
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hüseyin Bozkurt | -- | 4142 | 2024-03-06 09:46:39 | | | |
| 2 | Lindsay Dong | Meta information modification | 4142 | 2024-03-07 02:31:03 | | |
Video Upload Options
Much attention has been given to the use of microalgae to produce functional foods that have valuable bioactive chemicals, including essential amino acids, polyunsaturated fatty acids, vitamins, carotenoids, fiber, and minerals. Microalgal biomasses are increasingly being used to improve the nutritional values of foods because of their unique nutrient compositions that are beneficial to human health. Their protein content and amino acid composition are the most important components. The microalgal biomass used in the therapeutic supplement industry is dominated by bio-compounds like astaxanthin, β-carotene, polyunsaturated fatty acids like eicosapentaenoic acid and docosahexaenoic acid, and polysaccharides such as β-glucan. The popularity of microalgal supplements is growing because of the health benefits of their bioactive substances.
1. Introduction
1.1. Functional Food
1.2. Microalgae and Their Valuable Metabolites
1.3. Functional Food Products with Incorporation of Microalgae
2. Microalgae Food Application

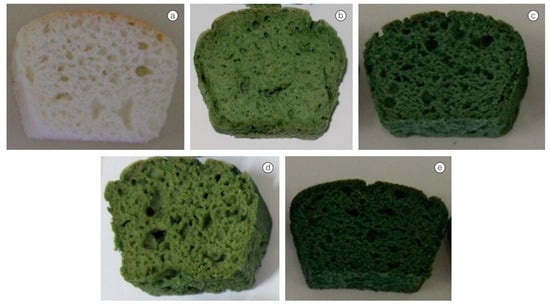
3.1. Challenges for Sensory Qualities of Food in Food Products with Microalgae
3.2. Food Safety and Potential Risks
3.3. Challenge in Ensuring the Stability of the Nutritional Content of Microalgae
3. Conclusions
References
- Zhou, J.; Liu, J.; Lin, D.; Gao, G.; Wang, H.; Guo, J.; Rao, P.; Ke, L. Boiling-Induced Nanoparticles and Their Constitutive Proteins from Isatis Indigotica Fort. Root Decoction: Purification and Identification. J. Tradit. Complement. Med. 2017, 7, 178–187.
- Health Canada. Standards of Evidence for Evaluating Foods with Health Claims: A Proposed Framework; Consultatıon Document; Health Canada: Ottawa, ON, USA, 2000; Available online: https://www.canada.ca/content/dam/hc-sc/migration/hc-sc/fn-an/alt_formats/hpfb-dgpsa/pdf/label-etiquet/consultation_doc-eng.pdf (accessed on 14 November 2023).
- Bagchi, D. Neutraceutical and Functional Food Regulations; Elsevier: New York, NY, USA, 2008.
- EFSA. Authority European Food Safety Authority, and European Centre for Disease Prevention and Control. In The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food-Borne Outbreaks in 2010; EFSA: Parma, Italy, 2015.
- Iwatani, S.; Yamamoto, N. Functional Food Products in Japan: A Review. Food Sci. Hum. Wellness 2019, 8, 96–101.
- Betoret, E.; Betoret, N.; Vidal, D.; Fito, P. Functional Foods Development: Trends and Technologies. Trends Food Sci. Technol. 2011, 22, 498–508.
- Shuba, E.S.; Kifle, D. Microalgae to Biofuels: ‘Promising’ Alternative and Renewable Energy, Review. Renew. Sustain. Energy Rev. 2018, 81, 743–755.
- Hosseinkhani, N.; McCauley, J.I.; Ralph, P.J. Key Challenges for the Commercial Expansion of Ingredients from Algae into Human Food Products. Algal Res. 2022, 64, 102696.
- Chen, C.; Tang, T.; Shi, Q.; Zhou, Z.; Fan, J. The Potential and Challenge of Microalgae as Promising Future Food Sources. Trends Food Sci. Technol. 2022, 126, 99–112.
