
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Shaozhong Zeng | -- | 3114 | 2024-03-06 09:29:32 | | | |
| 2 | Sirius Huang | Meta information modification | 3114 | 2024-03-08 01:51:29 | | |
Video Upload Options
Covalent organic frameworks (COFs) are constructed from small organic molecules through reversible covalent bonds, and are therefore considered a special type of polymer. Small organic molecules are divided into nodes and connectors based on their roles in the COF’s structure. The connector generally forms reversible covalent bonds with the node through two reactive end groups. The adjustment of the length of the connector facilitates the adjustment of pore size. Due to the diversity of organic small molecules and reversible covalent bonds, COFs have formed a large family since their synthesis in 2005. Among them, a type of COF containing redox active groups such as –C=O–, –C=N–, and –N=N– has received widespread attention in the field of energy storage. The ordered crystal structure of COFs ensures the ordered arrangement and consistent size of pores, which is conducive to the formation of unobstructed ion channels, giving these COFs a high-rate performance and a long cycle life. The voltage and specific capacity jointly determine the energy density of cathode materials.
1. Introduction
2. Active Functional Groups of COFs Materials
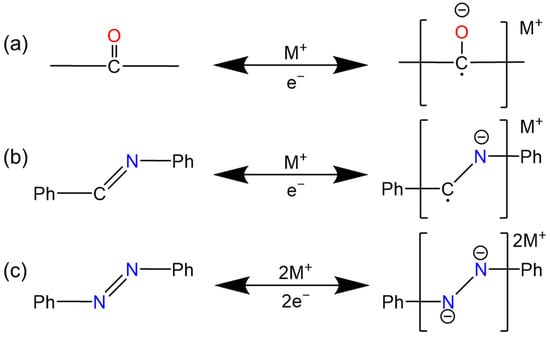
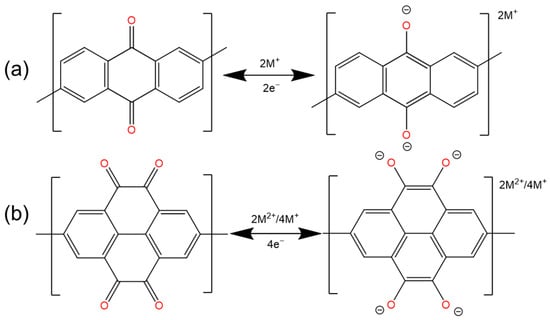
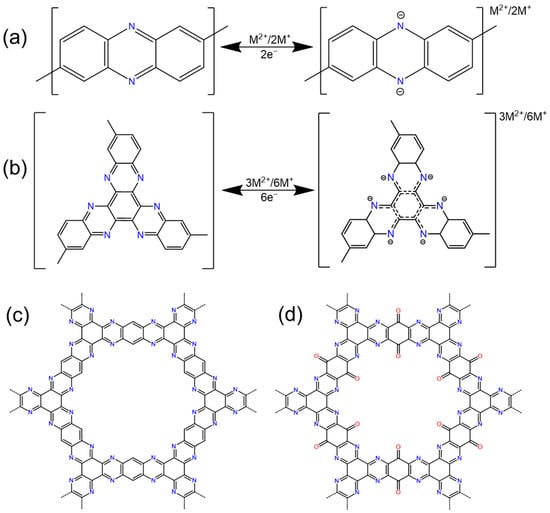
2.1. Quinones and Ketones
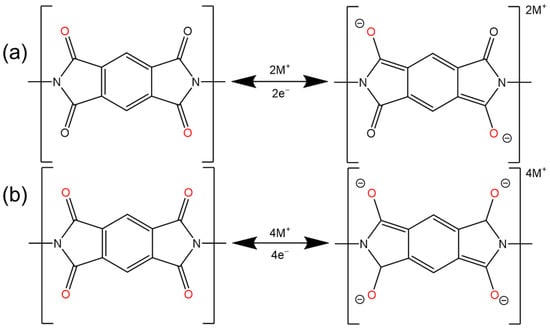
2.2. Imide
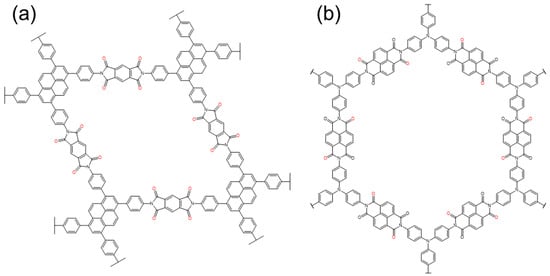
2.3. Imine and Azo
2.4. Pyrazine
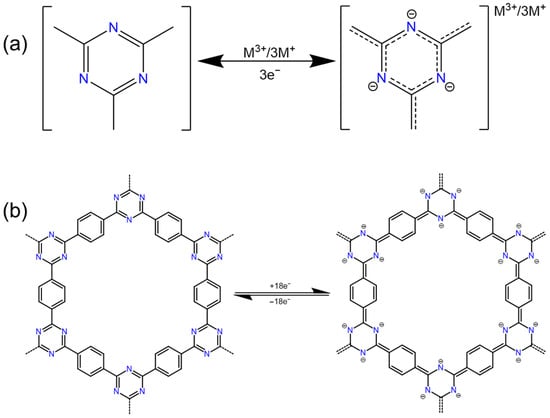
2.5. Triazine
References
- Cote, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Porous, crystalline, covalent organic frameworks. Science 2005, 310, 1166–1170.
