Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Li, X.; Jin, Y.; Zhu, N.; Yin, J.; Jin, L.Y. Pillar[n]arene-Based Supramolecular Polymers. Encyclopedia. Available online: https://encyclopedia.pub/entry/55913 (accessed on 07 February 2026).
Li X, Jin Y, Zhu N, Yin J, Jin LY. Pillar[n]arene-Based Supramolecular Polymers. Encyclopedia. Available at: https://encyclopedia.pub/entry/55913. Accessed February 07, 2026.
Li, Xu, Yan Jin, Nansong Zhu, Jinghua Yin, Long Yi Jin. "Pillar[n]arene-Based Supramolecular Polymers" Encyclopedia, https://encyclopedia.pub/entry/55913 (accessed February 07, 2026).
Li, X., Jin, Y., Zhu, N., Yin, J., & Jin, L.Y. (2024, March 06). Pillar[n]arene-Based Supramolecular Polymers. In Encyclopedia. https://encyclopedia.pub/entry/55913
Li, Xu, et al. "Pillar[n]arene-Based Supramolecular Polymers." Encyclopedia. Web. 06 March, 2024.
Copy Citation
The field of fluorescence sensing, leveraging various supramolecular self-assembled architectures constructed from macrocyclic pillar[n]arenes, has seen significant advancement in recent decades.
fluorescence sensing
environmental pollutants
pillar[n]arene
supramolecular
1. Introduction
Through noncovalent interactions, supramolecular chemistry unites components with different structures and functionalities [1][2][3][4][5][6][7][8], enabling the construction of dynamically reversible smart composites [9][10][11][12]. Increasing attention has been given to intelligent supramolecular structures formed by host–guest interactions, particularly for their stimuli-responsive properties and expansive application potential. As a vital segment of supramolecular chemistry, macrocyclic hosts have found extensive use across various domains. Presently, there are five prominent macrocyclic hosts, including cyclodextrins, calix[n]arenes, crown ethers, cucurbit[n]urils, and pillar[n]arenes. Each host can specifically accommodate different guest molecules by offering suitable cavities. Pillar[n]arenes, in particular, stand out due to their electron-rich and rigid cavities, symmetrical frame structure, and exceptional host–guest properties [13][14][15][16][17][18][19][20][21]. The positional modification of pillar[n]arenes endows them with distinct characteristics [22][23][24][25][26][27][28].
The coordination metal ions discussed in this review include Ag+, Hg2+, Cu2+, Zn2+, Pt2+, Fe3+, Al3+, Eu3+, and Tb3+. Host–guest complexes with metal coordination sites offer considerable scientific value, characterized by a structure resembling a pillar with many self-assembly driving forces, including cation–π, hydrophobic/hydrophilic, C–H–π, and π–π interactions, among others. Researchers categorize two types of supramolecular architectures with metal ion coordination sites: (i) functional pillar[n]arenes with metal coordination sites are initially designed and synthesized. The existing studies on pillar[n]arenes derive from the successful synthesis of pillar[5]arenes [29], followed by pillar[6]arenes [30]. Additionally, pillar[n]arenes with larger cavities (n = 6–15) can be synthesized from pillar[5]arenes by ring expansion, a process driven by kinetic control [31]. Due to the unique advantages of pillar[n]arenes, many novel functional pillar[n]arenes have emerged one after another [32][33]. The key factors for the design and synthesis of functional pillar[n]arene molecules mainly lie in the following two aspects: Firstly, the reaction time, concentration, temperature, feeding order, and other external conditions should be strictly controlled to minimize the production of various polymer byproducts when synthesizing pillar[n]arenes. Secondly, considering that the kind, position, and quantity of substituents on both sides of pillar[n]arenes have significant effects on their solubility, conformation, and host–guest properties, which directly influences the practical application of pillar[n]arenes, it is also challenging to design functional pillar[n]arenes. Pillar[n]arene derivatives reviewed in this paper mostly concentrate on the single- or dual-functional modifications of the macrocyclic host, including mono, double, and total substitution of the benzene rings. Pillar[n]arenes before functionalization often contain halogen atoms, azide groups, hydroxyl groups, or amino groups as the modification sites. Different reaction sites can adopt specific synthesis strategies to achieve high yield, easy separation, green synthesis, and other goals. The pillar[n]arenes can be also substituted by molecules (e.g., adenin, quinoline, acylhydrazone, thymine, pyridine, triazole, 1,3,4-oxadiazole, bis-2,2′:6′,2″-terpyridin, carboxyl, naphthalimide, thioacetylhydrazine, et al.) to possess the metal coordination ability. These functional pillar[n]arenes can further coordinate with metal ions in the presence or absence of guest molecules, generating supramolecular architectures with specific sensing capacities through multiple noncovalent interaction forces. (ii) Guest molecules with both host–guest recognition abilities and metal coordination sites are firstly designed and synthesized. The host–guest recognition groups are generally composed of quaternary ammonium groups, cyanogen groups, halogen atoms, or nitrogen heterocycles. Additionally, the involved coordination sites mainly consist of phenazine imidazole, thienyl functionalized diketopyrrolopyrrole, or multiple amide groups. After combining these functional groups into a small molecule, these guests can form complexes through host–guest interactions with pillar[n]arenes, which further coordinate with metal ions to generate supramolecular architectures with specific sensing capacities.
2. Single-Stimulus Responsive Sensors
Scheme 1 lists the molecular structures of the guest molecules and pillar[n]arenes that are discussed in this section.