- Becker, E.W. Micro-Algae as a Source of Protein. Biotechnol. Adv. 2007, 25, 207–210.
- Matos, J.; Cardoso, C.; Bandarra, N.M.; Afonso, C. Microalgae as Healthy Ingredients for Functional Food: A Review. Food Funct. 2017, 8, 2672–2685.
- Gomez-Zavaglia, A.; Prieto Lage, M.A.; Jimenez-Lopez, C.; Mejuto, J.C.; Simal-Gandara, J. The Potential of Seaweeds as a Source of Functional Ingredients of Prebiotic and Antioxidant Value. Antioxidants 2019, 8, 406.
- Lafarga, T.; Fernández-Sevilla, J.M.; González-López, C.; Acién-Fernández, F.G. Spirulina for the Food and Functional Food Industries. Food Res. Int. 2020, 137, 109356.
- Çelekli, A.; Yavuzatmaca, M.; Bozkurt, H. Modeling of Biomass Production by Spirulina platensis as Function of Phosphate Concentrations and PH Regimes. Bioresour. Technol. 2009, 100, 3625–3629.
- Çelekli, A.; Arslanargun, H.; Soysal, Ç.; Gültekin, E.; Bozkurt, H. Biochemical Responses of Filamentous Algae in Different Aquatic Ecosystems in South East Turkey and Associated Water Quality Parameters. Ecotoxicol. Environ. Saf. 2016, 133, 403–412.
- Lafarga, T. Effect of Microalgal Biomass Incorporation into Foods: Nutritional and Sensorial Attributes of the End Products. Algal Res. 2019, 41, 101566.
- Markou, G.; Chatzipavlidis, I.; Georgakakis, D. Carbohydrates Production and Bio-Flocculation Characteristics in Cultures of Arthrospira (Spirulina) platensis: Improvements through Phosphorus Limitation Process. BioEnergy Res. 2012, 5, 915–925.
- Çelekli, A.; Gültekin, E.; Bozkurt, H. Morphological and Biochemical Responses of Spirogyra Setiformis Exposed to Cadmium. Clean-Soil Air Water 2016, 44, 256–262.
- Colla, L.M.; Reinehr, C.O.; Reichert, C.; Costa, J.A.V. Production of Biomass and Nutraceutical Compounds by Spirulina platensis under Different Temperature and Nitrogen Regimes. Bioresour. Technol. 2007, 98, 1489–1493.
- Ogbonda, K.H.; Aminigo, R.E.; Abu, G.O. Influence of Temperature and PH on Biomass Production and Protein Biosynthesis in a Putative Spirulina sp. Bioresour. Technol. 2007, 98, 2207–2211.
- Abeyrathne, E.D.N.S.; Lee, H.Y.; Ahn, D.U. Egg White Proteins and Their Potential Use in Food Processing or as Nutraceutical and Pharmaceutical Agents—A Review. Poult. Sci. 2013, 92, 3292–3299.
- Huang, X.; Ahn, D.U. How Can the Value and Use of Egg Yolk Be Increased? J. Food Sci. 2019, 84, 205–212.
- Bohrer, B.M. Nutrient Density and Nutritional Value of Meat Products and Non-Meat Foods High in Protein. Trends Food Sci. Technol. 2017, 65, 103–112.
- Markou, G.; Arapoglou, D.; Eliopoulos, C.; Balafoutis, A.; Taddeo, R.; Panara, A.; Thomaidis, N. Cultivation and Safety Aspects of Arthrospira platensis (Spirulina) Grown with Struvite Recovered from Anaerobic Digestion Plant as Phosphorus Source. Algal Res. 2019, 44, 101716.
- Moughan, P.J. Digestion and Absorption of Proteins and Peptides. In Designing Functional Foods; Elsevier: Amsterdam, The Netherlands, 2009; pp. 148–170.
