1. Complications in MCS
1.1. Patient Selection and Challenges in MCS Therapy
Patient selection is a crucial factor in determining MCS suitability, risk of complications and patient outcome.
Three main patient categories are considered, including basal characteristics, comorbidities and cardiac conditions. Basal characteristics encompass an assessment of the patient’s age (<75) and overall physical health, with younger healthier patients generally having lower mortality rates. Comorbidities include factors which also negatively impact patient outcomes, such as a high BMI (>40), diabetes (with poor glycemic control), a cancer diagnosis with <1-year survival, irreversible liver dysfunction, kidney disease and renal dysfunction (with a glomerular filtration rate [GFR] of <30). Cardiac conditions are also examined for device suitability, including an assessment of the patient’s left and right ventricular size and function. The presence of valve disease is also considered, with severe mitral stenosis and mild aortic regurgitation classified as contraindications. A neurological and cognitive assessment will also be conducted, since moderate–severe cognitive impairment and dementia are contraindications for MCS
[1]. Together, these considerations enable healthcare providers to make informed decisions regarding patient suitability for device implantation, aiming to improve patient care. Although these categories are considered, the use of MCS therapy is not without some challenges.
While durable MCS devices offer numerous advantages, they can be associated with high levels of shear stress. Some shear stress is necessary to prevent coagulation, however, excessive levels of shear stress can lead to downstream blood damage
[2]. Despite careful patient selection, there are various complications linked to MCS devices, including stroke, pump thrombosis and GI bleeding. Typically, the risk for complications tends to be the highest in the early post-operative period and gradually reduces thereafter
[3]. Nevertheless, rates vary from device to device, suggesting that device design and flow patterns may be a factor in the development of these complications and could affect patient outcomes (
Table 1). In
Table 1 the researchers clarify distinctions between devices, especially those sharing the same flow types, highlighting that flow dynamics alone cannot fully explain the observed differences. Therefore, it is important to determine other differentiating factors, including device designs, to enable comparisons among all individual devices through generations. This approach allows for a comprehensive evaluation rather than grouping devices solely based on flow characteristics.
Table 1. Complication Rates in Patients with Implantable MCS Devices.
| Device Name |
Haemorrhagic Stroke |
Ischaemic Stroke |
Pump Thrombosis |
GI Bleeding |
References |
| Novacor |
9.0% |
15.0% |
N/A |
20.0% |
[4][5] |
| HeartMate (I) HVE |
8.0% |
7.0% |
0% |
6.5% |
[6][7] |
| CardioWest |
0.0–2.3% |
2.0–2.3% |
N/A |
4.0% |
[8][9] |
| Jarvik 2000 |
Overall stroke rate: 20.5% |
1.2% |
10.8–14% |
[10][11] |
| HeartMate II |
4.0–9.3% |
8.1–13.4% |
10.7–13.9% |
19–34.2% |
[2][12][13][14][15][16] |
| EVAHEART |
6.6–13.5% |
17.7–20.0% |
1.0% |
0.0% |
[17][18] |
| INCOR |
14.3% |
2.4% |
0.0% |
0.0% |
[19] |
| VentrAssist |
8% |
16% |
15% |
12% |
[20][21] |
| HVAD |
8–14.9% |
4.9–17.6% |
6.4–14% |
35.1 |
[12][22][23] |
| HeartMate 3 |
1.5–4.2% |
3.90–6.3% |
0–1.4% |
6.1–24.5% |
[2][13][24][25] |
1.2. Strokes
Strokes remain one of the leading causes of death in VAD patients
[2]. The types of strokes that affect MCS patients include ischaemic and haemorrhagic. An ischaemic stroke is caused by a blocked blood vessel that results in reduced oxygen to the brain tissue. Whereas a haemorrhagic stroke is caused either by a ruptured blood vessel in the brain or a haemorrhagic transformation following an ischaemic stroke
[27]. Numerous patient-specific and device-specific risk factors can increase the incidence of stroke. Patient-specific factors include patient lifestyle, demographics, co-morbidities and pre-existing conditions. Device-related risk factors include antithrombotic/anticoagulation regimes, thrombosis and incidence of non-surgical bleeding events
[28]. Strategies to reduce the risk of stroke include lifestyle modifications and administration of anticoagulants, such as warfarin. While anticoagulants reduce the risk of thromboembolic events, it is worth noting that increased anticoagulation can also increase the risk of GI bleeding. The incidence of stroke also increases following GI bleeds and is related to the reduced anticoagulation regime in response to the bleeding episode. This complex interplay highlights the challenges in managing the fine line between strokes and bleeding events
[29]. There are some limitations associated with traditional anticoagulants like warfarin, including frequent assessments of time spent in therapeutic range (TTR), as well as international normalized ratio (INR) measurements to maintain patients within a target range of 2.0–3.0. Deviations from this range could increase the risk of bleeding. As such, there is research into novel anticoagulants known as direct oral anticoagulants (DOACs), that inhibit thrombin (dabigatran) or Factor Xa (apixaban and rivaroxaban)
[30][31]. One multi-centre, phase II, randomised clinical trial (RE-ALIGN) was conducted to investigate the impact of dabigatran compared to warfarin in patients with mechanical heart valves. However, the trial was prematurely terminated due to the presence of increased thrombosis and bleeding events in patients prescribed with dabigatran. This study suggests that not all anticoagulants are suitable for MCS and highlights the possible dangers associated with the use of DOACs in device patients. As such, caution is urged with the use of DOACs in the future until more reliable data are available