- Tao, S.S.; Jiang, D.L. Covalent Organic Frameworks for Energy Conversions: Current Status, Challenges, and Perspectives. CCS Chem. 2021, 3, 2003–2024.
- Wang, H.; Zeng, Z.; Xu, P.; Li, L.; Zeng, G.; Xiao, R.; Tang, Z.; Huang, D.; Tang, L.; Lai, C.; et al. Recent progress in covalent organic framework thin films: Fabrications, applications and perspectives. Chem. Soc. Rev. 2019, 48, 488–516.
- Xu, F.; Jin, S.; Zhong, H.; Wu, D.; Yang, X.; Chen, X.; Wei, H.; Fu, R.; Jiang, D. Electrochemically active, crystalline, mesoporous covalent organic frameworks on carbon nanotubes for synergistic lithium-ion battery energy storage. Sci. Rep.-UK 2015, 5, 8225.
- Haldar, S.; Schneemann, A.; Kaskel, S. Covalent Organic Frameworks as Model Materials for Fundamental and Mechanistic Understanding of Organic Battery Design Principles. J. Am. Chem. Soc. 2023, 145, 13494–13513.
- Wei, S.; Wang, J.; Li, Y.; Fang, Z.; Wang, L.; Xu, Y. Recent progress in COF-based electrode materials for rechargeable metal-ion batteries. Nano Res. 2023, 16, 6753–6770.
- Sun, J.L.; Xu, Y.F.; Lv, Y.Q.; Zhang, Q.C.; Zhou, X.S. Recent Advances in Covalent Organic Framework Electrode Materials for Alkali Metal-Ion Batteries. CCS Chem. 2023, 5, 1259–1276.
- Zhong, M.; Liu, M.; Li, N.; Bu, X.H. Recent advances and perspectives of metal/covalent-organic frameworks in metal-air batteries. J. Energy Chem. 2021, 63, 113–129.
- Sang, P.; Chen, Q.; Wang, D.Y.; Guo, W.; Fu, Y. Organosulfur Materials for Rechargeable Batteries: Structure, Mechanism, and Application. Chem. Rev. 2023, 123, 1262–1326.
- Kong, L.; Liu, M.; Huang, H.; Xu, Y.; Bu, X.H. Metal/Covalent-Organic Framework Based Cathodes for Metal-Ion Batteries. Adv. Energy Mater. 2021, 12, 2100172.
- Lu, Y.; Chen, J. Prospects of organic electrode materials for practical lithium batteries. Nat. Rev. Chem. 2020, 4, 127–142.
- Shea, J.J.; Luo, C. Organic Electrode Materials for Metal Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 5361–5380.
- Zhao, Q.; Lu, Y.; Chen, J. Advanced Organic Electrode Materials for Rechargeable Sodium-Ion Batteries. Adv. Energy Mater. 2017, 7, 22.
- Liang, Y.L.; Tao, Z.L.; Chen, J. Organic Electrode Materials for Rechargeable Lithium Batteries. Adv. Energy Mater. 2012, 2, 742–769.
- Li, L.H.; Yang, H.H.; Wang, X.; Ma, Y.H.; Ou, W.Z.; Peng, H.; Ma, G.F. An anthraquinone-based covalent organic framework for highly reversible aqueous zinc-ion battery cathodes. J. Mater. Chem. A 2023, 11, 26221–26229.
- Liang, Y.L.; Zhang, P.; Yang, S.Q.; Tao, Z.L.; Chen, J. Fused Heteroaromatic Organic Compounds for High-Power Electrodes of Rechargeable Lithium Batteries. Adv. Energy Mater. 2013, 3, 600–605.
- Wu, S.; Wang, W.; Li, M.; Cao, L.; Lyu, F.; Yang, M.; Wang, Z.; Shi, Y.; Nan, B.; Yu, S.; et al. Highly durable organic electrode for sodium-ion batteries via a stabilized alpha-C radical intermediate. Nat. Commun. 2016, 7, 13318.
- Williams, D.L.; Byrne, J.J.; Driscoll, J.S. A High Energy Density Lithium/Dichloroisocyanuric Acid Battery System. J. Electrochem. Soc. 1969, 116, 2.