- Kazir, M.; Abuhassira, Y.; Robin, A.; Nahor, O.; Luo, J.; Israel, A.; Golberg, A.; Livney, Y.D. Extraction of Proteins from Two Marine Macroalgae, Ulva Sp. and Gracilaria Sp., for Food Application, and Evaluating Digestibility, Amino Acid Composition and Antioxidant Properties of the Protein Concentrates. Food Hydrocoll. 2019, 87, 194–203.
- Niccolai, A.; Zittelli, G.C.; Rodolfi, L.; Biondi, N.; Tredici, M.R. Microalgae of Interest as Food Source: Biochemical Composition and Digestibility. Algal Res. 2019, 42, 101617.
- Watts, S.A.; Lawrence, A.L.; Lawrence, J.M. Nutrition. In Developments in Aquaculture and Fisheries; Elsevier: Amsterdam, The Netherlands, 2013; Volume 38, pp. 155–169.
- Soni, R.A.; Sudhakar, K.; Rana, R.S. Spirulina–From Growth to Nutritional Product: A Review. Trends Food Sci. Technol. 2017, 69, 157–171.
- Gün, D.; Çelekli, A.; Bozkurt, H.; Kaya, S. Optimization of Biscuit Enrichment with the Incorporation of Arthrospira platensis: Nutritional and Sensory Approach. J. Appl. Phycol. 2022, 34, 1555–1563.
- Vonshak, A. Spirulina platensis Arthrospira: Physiology, Cell-Biology and Biotechnology; CRC Press: Boca Raton, FL, USA, 2002; ISBN 1482272970.
- Tudor, C.; Gherasim, E.C.; Dulf, F.V.; Pintea, A. In Vitro Bioaccessibility of Macular Xanthophylls from Commercial Microalgal Powders of Arthrospira platensis and Chlorella pyrenoidosa. Food Sci. Nutr. 2021, 9, 1896–1906.
- Rani, K.; Sandal, N.; Sahoo, P.K. A Comprehensive Review on Chlorella-Its Composition, Health Benefits, Market and Regulatory Scenario. Pharma Innov. J. 2018, 7, 584–589.
- Safi, C.; Zebib, B.; Merah, O.; Pontalier, P.-Y.; Vaca-Garcia, C. Morphology, Composition, Production, Processing and Applications of Chlorella vulgaris: A Review. Renew. Sustain. Energy Rev. 2014, 35, 265–278.
- da Silva, M.R.O.B.; Moura, Y.A.S.; Converti, A.; Porto, A.L.F.; Marques, D.d.A.V.; Bezerra, R.P. Assessment of the Potential of Dunaliella Microalgae for Different Biotechnological Applications: A Systematic Review. Algal Res. 2021, 58, 102396.
- Borowitzka, M.A. Dunaliella: Biology, Production, and Markets. In Handbook of Microalgal Culture; Wiley: Hoboken, NJ, USA, 2013; pp. 359–368.
- Monte, J.; Ribeiro, C.; Parreira, C.; Costa, L.; Brive, L.; Casal, S.; Brazinha, C.; Crespo, J.G. Biorefinery of Dunaliella salina: Sustainable Recovery of Carotenoids, Polar Lipids and Glycerol. Bioresour. Technol. 2020, 297, 122509.
- Çelekli, A.; Dönmez, G. Bir Dunaliella Türünün Gelişimine ve β-Karoten Üretimine PH ve Tuz Konsantrasyonlarının Etkisi. Ege J. Fish. Aquat. Sci. 2001, 18, 79–86. Available online: http://www.egejfas.org/en/download/article-file/58155 (accessed on 14 November 2023).
- Torres-Tiji, Y.; Fields, F.J.; Mayfield, S.P. Microalgae as a Future Food Source. Biotechnol. Adv. 2020, 41, 107536.
- Onorato, C.; Rösch, C. Comparative Life Cycle Assessment of Astaxanthin Production with Haematococcus pluvialis in Different Photobioreactor Technologies. Algal Res. 2020, 50, 102005.