[32]. Gender differences have been also observed in stroke rates after VAD implantation. For instance, female HMII patients showed increased stroke rates compared to male patients, despite similarities in blood pressure, INR and platelet counts
[27][33]. These discrepancies may be due to age, life span, genetics, hormonal (mainly oestrogen) and additional unknown factors that require research to provide a more complete understanding
[34]. Furthermore, there are differences in stroke rates between different devices. Jarvik 2000 patients experience stroke rates as high as 20.5%, compared with the lower stroke rates of 6.3% in HM3 patients after two years. This difference could be due to the axial flow used by the Jarvik 2000
[2][10][33]. However, not all patients with centrifugal flow devices have a low incidence of strokes, as observed with the HVAD (14.9–17.6%) (
Table 1)
[35]. Consequently, in 2021, the distribution of the HeartWare HVAD was terminated due to device issues and high levels of neurological adverse events that increased the risk of mortality among patients
[36]. In contrast to device design, an investigation was conducted into the influence of outflow cannula alignment on the risk of stroke among VAD patients. This study revealed that patients with a VAD outflow cannula to aortic angle of <37.5°, or a graft diameter of anastomosis of <1.5 cm, had a significantly higher incidence of stroke
[37]. As such, additional studies are required to investigate the optimal cannula alignment and the mechanisms involved in the development of strokes in VAD patients, to ultimately reduce both morbidity and mortality.
1.3. Pump Thrombosis
Pump thrombosis is characterised by the formation of a thrombus within the flow path of a device, which also includes the inflow cannula and outflow graft. When pump thrombosis occurs, it requires immediate intervention to prevent consequences such as device failure, further thromboembolic events and death (
Figure 1). If the initial thrombolytic treatment is unsuccessful, device replacement may be necessary to save a patient’s life. However, this occurrence can substantially decrease the overall cost-effectiveness of MCS therapy
[26]. As such, pump thrombosis is an adverse event that requires careful attention and the implementation of effective strategies to overcome. The exact cause of pump thrombosis remains unknown, although there are common factors to consider: the device, the patient and clinical management. Device factors include the type of flow, bearing and biomaterials used. Patient factors include sex, age and body mass index (BMI), whereas clinical factors include the management of anticoagulation/antithrombotic therapies, pump and cannula positioning and device operating speed. Pump thrombosis manifests clinically in MCS patients as an increase in pump power, with a reduction in pulsatility and blood pressure. Stagnant blood zones also pose a substantial risk that could increase the occurrence of pump thrombosis. Currently, there is no single biomarker that can be used to predict thrombosis with certainty. Platelets play a key role in this multifactorial process, however, changes in platelet receptor expression are insufficient for the identification of thrombosis. As such, ongoing research is underway to identify additional biomarkers for predicting thrombus formation in MCS patients
[38]. A multi-centre study, known as PREVENT (PREVENTtion of HeartMate II Pump Thrombosis Through Clinical Management), was conducted with 300 patients and aimed to reduce the occurrence of pump thrombosis. The signs and symptoms of pump thrombosis were classified as the presence of clinical hemolysis, worsening of heart failure and abnormal pump parameters. The study suggested that pump thrombosis can be reduced by following a protocol of structured device implantation, which includes the presence of an optimal pump pocket and adequate positioning of the pump, inflow and outflow cannulas. Afterwards, they recommend adequate anticoagulation/antithrombotic medications and optimal device speed. The results showed that these interventions are feasible and can successfully reduce the risk of pump thrombosis in many patients
[39]. Despite these findings being for HMII patients, they may be useful with additional devices. Furthermore, the MOMENTUM 3 trial also yielded valuable insights, demonstrating that pump thrombosis rates were significantly lower in the centrifugal HM3 device compared to its predecessor, the axial HMII (1.4% (
n = 7/516) versus 13.9% (
n = 70/512), respectively). However, these findings do not imply that axial flow is directly related to pump thrombosis. For instance, axial flow devices such as the Jarvik 2000 and Incor have similar rates of pump thrombosis to the HM3 (0–1.2%)
[8][19]. Furthermore, pump thrombosis rates range from 6.4–14% with the HVAD, despite its centrifugal flow (
Table 1)
[12][22][23]. The intricacies of device design and flow type are principal factors in the development of thrombosis. Consequently, tailoring strategies to manage pump thrombosis might also require device-specific approaches. Device evaluation is therefore required to ensure the safety and haemocompatibility of future devices, whilst acknowledging the interplay between the design and the associated risks. This suggests that one verified technique may not translate to patients with another device, highlighting the need for rigorous testing.