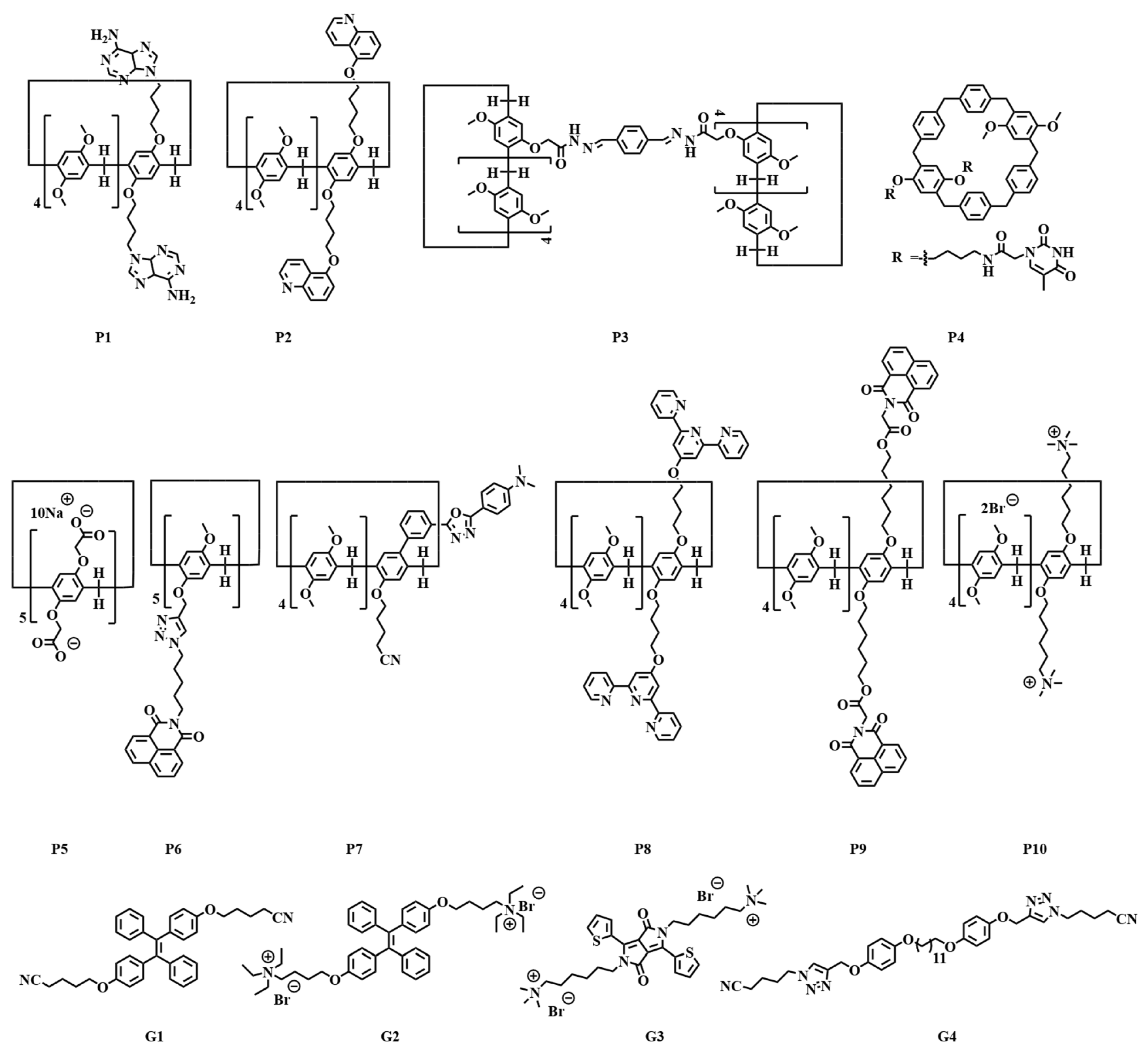
Scheme 1. Molecular structures of pillar[n]arenes and guests in the section of single-stimulus responsive fluorescence sensors.
A prime example is the widespread concern over environmental pollution caused by heavy metal ions. Silver(I) (Ag+) ions are known for their excellent electrical and thermal conductivity, ductility, and stability. However, excessive silver can disrupt active enzymes by binding to sulfhydryl groups in proteins, leading to their accumulation in the food chain. Therefore, developing economical, simple, environmentally friendly, and efficient materials for detecting and removing Ag+ ions is crucial.
In 2022, Wang W.M. and Yang Y.W. designed a stable supramolecular system utilizing host–guest interactions and coordination effects [34]. They employed two adenine binding sites in pillar[5]arene (P1) and tetraphenylvinyl (TPE, G1) functionalized with cyano groups, which possess AIE properties (Figure 1). Spherical supramolecular aggregates formed via 1:1 coordination between P1’s adenines and Ag+ ions. The synergistic effect of coordination between P1 and Ag+, coupled with the host–guest interaction between P1 and G1, resulted in a crosslinked P1⊂G1@Ag+ assembly. This assembly triggered the restriction of intramolecular rotation (RIR) and SAIEE mechanisms. The fluorescence lifetime and fluorescence quantum yield of P1⊂G1@Ag+ were 4.60 ns and 58.38%, respectively, with the rate constant of nonradiative decay being half that of P1⊂G1. Under 365 nm UV lamp irradiation, the assembly displayed bright blue-green fluorescence upon Ag+ ion addition, permitting effective Ag+ ion adsorption and sensitive detection. Moreover, the supramolecular assembly could be easily processed without a decline in activity, offering a useful tool for practical applications.