- Brotosudarmo, T.H.P.; Limantara, L.; Setiyono, E. Heriyanto Structures of Astaxanthin and Their Consequences for Therapeutic Application. Int. J. Food Sci. 2020, 2020, 2156582.
- Ren, Y.; Deng, J.; Huang, J.; Wu, Z.; Yi, L.; Bi, Y.; Chen, F. Using Green Alga Haematococcus pluvialis for Astaxanthin and Lipid Co-Production: Advances and Outlook. Bioresour. Technol. 2021, 340, 125736.
- Shah, M.M.R.; Liang, Y.; Cheng, J.J.; Daroch, M. Astaxanthin-Producing Green Microalga Haematococcus pluvialis: From Single Cell to High Value Commercial Products. Front. Plant Sci. 2016, 7, 531.
- Khoo, K.S.; Lee, S.Y.; Ooi, C.W.; Fu, X.; Miao, X.; Ling, T.C.; Show, P.L. Recent Advances in Biorefinery of Astaxanthin from Haematococcus pluvialis. Bioresour. Technol. 2019, 288, 121606.
- Soto-Sierra, L.; Stoykova, P.; Nikolov, Z.L. Extraction and Fractionation of Microalgae-Based Protein Products. Algal Res. 2018, 36, 175–192.
- Pulz, O.; Gross, W. Valuable Products from Biotechnology of Microalgae. Appl. Microbiol. Biotechnol. 2004, 65, 635–648.
- Becker, W. Microalgae for Aquaculture: The Nutritional Value of Microalgae for Aquaculture. In Handbook of Microalgal Culture: Biotechnology and Applied Phycology; Richmond, A., Ed.; John Wiley & Sons: Oxford, UK, 2004.
- De Luca, M.; Pappalardo, I.; Limongi, A.R.; Viviano, E.; Radice, R.P.; Todisco, S.; Martelli, G.; Infantino, V.; Vassallo, A. Lipids from Microalgae for Cosmetic Applications. Cosmetics 2021, 8, 52.
- Lafarga, T.; Rodríguez-Bermúdez, R.; Morillas-España, A.; Villaró, S.; García-Vaquero, M.; Morán, L.; Sánchez-Zurano, A.; González-López, C.V.; Acién-Fernández, F.G. Consumer Knowledge and Attitudes towards Microalgae as Food: The Case of Spain. Algal Res. 2021, 54, 102174.
- Plaza, M.; Herrero, M.; Cifuentes, A.; Ibanez, E. Innovative Natural Functional Ingredients from Microalgae. J. Agric. Food Chem. 2009, 57, 7159–7170.
- Hassan, S.M.; Ashour, M.; Soliman, A.A.F.; Hassanien, H.A.; Alsanie, W.F.; Gaber, A.; Elshobary, M.E. The Potential of a New Commercial Seaweed Extract in Stimulating Morpho-Agronomic and Bioactive Properties of Eruca vesicaria (L.) Cav. Sustainability 2021, 13, 4485.
- Ma, H.; Xiong, H.; Zhu, X.; Ji, C.; Xue, J.; Li, R.; Ge, B.; Cui, H. Polysaccharide from Spirulina platensis Ameliorates Diphenoxylate-Induced Constipation Symptoms in Mice. Int. J. Biol. Macromol. 2019, 133, 1090–1101.
- The European Commission. Commission Regulation (EU) 2015/1933 of 27 October 2015 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels for Polycyclic Aromatic Hydrocarbons in Cocoa Fibre, Banana Chips, Food Supplements, Dried Herbs and Dried Spices. Off. J. Eur. Union 2015, 282, 11–13. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32015R1933 (accessed on 14 November 2023).
- Matos, J.; Cardoso, C.L.; Falé, P.; Afonso, C.M.; Bandarra, N.M. Investigation of Nutraceutical Potential of the Microalgae Chlorella vulgaris and Arthrospira platensis. Int. J. Food Sci. Technol. 2020, 55, 303–312.