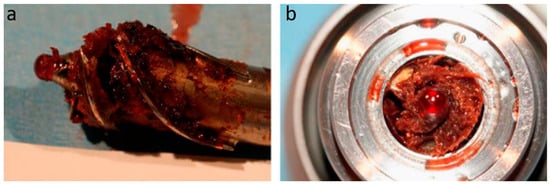
Figure 1. Pump Thrombosis. HeartMate II with pump thrombosis. (
a) The HeartMate II impeller with a red clot (blood only). (
b) Cross-section of the HeartMate II motor with pump thrombosis. Image obtained with permission and modified from Elsevier and Uriel et al., 2014
[40].
1.4. Strategies to Overcome Pump Thrombosis
To optimise the efficiency and cost-effectiveness of durable MCS device therapy, it is crucial to minimise the risk of thrombosis. While aspirin has demonstrated its effectiveness in reducing pump thrombosis, device design is a crucial factor to consider. In HVAD patients, 325 mg of aspirin was necessary to successfully reduce the occurrence of pump thrombosis and device malfunction
[23]. Several studies have also shown that a reduction or discontinuation of aspirin in HMII patients with bleeding complications increased the risk of thromboembolic events within this patient population
[41][42]. Conversely, HM3 patients have benefited from advancements in device design, leading to a near removal of pump thrombosis occurrence
[43]. Consequently, aspirin would have little effect on pump thrombosis in HM3 patients and may only increase the risk of bleeding complications. A small multicentre study involving HM3 patients has revealed promising results, whereby individuals who continued anticoagulation treatment, but discontinued aspirin treatment, experienced no pump thrombosis or thromboembolic events. However, out of the 23 patients who remained on aspirin, 9 encountered episodes of bleeding
[44]. Thus, aspirin may not be necessary for all VAD patients, emphasizing the need for personalised treatment strategies to balance the risk of thrombosis and bleeding-related complications. Achieving this balance requires the meticulous evaluation and vigilant monitoring of aspirin use, including considerations of dosage and duration. Moreover, the close monitoring of patients’ clinical status and observations for potential signs of GI bleeding are essential to optimise patient care. Ongoing research is crucial for an enhanced understanding of the impact of aspirin on patient outcomes. The recently completed Antiplatelet Removal and Hemocompatibility Events with the HeartMate 3 Pump (ARIES HM3) clinical trial marks an important milestone for understanding the role of aspirin, particularly in HM3 patients on a vitamin K antagonist. This double-blind, randomised and placebo-controlled study provides valuable insight into the clinical benefits of personalised treatment approaches for pump thrombosis and improved patient outcomes
[45][46]. The multi-centre trial revealed that patients who did not receive aspirin as part of their antithrombotic regimen had a significant reduction (34%) in bleeding, but no raised incidence of stroke or thromboembolic events
[47]. Therefore, omitting aspirin in HM3 patients with a vitamin K antagonist could be a safe and effective strategy, potentially reducing hospitalization rates and the cost of care due to reduced complications.