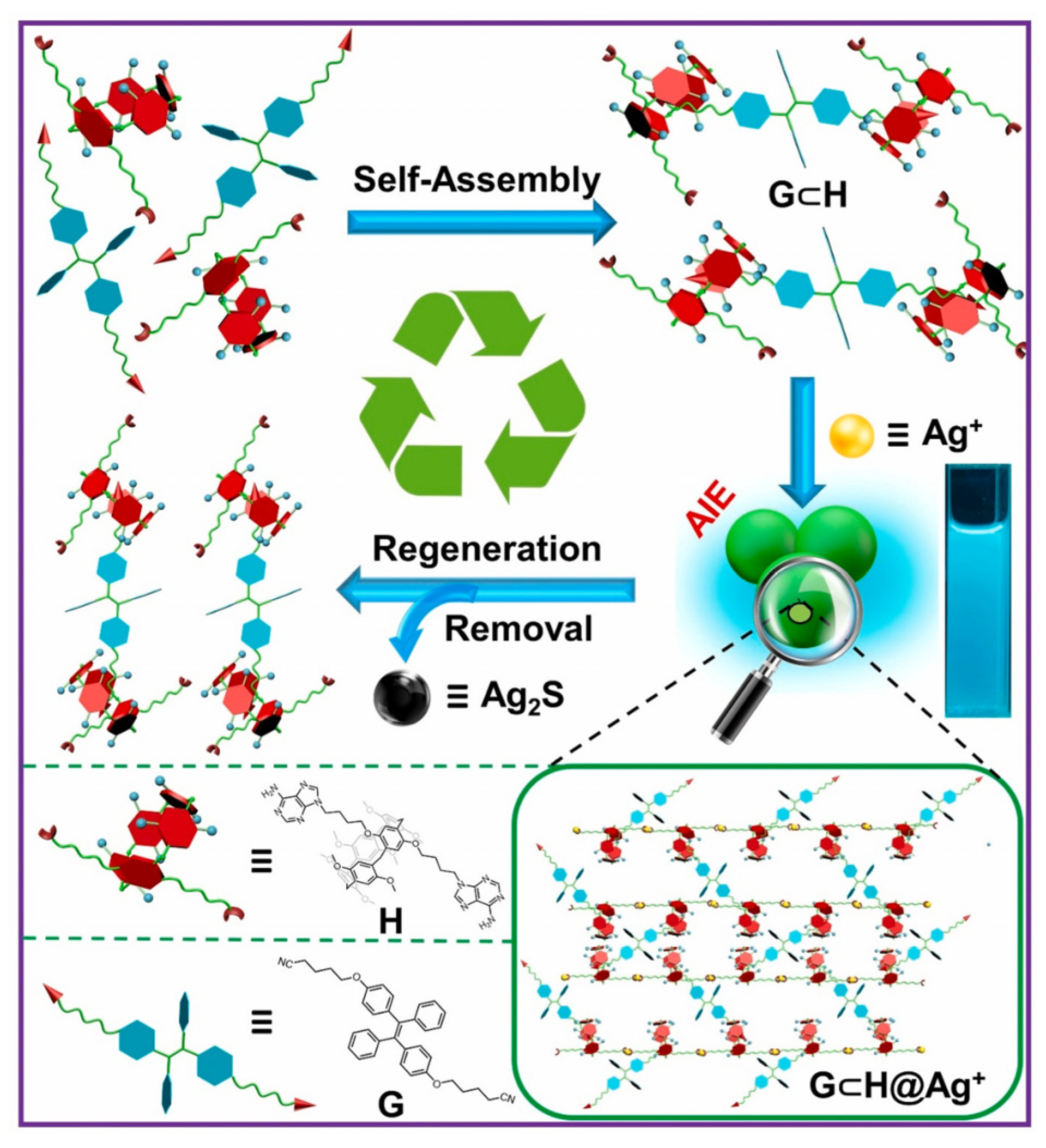
Figure 1. Schematic diagram showing how to detect and remove Ag+ ions from water using the supramolecular assembly and the assembly regeneration process.
The need for effective devices for sensitive toxic gas detection is also paramount. For instance, hydrazine hydrate (DH) is extensively used in synthesizing pesticides and medicines, fuels for satellites and rockets, and as a preservation agent in nuclear and power facilities. However, DH is very harmful, causing severe damage to the skin and central organs upon human absorption. Consequently, developing flexible, fast, and sensitive materials and methods for DH detection is critically important.
A year later, Lin Q.’s group developed a metallic gel by coordinating the nitrogen atom of 5-hydroxyquinoline functionalized pillar[5]arene (P2) with Ag+ ions [35]. P2 formed a one-dimensional coordination polymer through interaction with Ag+ ions via the N atoms on the quinoline groups (Figure 2). Adjacent coordination polymers were interconnected through π–π interactions between the P2 groups, facilitating the metallogel’s formation. The mean squared displacement of the gel P2 was lower than that of the P2-Ag gel, indicating that the inclusion of Ag+ improved the flexibility of the P2-Ag gel. The supramolecular assembly was further analyzed from a microscopic morphology perspective. P2 alone exhibited a lamellar structure. After adding Ag+ ions, the resultant metallogel P2-Ag transformed into a folded membrane structure, which is attributable to the coordination bond between Ag+ ions and P2. When exposed to DH vapor, the folded membrane structure of P2-Ag altered into a microspherical structure, suggesting the disruption of Ag+ ion coordination and the formation of a microspherical structure by P2 based on the hydrophobic effect. This gel enabled multichannel sensitive detection of DH through visual, fluorescence, and electrochemical means. DH disrupted the coordination by reducing Ag+ ions under DMSO/H2O conditions (fw = 20%), leading to the collapse and fluorescence quenching of the metallogel. The lowest critical gelation concentration (CGC) was 8% (w/v, 10 mg/mL = 1%). The gel–sol transition temperature was 62–63 °C. Multichannel detection of DH could be conveniently and efficiently realized in both water and air through sound and light alarms. The LOD reached 0.10 mg/m3 in air and 2.68 × 10−8 mol/L in water, below the US Environmental Protection Agency’s standard for drinking water.