- Barta, D.G.; Coman, V.; Vodnar, D.C. Microalgae as Sources of Omega-3 Polyunsaturated Fatty Acids: Biotechnological Aspects. Algal Res. 2021, 58, 102410.
- Hashimoto, M.; Maekawa, M.; Katakura, M.; Hamazaki, K.; Matsuoka, Y. Possibility of Polyunsaturated Fatty Acids for the Prevention and Treatment of Neuropsychiatric Illnesses. J. Pharmacol. Sci. 2014, 124, 294–300.
- Nestel, P.; Clifton, P.; Colquhoun, D.; Noakes, M.; Mori, T.A.; Sullivan, D.; Thomas, B. Indications for Omega-3 Long Chain Polyunsaturated Fatty Acid in the Prevention and Treatment of Cardiovascular Disease. Heart Lung Circ. 2015, 24, 769–779.
- Ho, S.-H.; Chen, C.-Y.; Chang, J.-S. Effect of Light Intensity and Nitrogen Starvation on CO2 Fixation and Lipid/Carbohydrate Production of an Indigenous Microalga Scenedesmus Obliquus CNW-N. Bioresour. Technol. 2012, 113, 244–252.
- Matsui, M.S.; Muizzuddin, N.; Arad, S.; Marenus, K. Sulfated Polysaccharides from Red Microalgae Have Antiinflammatory Properties in Vitro and In Vivo. Appl. Biochem. Biotechnol. 2003, 104, 13–22.
- Pradhan, B.; Patra, S.; Nayak, R.; Behera, C.; Dash, S.R.; Nayak, S.; Sahu, B.B.; Bhutia, S.K.; Jena, M. Multifunctional Role of Fucoidan, Sulfated Polysaccharides in Human Health and Disease: A Journey under the Sea in Pursuit of Potent Therapeutic Agents. Int. J. Biol. Macromol. 2020, 164, 4263–4278.
- de Jesus Raposo, M.F.; De Morais, R.M.S.C.; de Morais, A.M.M.B. Bioactivity and Applications of Sulphated Polysaccharides from Marine Microalgae. Mar. Drugs 2013, 11, 233–252.
- Rajasekar, P.; Palanisamy, S.; Anjali, R.; Vinosha, M.; Elakkiya, M.; Marudhupandi, T.; Tabarsa, M.; You, S.; Prabhu, N.M. Isolation and Structural Characterization of Sulfated Polysaccharide from Spirulina platensis and Its Bioactive Potential: In Vitro Antioxidant, Antibacterial Activity and Zebrafish Growth and Reproductive Performance. Int. J. Biol. Macromol. 2019, 141, 809–821.
- Liang, M.-H.; Zhu, J.; Jiang, J.-G. Carotenoids Biosynthesis and Cleavage Related Genes from Bacteria to Plants. Crit. Rev. Food Sci. Nutr. 2018, 58, 2314–2333.
- Lisboa, C.R.; Pereira, A.; Ferreira, S.P.; Costa, J.A.V. Utilisation of Spirulina sp. and Chlorella pyrenoidosa Biomass for the Productionof Enzymatic Protein Hydrolysates. Int. J. Eng. Res. Appl. 2014, 5, 29–38.
- Hoseini, S.M.; Khosravi-Darani, K.; Mozafari, M.R. Nutritional and Medical Applications of Spirulina Microalgae. Mini Rev. Med. Chem. 2013, 13, 1231–1237.
- Christaki, E.; Florou-Paneri, P.; Bonos, E. Microalgae: A Novel Ingredient in Nutrition. Int. J. Food Sci. Nutr. 2011, 62, 794–799.
- Ambrosi, M.A.; Reinehr, C.O.; Bertolin, T.E.; Costa, J.A.V.; Colla, L.M. Propriedades de Saúde de Spirulina spp. Rev. Ciências Farm. Básica e Apl. 2008, 29, 109–117.
