of indazolium trans- (KP1019) bound to human serum albumin reveals two ruthenium binding sites and provides insights into the drug binding mechanism. J. Med. Chem. 2016, 59, 5894–5903.
- Neuditschko, B.; Legin, A.A.; Baier, D.; Schintlmeister, A.; Reipert, S.; Wagner, M.; Keppler, B.K.; Berger, W.; Meier-Menches, S.M.; Gerner, C. Interaction with ribosomal proteins accompanies stress induction of the anticancer metallodrug BOLD-100/KP1339 in the endoplasmic reticulum. Angew. Chem. Int. Ed. Engl. 2021, 60, 5063–5068.
- Alessio, E.; Messori, L. NAMI-A and KP1019/1339, two iconic ruthenium anticancer drug candidates face-to-face: A case story in medicinal inorganic chemistry. Molecules 2019, 24, 1995.
- Hinton, S.R.; Corpuz, E.L.; Holman, K.L.M.; Meyer, S.C. A split β-lactamase sensor for the detection of DNA modification by cisplatin and ruthenium-based chemotherapeutic drugs. J. Inorg. Biochem. 2022, 236, 111986.
- Rahman, K.M.M.; Giram, P.; Foster, B.A.; You, Y. Photodynamic therapy for bladder cancers, a focused review. Photochem. Photobiol. 2023, 99, 420–436.
- Murray, B.S.; Babak, M.V.; Hartinger, C.G.; Dyson, P.J. The Development of RAPTA Compounds for the Treatment of Tumors. Coord. Chem. Rev. 2016, 306, 86–114.
- Casini, A.; Gabbiani, C.; Sorrentino, F.; Rigobello, M.P.; Bindoli, A.; Geldbach, T.J.; Marrone, A.; Re, N.; Hartinger, C.G.; Dyson, P.J.; et al. Emerging Protein Targets For Anticancer Metallodrugs: Inhibition of thioredoxin reductase and cathepsin B by antitumor ruthenium(II)−arene compounds. J. Med. Chem. 2008, 51, 6773–6781.
- Aird, R.E.; Cummings, J.; Ritchie, A.A.; Muir, M.; Morris, R.E.; Chen, H.; Sadler, P.J.; Jodrell, D.I. In vitro and in vivo activity and cross resistance profiles of novel ruthenium (II) organometallic arene complexes in human ovarian cancer. Br. J. Cancer 2002, 86, 1652–1657.
- Romero-Canelon, I.; Sadler, P.J. Next-generation metal anticancer complexes: Multitargeting via redox modulation. Inorg. Chem. 2013, 52, 12276–12291.
- Scolaro, C.; Bergamo, A.; Brescacin, L.; Delfino, R.; Cocchietto, M.; Laurenczy, G.; Geldbach, T.J.; Sava, G.; Dyson, P.J. In vitro and in vivo evaluation of ruthenium(II)-arene PTA complexes. J. Med. Chem. 2005, 48, 4161–4171.
- Morris, R.E.; Aird, R.E.; Murdoch, P.D.; Chen, H.M.; Cummings, J.; Hughes, N.D.; Parsons, S.; Parkin, A.; Boyd, G.; Jodrell, D.I.; et al. Inhibition of cancer cell growth by ruthenium(II) arene complexes. J. Med. Chem. 2001, 44, 3616–3621.
- Habtemariam, A.; Melchart, M.; Fernandez, R.; Parsons, S.; Oswald, I.D.; Parkin, A.; Fabbiani, F.P.; Davidson, J.E.; Dawson, A.; Aird, R.E.; et al. Structure-activity relationships for cytotoxic ruthenium(II) arene complexes containing N,N-, N,O-, and O,O-chelating ligands. J. Med. Chem. 2006, 49, 6858–6868.
- Swaminathan, S.; Haribabu, J.; Balakrishnan, N.; Vasanthakumar, P.; Karvembu, R. Piano stool Ru(II)-arene complexes having three monodentate legs: A comprehensive review on their development as anticancer therapeutics over the past decade. Coord. Chem. Rev. 2022, 459, 214403.