1.5. Gastrointestinal (GI) Bleeding
GI bleeding encompasses a range of clinical manifestations, including haematemesis (vomiting blood), haematochezia (blood in stools), bleeding identified during colonoscopy and/or a reduction in haemoglobin and HCT levels, often necessitating blood transfusion
[35]. Research indicates that up to 24.5% of HM3 patients still experience GI bleeding events
[13][48][49][50][51]. There appears to be a relationship between the loss of pulsatility and an increase in bleeding in MCS device patients. A retrospective analysis revealed that VAD patients with pulsatile devices (HeartMate XVE,
n = 109) had lower rates of GI bleeding after one year. While GI bleeding events were 6.5% and 21.8% for pulsatile and non-pulsatile, respectively
[6]. Moreover, further studies demonstrate that survival is higher in HM3 patients (74.7%) compared with patients with a pulsatile device (24%) after two years
[7][13]. However, variations in bleeding rates exist even between devices that utilise the same flow type. For instance, devices with centrifugal flow, such as the HVAD (35.1%), HM3 (24.5%) and VentrAssist (12%) exhibit varying rates of GI bleeding. Similarly, axial devices like the HMII (19–34.2%) and Jarvik 2000 (10.8–14%) also show differences in GI bleeding rates
[38][48]. Therefore, these discrepancies could suggest that device design plays a role in the development of GI bleeding. In contrast, there were no occurrences of GI bleeding with the EVAHEART (centrifugal) or INCOR (axial), although this could be due to small patient numbers
[12][13][17][18][19][20][21][52].
1.6. Risk Factors for Gastrointestinal Bleeding
The risk factors associated with increased GI bleeding are multifaceted and encompass various aspects. These factors include vWF degradation, platelet dysfunction, the presence of angiodysplasia and arteriovenous malformations (AVMs). Clinical factors that can increase the risk of GI bleeding include antithrombotic and anticoagulant medication, increased INR, a history of previous bleeding and device flow type
[53]. Patients who had experienced GI bleeding were also more likely to develop stroke compared to those who did not have any bleeding
[54]. Thus, further research can gain insights into the underlying mechanisms of GI bleeding and lead to the identification of strategies to mitigate this risk in MCS patients. Perioperative bleeding can also occur in MCS patients and is associated with higher mortality rates and an increased risk of re-bleeding episodes. High right atrial pressure was identified as a risk factor for perioperative bleeding, therefore, careful management before MCS device implantation could potentially reduce the risk of bleeding complications in these patients. This approach can improve patient outcomes and the overall success of device implantation
[55]. The integration of smart, real-time monitoring for relevant parameters further enhances the ability to detect and promptly respond to bleeding episodes. Rapid responses to GI bleeding events are vital for effective and timely management. Furthermore, several recent studies have used bio-banked samples from the PREVENT study to analyse pre- and post-operative serum from HMII patients. Patients with pre-operative elevations in angiopoietin-2 and TNF-α (tumour necrosis factor-alpha) were more likely to experience bleeding events following device implantation
[56]. In addition, increased thrombin formation post-implantation was found to be associated with increased bleeding episodes
[57]. As such, clinicians can use these findings to implement additional parameters to haematology testing to facilitate the identification of high-risk bleeders, paving the way for tailored patient management. This integration could enhance patient care and optimise outcomes for MCS device patients. Extensive research is essential to gain a comprehensive understanding of these factors and their impact on patient outcomes, especially before implantation, to prevent perioperative bleeding episodes. Implementing strategies for the prediction, prevention, early detection and management of GI bleeding at any stage is crucial. This includes routine blood tests, imaging techniques, endoscopic interventions or adjustments in device settings to mitigate the risk of GI bleeding.