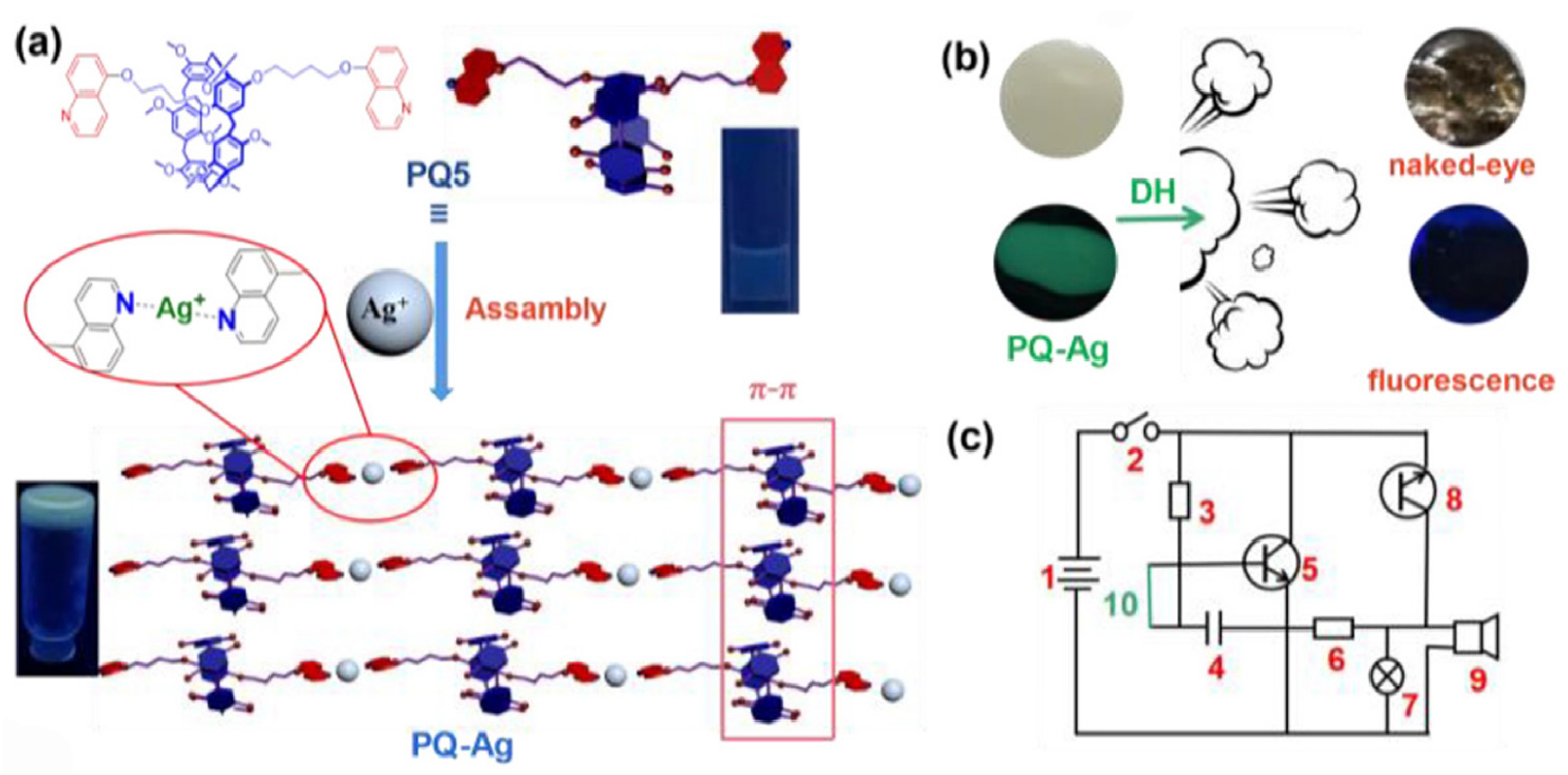
Figure 2. (a) A cartoon depicting the metallo-supramolecular polymer gel’s assembly method, (b) the PQ-Ag’s multichannel DH detection, and (c) the DH alarm circuit schematic diagram (1: electric source; 2: switch; 3: resistance; 4: electric capacity; 5: NPN triode; 6: resistance; 7: bulb; 8: PNP triode; 9: buzzer; 10: gel probe).
Mercury (Hg2+), among the most hazardous heavy metals, poses significant health and environmental risks. Exposure to Hg2+ ions, even in minute concentrations, presents a potential hazard to humans. For instance, in 2018, the group led by Lin Q. designed and synthesized another sensor based on pillararene AIEgens, which utilized bi-pillar[5]arene-based assemblies incorporating advanced AIEgens (P3) [36]. The assembly was driven by intermolecular hydrogen bonding (such as –N–H⋯C=O– and –C–H⋯N=C–), π–π stacking interactions, and hydrophobic effects. The Tyndall effect was observed in a 30% aqueous solution, with the critical aggregation fraction of water being 24% for P3, which exhibited a fluorescence quantum yield of 21%. P3 formed a sharp rod-shaped structure in DMSO/H2O (fw = 50%). The aggregated P3 was disassembled at low concentrations or high temperatures. A 1:2 complex was formed by binding P3 with Hg2+ ions (binding constant: 2.50 × 103 L2/mol2). The coordination between the Hg2+ ions and the P3 acylhydrazone group served as the basis for the sensing mechanism. To explore practical applications, a glass sheet was submerged in a high concentration of P3 to generate a film. This film enabled the convenient detection of Hg2+ ions in water, effectively separating and sensitively detecting them with an LOD of 4.30 × 10−8 mol/L. This innovative bi-pillar[5]arene AIEgen could pave the way for new designs and developments in pillar[n]arene AIEgens.
The pillar[n]arenes include two types: pillar[5]arene and pillar[6]arene [37]. The latter was developed by Yang Y.W.’s group. They utilized the strong interactions between thymine (T) and Hg2+ ions. Building on this, Dai D.H. and Yang Y.W. constructed a crosslinked supramolecular polymer through host–guest interactions, utilizing a TPE-bridged bis(quaternary ammonium) guest (G2) with AIE characteristics and a newly constructed [2]biphenyl-extended pillar[6]arene with two thymine sites as arms (P4) (Figure 3). The thymine groups’ close T-Hg2+-T coupling with the Hg2+ ions led to the formation of spherical assemblies with an average diameter of 164 nm. Fluorescent emission occurred immediately upon the addition of Hg2+ ions. The introduction of Hg2+ ions into the supramolecular system initiated supramolecular SAIEE. With its integrated mode of operation, great selectivity, and a quick adsorption rate (removal efficiency: 90%), this supramolecular polymer effectively accomplished the real-time detection and removal of Hg2+ ions from water. The assembly of P4 and G2 demonstrated excellent recyclability, maintaining effectiveness over more than five cycles of the removal process.