- Gharibzahedi, S.M.T.; Jafari, S.M. The Importance of Minerals in Human Nutrition: Bioavailability, Food Fortification, Processing Effects and Nanoencapsulation. Trends Food Sci. Technol. 2017, 62, 119–132.
- Shamah, T.; Villalpando, S. The Role of Enriched Foods in Infant and Child Nutrition. Br. J. Nutr. 2006, 96, S73–S77.
- Costa, J.A.V.; De Morais, M.G. The Role of Biochemical Engineering in the Production of Biofuels from Microalgae. Bioresour. Technol. 2011, 102, 2–9.
- Barsanti, L.; Gualtieri, P. Algae: Anatomy, Biochemistry, and Biotechnology; CRC Press: Boca Raton, FL, USA, 2005; ISBN 0429095813.
- Iyer, U.M.; Dhruv, S.A.; Mani, I.U.; Gershwin, M.E.; Belay, A. Spirulina in Human Nutrition and Health. In Spirulina in Human Nutrition and Health; CRC Press: Boca Raton, FL, USA, 2008; p. 312.
- Chu, W.-L. Biotechnological Applications of Microalgae. IeJSME 2012, 6, S24–S37.
- Martelli, G.; Folli, C.; Visai, L.; Daglia, M.; Ferrari, D. Thermal Stability Improvement of Blue Colorant C-Phycocyanin from Spirulina platensis for Food Industry Applications. Process Biochem. 2014, 49, 154–159.
- Vílchez, C.; Forján, E.; Cuaresma, M.; Bédmar, F.; Garbayo, I.; Vega, J.M. Marine Carotenoids: Biological Functions and Commercial Applications. Mar. Drugs 2011, 9, 319–333.
- Zouari, N.; Abid, M.; Fakhfakh, N.; Ayadi, M.A.; Zorgui, L.; Ayadi, M.; Attia, H. Blue-Green Algae (Arthrospira platensis) as an Ingredient in Pasta: Free Radical Scavenging Activity, Sensory and Cooking Characteristics Evaluation. Int. J. Food Sci. Nutr. 2011, 62, 811–813.
- Koli, D.K.; Rudra, S.G.; Bhowmik, A.; Pabbi, S. Nutritional, Functional, Textural and Sensory Evaluation of Spirulina Enriched Green Pasta: A Potential Dietary and Health Supplement. Foods 2022, 11, 979.
- Li, H.-B.; Jiang, Y.; Chen, F. Isolation and Purification of Lutein from the Microalga Chlorella vulgaris by Extraction after Saponification. J. Agric. Food Chem. 2002, 50, 1070–1072.
- Fradique, M.; Batista, A.P.; Nunes, M.C.; Gouveia, L.; Bandarra, N.M.; Raymundo, A. Incorporation of Chlorella vulgaris and Spirulina maxima Biomass in Pasta Products. Part 1: Preparation and Evaluation. J. Sci. Food Agric. 2010, 90, 1656–1664.
- Fernandes, B.; Dragone, G.; Abreu, A.P.; Geada, P.; Teixeira, J.; Vicente, A. Starch Determination in Chlorella vulgaris—A Comparison between Acid and Enzymatic Methods. J. Appl. Phycol. 2012, 24, 1203–1208.
- Gouveia, L.; Batista, A.P.; Miranda, A.; Empis, J.; Raymundo, A. Chlorella vulgaris Biomass Used as Colouring Source in Traditional Butter Cookies. Innov. Food Sci. Emerg. Technol. 2007, 8, 433–436.
- Jaeschke, D.P.; Teixeira, I.R.; Marczak, L.D.F.; Mercali, G.D. Phycocyanin from Spirulina: A Review of Extraction Methods and Stability. Food Res. Int. 2021, 143, 110314.
- de Morais, M.G.; de Miranda, M.Z.; Costa, J.A.V. Biscoitos de Chocolate Enriquecidos Com Spirulina platensis: Características Físicoquímicas, Sensoriais e Digestibilidade. Aliment. E Nutr. Araraquara 2009, 17, 323–328.