- Hildebrandt, J.; Häfner, N.; Kritsch, D.; Görls, H.; Dürst, M.; Runnebaum, I.B.; Weigand, W. Highly cytotoxic osmium(II) compounds and their ruthenium(II) analogues targeting ovarian carcinoma cell lines and evading cisplatin resistance mechanisms. Int. J. Mol. Sci. 2022, 23, 4976.
- Lu, Y.; Zhu, D.; Le, Q.; Wang, Y.; Wang, W. Ruthenium-based antitumor drugs and delivery systems from monotherapy to combination therapy. Nanoscale 2022, 14, 16339–16375.
- Milović, E.; Janković, N.; Petronijević, J.; Joksimović, N.; Kosanić, M.; Stanojković, T.; Matić, I.; Grozdanić, N.; Klisurić, O.; Stefanović, S. Synthesis, characterization, and biological evaluation of tetrahydropyrimidines: Dual-activity and mechanism of action. Pharmaceutics 2022, 14, 2254.
- Xu, Y.; Wang, F.; Guo, H.; Wang, S.; Ni, S.; Zhou, Y.; Wang, Z.; Bao, H.; Wang, Y. Antitussive and anti-inflammatory dual-active agents developed from natural product lead compound 1-methylhydantoin. Molecules 2019, 24, 2355.
- Hegazy, G.E.; Abu-Serie, M.M.; Abo-Elela, G.M.; Ghozlan, H.; Sabry, S.A.; Soliman, N.A.; Abdel-Fattah, Y.R.R. In vitro dual (anticancer and antiviral) activity of the carotenoids produced by haloalkaliphilic archaeon Natrialba sp. M6. Sci. Rep. 2020, 10, 5986.
- Aldea, M.; Michot, J.-M.; Danlos, F.-X.; Ribas, A.; Soria, J.-C. Repurposing of anticancer drugs expands possibilities for antiviral and anti-inflammatory discovery in COVID-19. Cancer Discov. 2021, 11, 1336–1344.
- Trondl, R.; Heffeter, P.; Kowol, C.R.; Jakupec, M.A.; Berger, W.; Keppler, B.K. NKP-1339, the first ruthenium-based anticancer drug on the edge to clinical application. Chem. Sci. 2014, 5, 2925–2932.
- Meier-Menches, S.M.; Gerner, C.; Berger, W.; Hartinger, C.G.; Keppler, B.K. Structure-activity relationships for ruthenium and osmium anticancer agents towards clinical development. Chem. Soc. Rev. 2018, 47, 909–928.
- Pötsch, I.; Baier, D.; Keppler, B.K.; Berger, W. Challenges and chances in the preclinical to clinical translation of anticancer metallodrugs. RSC Metallobiol. 2019, 14, 308–347.
- Burris, H.A.; Bakewell, S.; Bendell, J.C.; Infante, J.; Jones, S.F.; Spigel, D.R.; Weiss, G.J.; Ramanathan, R.K.; Ogden, A.; Von Hoff, D.; et al. Safety and activity of IT-139, a ruthenium-based compound, in patients with advanced solid tumours: A First-in-human, open-label, dose-escalation phase I study with expansion cohort. ESMO Open 2016, 1, e000154.
- Farkas, E.; Marmion, C.J. (Eds.) Targeted Metallo-Drugs: Design, Development, and Modes of Action; CRC Press: Boca Raton, FL, USA, 2023; ISBN 9781032223308.
- Spratlin, J.L.; O’Kane, G.; Goodwin, R.A.; McWhirter, E.; Thompson, D.; Halani, K.; Jones, M.; Snow, M.; McAllister, E.R.; Machado, A.; et al. BOLD-100-001 (TRIO039): A phase 1b dose-escalation study of BOLD-100 in combination with FOLFOX chemotherapy in patients with advanced gastrointestinal solid cancers: Interim safety, tolerability, and efficacy. J. Clin. Oncol. 2022, 40 (Suppl. S16), 3031.