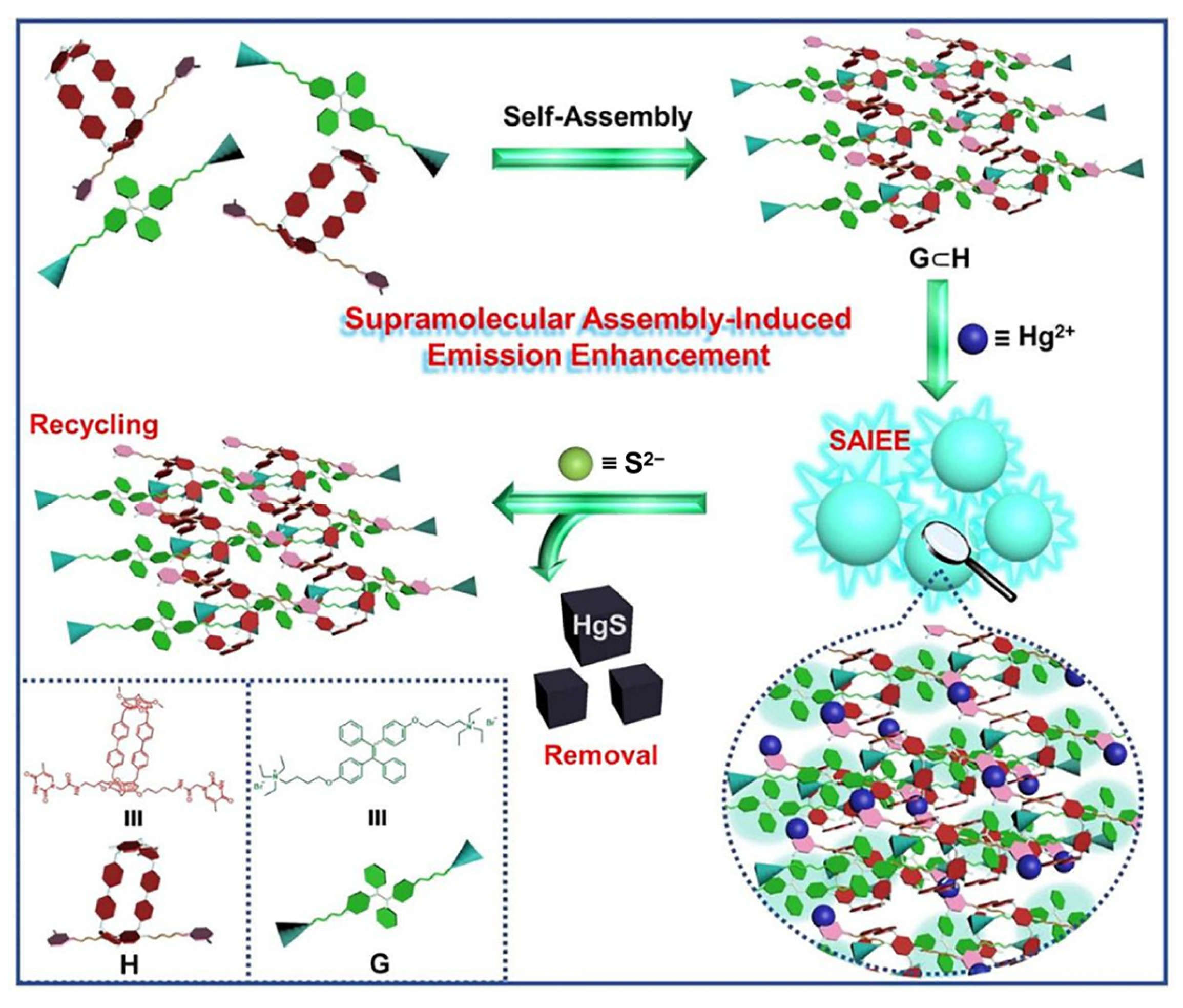
Figure 3. Schematic illustration showing how to detect and remove Hg2+ ions from water using the regeneration–recycling process and the “switch-on” fluorescence of supramolecular polymers.
Beyond the contributions of the previously mentioned research groups, a self-assembly consisting of carboxylatepillar[5]arene sodium salts (P5) and a diketopyrrole-bridged bis-quaternary ammonium guest (G3) was reported by Jiang X.M. and Cao D.R. [38]. Adding P5 to a G3 aqueous solution resulted in the morphological transformation of multilayer nanostructures (Figure 4). This host–guest complex exhibited multiple forces, including electrostatic, hydrophobic interactions, and π–π stacking interactions in aqueous solutions. The developed supramolecular system effectively detected and removed Hg2+ in real environmental water samples. The Hg2+ ions demonstrated synergistic interactions, including coordination with G3 and P5 and the Hg2+-cavity, forming a crosslinked network of P5⊂G3@Hg2+. The method exhibited good selectivity with a low LOD of 7.17 × 10−7 mol/L. Furthermore, the quenched fluorescence could be recovered post-treatment with Na2S, exhibiting a reversible process.
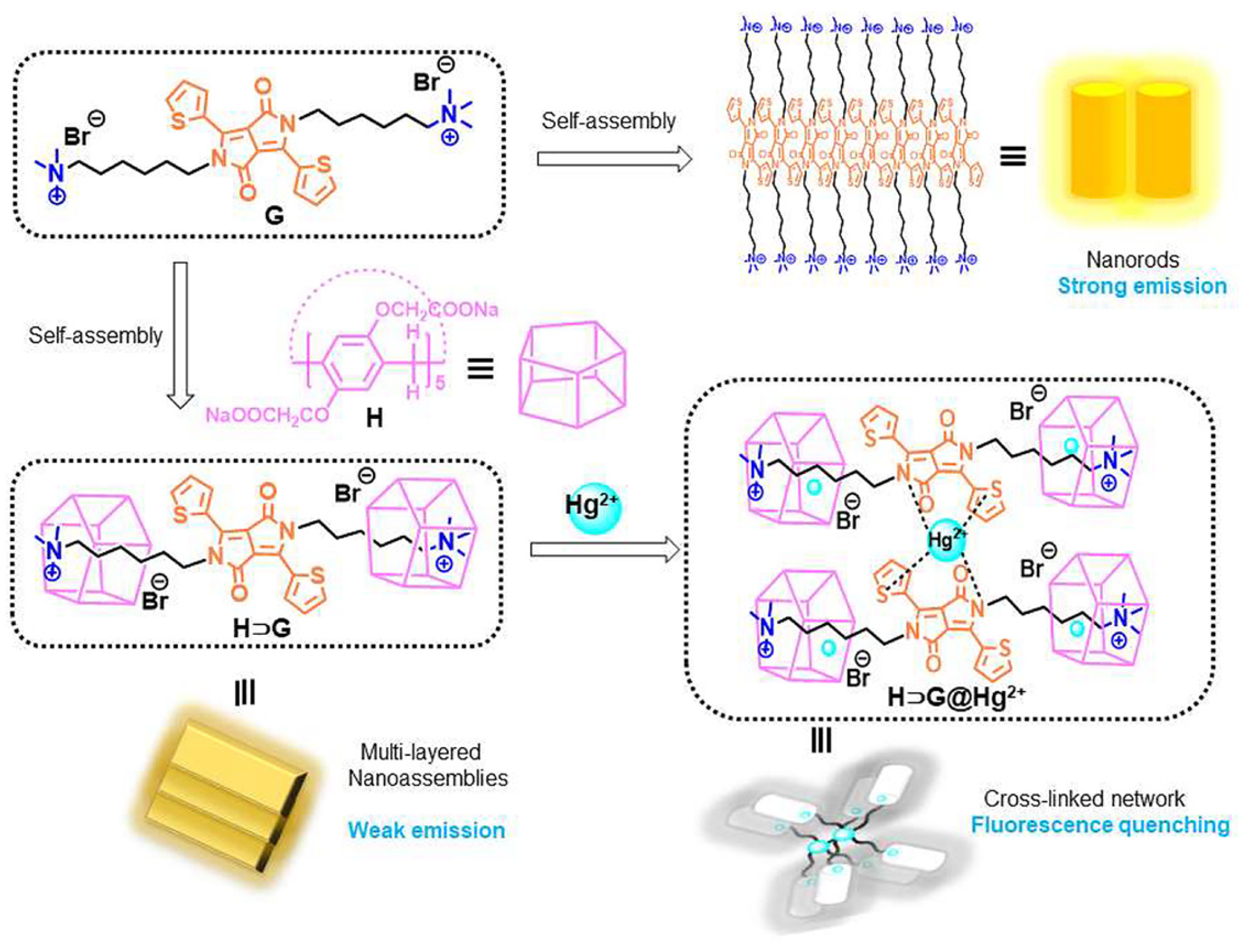
Figure 4. Schematic representations of the self-assembly of carboxylatopillar[5]arene sodium salts in the presence and absence of Hg2+ ions in water, as well as chemical structures and cartoon representations of the diketopyrrolopyrrole-bridged bis(quaternary ammonium) guest.