- Çelekli, A.; Alslibi, Z.A.; Bozkurt, H. Influence of Incorporated Spirulina platensis on the Growth of Microflora and Physicochemical Properties of Ayran as a Functional Food. Algal Res. 2019, 44, 101710.
- Çelekli, A.; Alslibi, Z.A.; Bozkurt, H. Boosting Effects of Spirulina platensis, Whey Protein, and Probiotics on the Growth of Microflora and the Nutritional Value of Ayran. Eng. Rep. 2020, 2, e12235.
- Figueira, F.d.S.; Crizel, T.d.M.; Silva, C.R.; Salas-Mellado, M.d.l.M. Pão Sem Glúten Enriquecido Com a Microalga Spirulina platensis. Brazilian J. Food Technol. 2011, 14, 308–316.
- Rabelo, S.F.; Lemes, A.C.; Takeuchi, K.P.; Frata, M.T.; de Carvalho, J.C.M.; Danesi, E.D.G. Development of Cassava Doughnuts Enriched with Spirulina platensis Biomass. Brazilian J. Food Technol. 2013, 16, 42–51.
- Beheshtipour, H.; Mortazavian, A.M.; Haratian, P.; Darani, K.K. Effects of Chlorella vulgaris and Arthrospira platensis Addition on Viability of Probiotic Bacteria in Yogurt and Its Biochemical Properties. Eur. Food Res. Technol. 2012, 235, 719–728.
- Marti-Quijal, F.J.; Zamuz, S.; Tomašević, I.; Rocchetti, G.; Lucini, L.; Marszałek, K.; Barba, F.J.; Lorenzo, J.M. A Chemometric Approach to Evaluate the Impact of Pulses, Chlorella and Spirulina on Proximate Composition, Amino Acid, and Physicochemical Properties of Turkey Burgers. J. Sci. Food Agric. 2019, 99, 3672–3680.
- De Marco, E.R.; Steffolani, M.E.; Martínez, C.S.; León, A.E. Effects of Spirulina Biomass on the Technological and Nutritional Quality of Bread Wheat Pasta. LWT-Food Sci. Technol. 2014, 58, 102–108.
- Food Ingredients New-Look Microalgae: Newly Approved Chlorella vulgaris Powders Accentuates Ice Creams, Shakes, Cakes and Pasta. Available online: https://www.foodingredientsfirst.com/news/new-look-microalgae-newly-approved-Chlorella-vulgaris-powders-accentuates-ice-creams-shakes-and-pasta.html (accessed on 13 February 2022).
- Wu, G.; Zhuang, D.; Chew, K.W.; Ling, T.C.; Khoo, K.S.; Van Quyen, D.; Feng, S.; Show, P.L. Current Status and Future Trends in Removal, Control, and Mitigation of Algae Food Safety Risks for Human Consumption. Molecules 2022, 27, 6633.
- Cai, Y.; Lim, H.R.; Khoo, K.S.; Ng, H.-S.; Cai, Y.; Wang, J.; Chan, A.T.-Y.; Show, P.L. An Integration Study of Microalgae Bioactive Retention: From Microalgae Biomass to Microalgae Bioactives Nanoparticle. Food Chem. Toxicol. 2021, 158, 112607.
- Cuellar-Bermudez, S.P.; Aguilar-Hernandez, I.; Cardenas-Chavez, D.L.; Ornelas-Soto, N.; Romero-Ogawa, M.A.; Parra-Saldivar, R. Extraction and Purification of High-value Metabolites from Microalgae: Essential Lipids, Astaxanthin and Phycobiliproteins. Microb. Biotechnol. 2015, 8, 190–209.
- Vieira, M.V.; Pastrana, L.M.; Fuciños, P. Microalgae Encapsulation Systems for Food, Pharmaceutical and Cosmetics Applications. Mar. Drugs 2020, 18, 644.