- Spratlin, J.; O’Kane, G.; Oh, D.Y.; Rha, S.Y.; McWhirter, E.; Elimova, E.; Kavan, P.; Choi, M.K.; Kim, D.W.; Goodwin, R.; et al. Abstract CT149: BOLD-100-001 (TRIO039): A phase 1b/2a dose-escalation study of BOLD-100 in combination with FOLFOX chemotherapy in patients with pre-treated advanced colorectal cancer: Interim efficacy, safety and tolerability analysis. Cancer Res. 2023, 83 (Suppl. S8), CT149.
- Park, B.J.; Raha, P.; Pankovich, J.; Bazett, M. Utilization of cancer cell line screening to elucidate the anticancer activity and biological pathways related to the ruthenium-based therapeutic BOLD-100. Cancers 2022, 15, 28.
- Labach, D.S.; Kohio, H.P.; Tse, E.A.; Paparisto, E.; Friesen, N.J.; Pankovich, J.; Bazett, M.; Barr, S.D. The metallodrug BOLD-100 is a potent inhibitor of SARS-CoV-2 replication and has broad-acting antiviral activity. Biomolecules 2023, 13, 1095.
- Bakewell, S.; Conde, I.; Fallah, Y.; McCoy, M.; Jin, L.; Shajahan-Haq, A.N. Inhibition of DNA repair pathways and induction of ROS are potential mechanisms of action of the small molecule inhibitor BOLD-100 in breast cancer. Cancers 2020, 12, 2647.
- Flocke, L.S.; Trondl, R.; Jakupec, M.A.; Keppler, B.K. Molecular mode of action of NKP-1339—A clinically investigated ruthenium-based drug—Involves ER- and ROS-related effects in colon carcinoma cell lines. Investig. New Drugs 2016, 34, 261–268.
- Schoenhacker-Alte, B.; Mohr, T.; Pirker, C.; Kryeziu, K.; Kuhn, P.S.; Buck, A.; Hofmann, T.; Gerner, C.; Hermann, G.; Koellensperger, G.; et al. Sensitivity towards the GRP78 inhibitor KP1339/IT-139 is characterized by apoptosis induction via caspase 8 upon disruption of ER homeostasis. Cancer Lett. 2017, 404, 79–88.
- Carlos, A.J.; Ha, D.P.; Yeh, D.W.; Van Krieken, R.; Tseng, C.C.; Zhang, P.; Gill, P.; Machida, K.; Lee, A.S. The chaperone GRP78 is a host auxiliary factor for SARS-CoV-2 and GRP78 depleting antibody blocks viral entry and infection. J. Biol. Chem. 2021, 296, 100759.
- Wernitznig, D.; Kiakos, K.; Del Favero, G.; Harrer, N.; Machat, H.; Osswald, A.; Jakupec, M.A.; Wernitznig, A.; Sommergruber, W.; Keppler, B.K. First-in-class ruthenium anticancer drug (KP1339/IT-139) induces an immunogenic cell death signature in colorectal spheroids in vitro. Metallomics 2019, 11, 1044–1048.
- Mucke, H.A. Patent highlights October–November 2021. Pharm. Pat. Anal. 2022, 11, 37–44.
- Ceramella, J.; Iacopetta, D.; Sinicropi, M.S.; Andreu, I.; Mariconda, A.; Saturnino, C.; Giuzio, F.; Longo, P.; Aquaro, S.; Catalano, A. Drugs for COVID-19: An update. Molecules 2022, 27, 8562.
- Lohitesh, K.; Chowdhury, R.; Mukherjee, S. Resistance a major hindrance to chemotherapy in hepatocellular carcinoma: An insight. Cancer Cell Int. 2018, 18, 44.
- Baier, D.; Schoenhacker-Alte, B.; Rusz, M.; Pirker, C.; Mohr, T.; Mendrina, T.; Kirchhofer, D.; Meier-Menches, S.M.; Hohenwallner, K.; Schaier, M.; et al. The anticancer ruthenium compound BOLD-100 targets glycolysis and generates a metabolic vulnerability towards glucose deprivation. Pharmaceutics 2022, 14, 238.