Copper ions (Cu2+) are also critical in various industries, and can result in soil contamination, bioaccumulation, and decreased agricultural production. Therefore, developing a selective and sensitive Cu2+ ion fluorescent chemical sensor, preferably a proportional chemical sensor, remains crucial in ion sensing research.
Pillar[5]arene was modified by Chang R. and Chang K.C. with five neighboring naphthalimide groups to form a new ligand, P6, for metal ion coordination, effectively serving as a ratiometric fluorescence sensor for Cu2+ ions in a CH2Cl2/CH3CN = 1/1 system [39]. This sensitivity was also observed in 10% aqueous methanol solutions. The introduction of naphthalimide groups enhanced intramolecular π–π stacking. P6 exhibited dual emission, comprising both the monomer and excimer emissions of the naphthalimide moieties. A synergistic interaction occurred between Cu2+ ions and the triazole groups on P6. P6 rapidly bound with Cu2+, maintaining stable fluorescence intensity. Upon complexing with Cu2+ ions, the excimer emission of ligand P6 was weakened, while the monomer emission intensified. The binding complexation ratio of P6 with Cu2+ ions was 1:1, with a binding constant of (3.39 ± 0.40) × 105 L2/mol2 and an LOD of 1.85 × 10−6 mol/L. Particles varying in diameters from (192 ± 65) to (206 ± 67) nm were produced. The relative fluorescence quantum yields of P1 and P1-Cu2+ were 0.13 and 0.11, respectively. The pillar[5]arene framework may be further functionalized in future studies to improve its selectivity for particular metal ions, or to modify it for use with different sensing platforms.
Liu S.Y. and Han J. developed another sensor for Cu2+ ions [40]. They designed a pillar[5]arene framework by functionalizing it with a cyanobutoxy moiety (P7) and a 1,3,4-oxadiazole subunit. This structure facilitated host–guest interactions between the electron-rich pillar[5]arene cavities and appropriately-sized neutral cyanobutoxy moieties, resulting in brush supramolecular polymers. Notably, the larger electron-deficient 1,3,4-oxadiazole groups remained outside the pillar[n]arene (P7) cavity after the creation of the host–guest inclusions, acting as a “brush” and enhancing the ability of the self-assembled supramolecular materials to interact with metal ions, such as Cu2+ ions. The critical aggregation concentration of P7 was 6.00 × 10−2 mol/L. These supramolecular brush-polymer architectures displayed distinct structural changes in response to fluorescence quenching after adding Cu2+ ions, suggesting a potential transformation into a crosslinked supramolecular network. The Irving–Williams order of stability might provide an explanation for the particular recognition of Cu2+. Therefore, this supramolecular brush polymer holds potential for application in metal cationic fluorescent chemical sensors.
In 2021, Chong H. et al. prepared a “three-component” supramolecular assembly by combining terpyridine attached pillar[5]arene (P8), cyano- and triazole-bearing alkyl chain (G4), and Zn2+ ions in a CHCl3 and CH3CN solvent system [41]. Terpyridine’s strong affinity for a variety of transition metal ions makes it an adaptable building block. The resulting composition exhibited a closely crosslinked porous morphology with nanoscale pore size. The P8 segment was incorporated into the cyano and triazole segment upon encapsulation. An organogel formed at a concentration of 1.00 mol/L. The polymerization was driven by host–guest interaction and metal-chelate cooperative forces. The P8 and Zn2+ assembly had a flake-like morphology. Nitrobenzene (picric acid, o-nitrobenzene, and phenol) was used as a sample. Of the three analytes, the assembly exhibited the highest sensitivity to picric acid, with an LOD of 1.66 × 10−4 mol/L. The quenching mechanism was believed to involve mixed processes of photo-induced electron transfer (PET) and fluorescence resonance energy transfer (FRET). This study represents a practical illustration of creating functional “multi-component” supramolecular systems with capabilities for explosive detection.