Additionally, elevated mechanical shear stress levels have been shown to activate platelets, prompting cytoskeletal changes to increase the surface area for binding
[58][59]. Continued exposure to mechanical shear stress causes platelet damage and impairs platelet function
[60]. Clinical studies further support this by showing that platelet function is compromised in MCS patients, resulting in prolonged platelet plug formation and impaired platelet aggregation
[61][62]. In fact, patients with MCS devices display a 2.5-fold reduction in platelet function compared to healthy controls, increasing the risk of GI bleeding
[62]. However, platelet dysfunction alone is not a reliable predictor of GI bleeding. Moreover, MCS-induced shear stress can also lead to the degradation of vWF, a large glycoprotein involved in clotting. High levels of shear stress results in two mechanisms of vWF degradation: firstly, vWF unravelling and an increased enzymatic-induced cleavage at the vWF A2 domain by ADAMTS13, and secondly, the mechanical degradation of vWF multimers
[24]. If required, device flow adjustments can also be made to minimise the risk of shear stress-induced complications. Further mechanisms could be involved in the development of bleeding events, including the absence of pulsatility
[53]. Pulsatile flow has been linked to reduced vWF degradation compared to continuous flow, revealing that pulsatility modulates the level of vWF degradation and can induce physiological vWF release from endothelial cells
[63]. Continued vWF degradation in MCS patients may lead to the development of acquired von Willebrand syndrome (aVWS) and an increased risk of GI bleeding
[64]. Despite significant vWF degradation in MCS patients, approximately 30.9% of these patients experienced GI bleeding, indicating that vWF degradation alone does not adequately predict or explain the exact cause of GI bleeding
[13][53][65]. The most common cause of GI bleeding in CF-VAD patients is due to AVMs, accounting for 61% of cases. A low pulsatility index, indicative of reduced pulse pressure, significantly increases GI bleeding at AVM sites
[50]. This low pulse pressure may also lead to hypoperfusion of the GI lining and mucosal ischemia, leading to the formation of immature vessels that are prone to bleeding. Increased intraluminal pressure coupled with reduced pulse pressure can also lead to angiodysplasia, mucosal hypoxia and, ultimately, GI bleeding
[6]. The pulsatile nature of physiological blood flow governs nitric oxide (NO) release from endothelial cells. NO is responsible for increasing vessel dilation for the regulation of blood pressure and flow. Thus, a CF device could reduce NO release, leading to lower blood pressure and flow within the GI tract, which may increase susceptibility to GI bleeding due to the development of angiodysplasia/AVMs
[6][49][66]. The interplay between pulse pressure, endothelial cell function and NO release highlights the complex mechanisms underlying GI bleeding in CF-VAD patients. Therefore, the mechanisms that govern GI bleeding require regular management and monitoring, encompassing aspects such as INR, platelet dysfunction, vWF breakdown, NO levels and AVM formation. This approach could help to increase safety and improve patient outcomes in MCS therapy. The multifactorial impact of MCS on the risk of patient GI bleeding should be forefront of the aims of the next generation of novel devices, aiming to overcome the impacts of low pulsatility and high levels of shear stress. Altogether, this information can be utilised to develop comprehensive patient risk profiling systems. These systems can then integrate patient- and device-related factors, allowing for the personalisation of management plans and accounting for individual variations in response to MCS therapy, such as vital signs and haematology data. Establishing techniques to identify high-risk individuals and predict bleeding remains a challenge, but clinicians must ensure regular long-term training and open communication to enhance patient safety.
2. The Financial Impact of MCS Device Complications
The increasing adoption of MCS therapy brings with it a consequential rise in the number of patients experiencing complications, which can have significant economic implications. These complications can increase the incremental cost-effectiveness ratio (ICER) of MCS devices, raising concerns about their impact on healthcare costs and resource allocation. It is essential to recognise that the cost-effectiveness and hospitalisation rates associated with MCS devices can vary across different countries and healthcare settings. This variation highlights the importance of assessing the economic implications of these devices in each specific context to ensure equitable healthcare delivery. By understanding the economic impacts, healthcare services can make informed decisions regarding the allocation of resources and consider the potential financial implications associated with increased MCS therapy. Currently, GI bleeding events and driveline infections cost GBP 6899 and GBP 7662 in England but cost USD 9990 and USD 13,681 in the USA
[67]. As such, the use of MCS devices still poses a challenge in providing equitable healthcare. It is of the utmost ethical and moral importance for all researchers to play a significant role in eradicating complications and improving patient quality of life, particularly considering the growing prevalence of these devices. Earlier studies demonstrate that MCS implantation is not yet as cost-effective compared to heart transplantation, but a reduction in complication rates and improvements in device design can improve overall costs
[68]. A recent analysis has shown that NHS England (National Health Service) has a willingness-to-pay threshold of GBP 50,000 per quality age life-year (QALY) for MCS device implantation. The ICER of MCS devices as destination therapy was placed at GBP 43,207 per QALY, which is less than the NHS threshold. With improvements in device design, MCS as destination therapy appears to be a cost-effective therapy for the NHS in the U.K
[69]. The rapid evolution of the next generation of devices aims to reduce the costs and complications associated with MCS therapy and to improve patient survival and quality of life. However, potential challenges could impact their widespread implementation, such as regulatory considerations, reimbursement issues and patient acceptance. Nevertheless, given the current financial implications, novel devices should enhance the cost-effectiveness and efficacy of future therapies. This can be achieved through ongoing research into improved product design, advanced technologies and patient assessments.