- Jang, M.; Kim, S.S.; Lee, J. Cancer cell metabolism: Implications for therapeutic targets. Exp. Mol. Med. 2013, 45, e45.
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218.
- Baier, D.; Mendrina, T.; Schoenhacker-Alte, B.; Pirker, C.; Mohr, T.; Rusz, M.; Regner, B.; Schaier, M.; Sgarioto, N.; Raynal, N.J.M.; et al. The lipid metabolism as target and modulator of BOLD-100 anticancer activity: Crosstalk with histone acetylation. Adv. Sci. 2023, 10, 2301939.
- Intravesical Photodynamic Therapy (PDT) in BCG Refractory/Intolerant Non-Muscle Invasive Bladder Cancer (NMIBC) Patients. Available online: https://clinicaltrials.gov/ct2/show/NCT03945162 (accessed on 11 October 2023).
- Kulkarni, G.; Richards, K.; Black, P.C.; Rendon, R.; Chin, J.; Shore, N.; Jayram, G.; Kramolowsky, E.; Saltzstein, D.; Agarwal, A.; et al. MP63-01 an interim analysis of a phase ii clinical study of intravesical photodynamic therapy in patients with bcg-unresponsive non-muscle invasive bladder cancer (NMIBC) carcinoma in-situ (CIS). J. Urol. 2023, 209 (Suppl. S4), e871.
- Chen, Q.; Ramu, V.; Aydar, Y.; Groenewoud, A.; Zhou, X.-Q.; Jager, M.J.; Cole, H.; Cameron, C.G.; McFarland, S.A.; Bonnet, S.; et al. TLD1433 photosensitizer inhibits conjunctival melanoma cells in zebrafish ectopic and orthotopic tumour models. Cancers 2020, 12, 587.
- Harada, Y.; Murayama, Y.; Takamatsu, T.; Otsuji, E.; Tanaka, H. 5-Aminolevulinic acid-induced protoporphyrin ix fluorescence imaging for tumor detection: Recent advances and challenges. Int. J. Mol. Sci. 2022, 23, 6478.
- Karges, J. Clinical development of metal complexes as photosensitizers for photodynamic therapy of cancer. Angew. Chem. Int. Ed. 2022, 61, e202112236.
- Swaminathan, S.; Karvembu, R. Dichloro Ru(II)-p-cymene-1,3,5-triaza-7-phosphaadamantane (RAPTA-C): A case study. ACS Pharm. Translat. Sci. 2023, 6, 982–996.
- Bashir, M.; Mantoo, I.A.; Arjmand, F.; Tabassum, S.; Yousuf, I. An overview of advancement of organoruthenium(II) complexes as prospective anticancer agents. Coord. Chem. Rev. 2023, 487, 215169.
- Rausch, M.; Dyson, P.J.; Nowak-Sliwinska, P. Recent considerations in the application of RAPTA-C for cancer treatment and perspectives for its combination with immunotherapies. Adv. Ther. 2019, 2, 1900042.
- Weiss, A.; Ding, X.; van Beijnum, J.R.; Wong, I.; Wong, T.J.; Berndsen, R.H.; Dormond, O.; Dallinga, M.; Shen, L.; Schlingemann, R.O.; et al. Rapid optimization of drug combinations for the optimal angiostatic treatment of cancer. Angiogenesis 2015, 18, 233–244.
- Coverdale, J.P.C.; Laroiya-McCarron, T.; Isolda Romero-Canelón, I. Designing ruthenium anticancer drugs: What have we learnt from the key drug candidates? Inorganics 2019, 7, 31.
- Weiss, A.; Berndsen, R.H.; Ding, X.; Ho, C.M.; Dyson, P.J.; Van Den Bergh, H.; Griffioen, A.W.; Nowak-Sliwinska, P. A streamlined search technology for identification of synergistic drug combinations. Sci. Rep. 2015, 5, 14508.