The role of Fe3+ ions as an essential metal element in human physiology is well-recognized. However, both deficiency and excess of Fe3+ ions beyond permissible limits can lead to severe health issues, such as anemia, tumorigenesis, organ dysfunction, and neurodegenerative diseases such as Alzheimer’s and Parkinson’s. Consequently, sensitive detection of Fe3+ ions is a critical concern in environmental and health monitoring. In 2018, Zhang Y.M. and Qi L. discovered that the pillar[5]arene structure (P9) could self-assemble into high-molecular-weight supramolecular π-gels with AIE properties [42]. Notably, the simplicity of the supramolecular system components enhanced its practical application value. Through π–π stacking interactions, P9 self-assembled into one-dimensional linear supramolecular π–gel chains. These π–gel chains interacted with the pillar[5]arene and naphthalimide to produce a two-dimensional supramolecular network. Subsequently, hydrophobic interactions compressed the two-dimensional network into microspheres. The CGC was 5% (w/v, 10 mg/mL = 1%). The gel–sol transition temperature was 43–45 °C. The LOD was 6.06 × 10−8 mol/L for Fe3+ ions. The gel demonstrated excellent recyclability and a 99.80% removal rate for Fe3+ ions in aqueous solutions. Additionally, the Fe3+ ion-coordinated supramolecular gel selectively sensed L-Cys with an LOD of 1.00 × 10−8 mol/L. This gel could be utilized in logic gates, offering significant advantages over previous logic devices in terms of reversibility, sensitivity, and prospective uses for ongoing transition metal and amino acid detection.
References
- Belmont-Sánchez, J.C.; Choquesillo-Lazarte, D.; García-Rubiño, M.E.; Matilla-Hernández, A.; Niclós-Gutiérrez, J.; Castiñeiras, A.; Frontera, A. Supramolecular nature of multicomponent crystals formed from 2,2′-thiodiacetic acid with 2,6-diaminopurine or N9-(2-Hydroxyethyl)adenine. Int. J. Mol. Sci. 2023, 24, 17381.
- Groschel, A.H.; Mueller, A.H.E. Self-assembly concepts for multicompartment nanostructures. Nanoscale 2015, 7, 11841–11876.
- Mujahid, A.; Afzal, A.; Dickert, F.L. Transitioning from Supramolecular Chemistry to Molecularly Imprinted Polymers in Chemical Sensing. Sensors 2023, 23, 7457.
- Liu, Q.; Sun, Z.; Dou, Y.; Kim, J.; Dou, X. Two-step self-assembly of hierarchically-ordered nanostructures. J. Mater. Chem. A 2015, 3, 11688–11699.
- Xu, L.; Wang, Z.; Wang, R.; Wang, L.; He, X.; Jiang, H.; Tang, H.; Cao, D.; Tang, B. A conjugated polymeric supramolecular network with aggregation-induced emission enhancement: An efficient light-harvesting system with an ultrahigh antenna effect. Angew. Chem. Int. Ed. 2020, 59, 9908–9913.
- An, D.; Shi, L.; Li, T.; Zhang, H.Y.; Chen, Y.; Hao, X.Q.; Song, M.P. Tailored supramolecular cage for efficient bio-labeling. Int. J. Mol. Sci. 2023, 24, 2147.
- Zhang, W.; Gao, C. Morphology transformation of self-assembled organic nanomaterials in aqueous solution induced by stimuli-triggered chemical structure changes. J. Mater. Chem. A 2017, 5, 16059–16104.
- Li, Z.; Hu, Y.; Wang, L.; Liu, H.F.; Ren, T.L.; Wang, C.; Li, D.L. Selective and accurate detection of nitrate in aquaculture water with surface-enhanced raman scattering (SERS) using gold nanoparticles decorated with β-cyclodextrins. Sensors 2024, 24, 1093.
- Jin, L.Y.; Bae, J.; Ryu, J.H.; Lee, M. Ordered nanostructures from the self-assembly of reactive coil-rod-coil molecules. Angew. Chem. Int. Ed. 2006, 45, 650–653.
- Pugliese, R. Structural and biomechanical properties of supramolecular nanofiber-based hydrogels in biomedicine. Biomedicines 2024, 12, 205.
- Roche, T.P.; Nedumpurath, P.J.; Karunakaran, S.C.; Schuster, G.B.; Hud, N.V. One-pot formation of pairing proto-RNA nucleotides and their supramolecular assemblies. Life 2023, 13, 2200.
- Croitoriu, A.; Chiriac, A.P.; Rusu, A.G.; Ghilan, A.; Ciolacu, D.E.; Stoica, I.; Nita, L.E. Morphological evaluation of supramolecular soft materials obtained through co-assembly processes. Gels 2023, 9, 886.
- Li, X.; Jin, Y.; Zhu, N.S.; Jin, L.Y. Applications of supramolecular polymers generated from pillararene-based molecules. Polymers 2023, 15, 4543.
- Lu, J.; Liu, P.; Deng, Y.; Zhu, N.; Jin, L.Y. Supramolecular nanoassemblies of rim-differentiated pillararene-rod-coil macromolecules via host-guest interactions for the sensing of cis-trans isomers of 1,4-diol-2-butene. J. Mol. Struct. 2023, 1291, 136054.
- Lu, P.; Cheng, J.; Li, Y.; Li, L.; Wang, Q.; He, C. Novel porous beta-cyclodextrin/pillararene copolymer for rapid removal of organic pollutants from water. Carbohyd. Polym. 2019, 216, 149–156.
- Li, Z.Y.; Zhang, Y.; Zhang, C.W.; Chen, L.J.; Wang, C.; Tan, H.W.; Yu, Y.H.; Li, X.P.; Yang, H.B. Cross-linked supramolecular polymer gels constructed from discrete multi-pillararene metallacycles and their multiple stimuli-responsive behavior. J. Am. Chem. Soc. 2014, 136, 8577–8589.
- Lou, X.Y.; Yang, Y.W. Pyridine-conjugated pillararene: From molecular crystals of blue luminescence to red-emissive coordination nanocrystals. J. Am. Chem. Soc. 2021, 143, 11976–11981.
- Zhu, H.; Li, Q.; Shi, B.; Xing, H.; Sun, Y.; Lu, S.; Shangguan, L.; Li, X.; Huang, F.; Stang, P.J. Formation of planar chiral platinum triangles via pillararene for circularly polarized luminescence. J. Am. Chem. Soc. 2020, 142, 17340–17345.
- Yan, H.; Yin, X.; Wang, D.; Han, T.; Tang, B.Z. Synergistically boosting the circularly polarized luminescence of functionalized pillararenes by polymerization and aggregation. Adv. Sci. 2023, 10, 2305149.
- Mei, Y.; Zhang, Q.W.; Gu, Q.; Liu, Z.; He, X.; Tian, Y. Pillararene-based fluorescent sensor array for biosensing of intracellular multi-neurotransmitters through host–guest recognitions. J. Am. Chem. Soc. 2022, 144, 2351–2359.
- Tuo, W.; Sun, Y.; Lu, S.; Li, X.; Sun, Y.; Stang, P.J. Pillararene-containing metallacycles and host-guest interaction caused aggregation-induced emission enhancement platforms. J. Am. Chem. Soc. 2020, 142, 16930–16934.
- Ogoshi, T.; Yamagishi, T.; Nakamoto, Y. Pillar-shaped macrocyclic hosts pillararenes: New key players for supramolecular chemistry. Chem. Rev. 2016, 116, 7937–8002.
- Lescop, C. Coordination-driven syntheses of compact supramolecular metallacycles toward extended metallo-organic stacked supramolecular assemblies. Acc. Chem. Res. 2017, 50, 885–894.
- St Onge, P.B.J.; Chen, T.C.; Langlois, A. Iron-coordinating π-conjugated semiconducting polymer: Morphology and charge transport in organic field-effect transistors. J. Mater. Chem. C 2020, 8, 8213–8223.
- Ni, X.L.; Xiao, X.; Cong, H. Cucurbituril-based coordination chemistry: From simple coordination complexes to novel poly-dimensional coordination polymers. Chem. Soc. Rev. 2013, 42, 9480–9508.
- Datta, S.; Saha, M.L.; Stang, P.J. Hierarchical assemblies of supramolecular coordination complexes. Acc. Chem. Res. 2018, 51, 2047–2063.
- Xia, D.; Wang, L.; Lv, X.; Chao, J.; Wei, X.; Wang, P. Dual-responsive Pseudorotaxane on the basis of a pH-Sensitive pillararene and its application in the fabrication of metallosupramolecular polypseudorotaxane. Macromolecules 2018, 51, 2716–2722.
- Liu, Y.; Shangguan, L.; Shi, B. A multi-responsive cross-linked supramolecular polymer network constructed by mussel yield coordination interaction and pillararene-based host–guest complexation. Chem. Commun. 2018, 54, 12230–12233.
- Ogoshi, T.; Kanai, S.; Fujinami, S.; Yamagishi, T.; Nakamoto, Y. Para-bridged symmetrical pillararenes: Their lewis acid catalyzed synthesis and host–guest property. J. Am. Chem. Soc. 2008, 130, 5022–5023.
- Cao, D.R.; Kou, Y.H.; Liang, J.Q.; Chen, J.J.; Wang, L.Y.; Meier, H. A facile and efficient preparation of pillararenes and a pillarquinone. Angew. Chem. Int. Ed. 2009, 48, 9721–9723.
- Ogoshi, T.; Ueshima, N.; Sakakibara, F.; Yamagishi, T.; Haino, T. Conversion from pillararene to pillararenes by ring expansion and encapsulation of C60 by pillararenes with nanosize. Org. Lett. 2014, 16, 2896–2899.
- Xiao, T.X.; Zhou, L.; Xu, L.X.; Zhong, W.W.; Zhao, W.; Sun, X.Q.; Elmes, R.B.P. Dynamic materials fabricated from water soluble pillararenes bearing triethylene oxide groups. Chin. Chem. Lett. 2019, 30, 271–276.
- Wu, J.R.; Wu, G.X.; Yang, Y.W. Pillararene-inspired macrocycles: From extended pillararenes to geminiarenes. Acc. Chem. Res. 2022, 55, 3191–3204.
- Wang, W.M.; Dai, D.; Wu, J.R.; Wang, C.; Wang, Y.; Yang, Y.W. Renewable supramolecular assembly-induced emission enhancement system for efficient detection and removal of silver(I). Dyes Pigment. 2022, 207, 110712.
- Jia, Y.; Guan, W.L.; Liu, J.; Hu, J.P.; Shi, B.; Yao, H.; Zhang, Y.M.; Wei, T.B.; Lin, Q. Novel conductive metallo-supramolecular polymer AIE gel for multi-channel highly sensitive detection of hydrazine hydrate. Chin. Chem. Lett. 2023, 34, 108082.
- Lin, Q.; Jiang, X.M.; Ma, X.Q.; Liu, J.; Yao, H.; Zhang, Y.M.; Wei, T.B. Novel bispillararene-based AIEgen and its’ application in mercury(II) detection. Sens. Actuat. B Chem. 2018, 272, 139–145.
- Dai, D.H.; Li, Z.; Yang, J.; Wang, C.Y.; Wu, J.R.; Wang, Y.; Zhang, D.M.; Yang, Y.W. Supramolecular assembly-induced emission enhancement for efficient mercury(II) detection and removal. J. Am. Chem. Soc. 2019, 141, 4756–4763.
- Jiang, X.; Wang, L.; Ran, X.; Tang, H.; Cao, D. Green, efficient detection and removal of Hg2+ by water-soluble fluorescent pillararene supramolecular self-assembly. Biosensors 2022, 12, 571.
- Chang, R.; Chen, C.Y.; Gao, L.; Li, Y.; Lee, Z.H.; Zhao, H.; Sue, A.C.; Chang, K.C. Highly selective Cu2+ detection with a naphthalimide-functionalised pillararene fluorescent chemosensor. Org. Biomol. Chem. 2024, 22, 745–752.
- Liu, S.; Wu, Q.; Zhang, T.; Zhang, H.; Han, J. Supramolecular brush polymers prepared from 1,3,4-oxadiazole and cyanobutoxy functionalised pillararene for detecting Cu2+. Org. Biomol. Chem. 2021, 19, 1287–1291.
- Chong, H.; Xu, Y.H.; Han, Y.; Yan, C.G.; Su, D.W.; Wang, C.Y. Pillararene-based “three-components” supramolecular assembly and the performance of nitrobenzene-based explosive fluorescence sensing. ChemistrySelect 2021, 6, 9363–9367.
- Zhang, Y.M.; Li, Y.F.; Zhong, K.P.; Qu, W.J.; Yao, H.; Wei, T.B.; Lin, Q. A bis-naphthalimide functionalized pillararene-based supramolecular π-gel acts as a multi-stimuli-responsive material. New J. Chem. 2018, 42, 16167–16173.
More
Information
Subjects:
Chemistry, Organic
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
739
Revisions:
2 times
(View History)
Update Date:
06 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