- Berndsen, R.H.; Weiss, A.; Abdul, U.K.; Wong, T.J.; Meraldi, P.; Griffioen, A.W.; Dyson, P.J.; Nowak-Sliwinska, P. Combination of ruthenium(II)-arene complex (RAPTA-C) and the epidermal growth factor receptor inhibitor erlotinib results in efficient angiostatic and antitumor activity. Sci. Rep. 2017, 7, 43005.
- Weiss, A.; Berndsen, R.H.; Dubois, M.; Müller, C.; Schibli, R.; Griffioen, A.W.; Dyson, P.J.; Nowak-Sliwinska, P. In vivo anti-tumor activity of the organometallic ruthenium(II)-arene complex (RAPTA-C) in human ovarian and colorectal carcinomas. Chem. Sci. 2014, 5, 4742–4748.
- Lu, M.; Wang, S.; Khine, Y.Y.; Hong, Y.; Zheng, J.; Lu, H.; Stenzel, M.H. Dual drug delivery system of RAPTA-C and paclitaxel based on fructose coated nanoparticles for metastatic cancer treatment. Biochem. Biophys. Res. Commun. 2023, 640, 134–141.
- Marzo, T.; Messori, L. A Role for metal-based drugs in fighting COVID-19 infection? The Case of Auranofin. ACS Med. Chem. Lett. 2020, 11, 1067–1068.
- De Paiva, R.E.F.; Marçal Neto, A.; Santos, I.A.; Jardim, A.C.G.; Corbi, P.P.; Bergamini, F.R.G. What is holding back the development of antiviral metallodrugs? A literature overview and implications for SARS-CoV-2 therapeutics and future viral outbreaks. Dalton Trans. 2020, 49, 16004–16033.
- Chuong, C.; DuChane, C.M.; Webb, E.M.; Rai, P.; Marano, J.M.; Bernier, C.M.; Merola, J.S.; Weger-Lucarelli, J. Noble metal organometallic complexes display antiviral activity against SARS-CoV-2. Viruses 2021, 13, 980.
- El-Lateef, H.M.A.; El-Dabea, T.; Khalaf, M.M.; Abu-Dief, A.M. Development of metal complexes for treatment of coronaviruses. Int. J. Mol. Sci. 2022, 23, 6418.
- Kojima, S.; Hasegawa, T.; Yonemura, T.; Sasaki, K.; Yamamoto, K.; Makimura, Y.; Takahashi, T.; Suzuki, T.; Suzuki, Y.; Kobayashi, K. Ruthenium complexes carrying a disialo complex-type oligosaccharide: Enzymatic synthesis and its application to a luminescent probe to detect influenza viruses. Chem. Commun. 2003, 11, 1250–1251.
- Wong, E.L.-M.; Sun, R.W.-Y.; Chung, N.P.-Y.; Lin, C.-L.S.; Zhu, N.; Che, C.-M. A mixed-valent ruthenium−oxo oxalato cluster Na7 with potent anti-HIV activities. J. Am. Chem. Soc. 2006, 128, 4938–4939.
- Gil-Moles, M.; Türck, S.; Basu, U.; Pettenuzzo, A.; Bhattacharya, S.; Rajan, A.; Ma, X.; Büssing, R.; Wölker, J.; Burmeister, H.; et al. Metallodrug profiling against SARS-CoV-2 target proteins identifies highly potent inhibitors of the S/ACE2 interaction and the Papain-like Protease PLpro. Chem. Eur. J. 2021, 27, 17928–17940.
- Janković, N.; Milović, E.; Jovanović, J.Đ.; Marković, Z.; Vraneš, M.; Stanojković, T.; Matić, I.; Crnogorac, M.Đ.; Klisurić, O.; Cvetinov, M. A new class of half-sandwich ruthenium complexes containing Biginelli hybrids: Anticancer and anti-SARS-CoV-2 activities. Chem. Biol. Interact. 2022, 363, 110025.