- Ma, D.; Zhao, H.; Cao, F.; Zhao, H.; Li, J.; Wang, L.; Liu, K. A carbonyl-rich covalent organic framework as a high-performance cathode material for aqueous rechargeable zinc-ion batteries. Chem. Sci. 2022, 13, 2385–2390.
- Xu, S.; Sun, M.; Wang, Q.; Wang, C. Recent progress in organic electrodes for zinc-ion batteries. J. Semicond. 2020, 41, 091704.
- Minakshi, M. Examining manganese dioxide electrode in KOH electrolyte using TEM technique. J. Electroanal. Chem. 2008, 616, 99–106.
- Xu, X.; Zhang, S.; Xu, K.; Chen, H.; Fan, X.; Huang, N. Janus Dione-Based Conjugated Covalent Organic Frameworks with High Conductivity as Superior Cathode Materials. J. Am. Chem. Soc. 2023, 145, 1022–1030.
- Gu, S.; Wu, S.; Cao, L.; Li, M.; Qin, N.; Zhu, J.; Wang, Z.; Li, Y.; Li, Z.; Chen, J.; et al. Tunable Redox Chemistry and Stability of Radical Intermediates in 2D Covalent Organic Frameworks for High Performance Sodium Ion Batteries. J. Am. Chem. Soc. 2019, 141, 9623–9628.
- Duan, J.; Wang, W.T.; Zou, D.G.; Liu, J.; Li, N.; Weng, J.Y.; Xu, L.P.; Guan, Y.; Zhang, Y.J.; Zhou, P.F. Construction of a Few-Layered COF@CNT Composite as an Ultrahigh Rate Cathode for Low-Cost K-Ion Batteries. ACS Appl. Mater. Interfaces 2022, 14, 31234–31244.
- Liu, Y.; Lu, Y.; Hossain Khan, A.; Wang, G.; Wang, Y.; Morag, A.; Wang, Z.; Chen, G.; Huang, S.; Chandrasekhar, N.; et al. Redox-Bipolar Polyimide Two-Dimensional Covalent Organic Framework Cathodes for Durable Aluminium Batteries. Angew. Chem. Int. Ed. 2023, 62, e202306091.
- Wang, G.; Chandrasekhar, N.; Biswal, B.P.; Becker, D.; Paasch, S.; Brunner, E.; Addicoat, M.; Yu, M.; Berger, R.; Feng, X. A Crystalline, 2D Polyarylimide Cathode for Ultrastable and Ultrafast Li Storage. Adv. Mater. 2019, 31, e1901478.
- Song, Z.; Zhan, H.; Zhou, Y. Polyimides: Promising energy-storage materials. Angew. Chem. Int. Ed. 2010, 49, 8444–8448.
- Cusin, L.; Peng, H.; Ciesielski, A.; Samori, P. Chemical Conversion and Locking of the Imine Linkage: Enhancing the Functionality of Covalent Organic Frameworks. Angew. Chem. Int. Ed. 2021, 60, 14236–14250.
- Luo, D.; Zhang, J.; Zhao, H.; Xu, H.; Dong, X.; Wu, L.; Ding, B.; Dou, H.; Zhang, X. Rational design of covalent organic frameworks with high capacity and stability as a lithium-ion battery cathode. Chem. Commun. 2023, 59, 6853–6856.
- Yang, D.H.; Yao, Z.Q.; Wu, D.H.; Zhang, Y.H.; Zhou, Z.; Bu, X.H. Structure-modulated crystalline covalent organic frameworks as high-rate cathodes for Li-ion batteries. J. Mater. Chem. A 2016, 4, 18621–18627.
- Shehab, M.K.; Weeraratne, K.S.; El-Kadri, O.M.; Yadavalli, V.K.; El-Kaderi, H.M. Templated Synthesis of 2D Polyimide Covalent Organic Framework for Rechargeable Sodium-Ion Batteries. Macromol. Rapid Commun. 2023, 44, e2200782.
- Wang, Z.; Li, Y.; Liu, P.; Qi, Q.; Zhang, F.; Lu, G.; Zhao, X.; Huang, X. Few layer covalent organic frameworks with graphene sheets as cathode materials for lithium-ion batteries. Nanoscale 2019, 11, 5330–5335.
- Bian, G.; Yin, J.; Zhu, J. Recent Advances on Conductive 2D Covalent Organic Frameworks. Small 2021, 17, e2006043.
- An, Y.K.; Tan, S.S.; Liu, Y.; Zhu, K.; Hu, L.; Rong, Y.G.; An, Q.Y. Designs and applications of multi-functional covalent organic frameworks in rechargeable batteries. Energy Storage Mater. 2021, 41, 354–379.
- Mighani, H. Schiff Base polymers: Synthesis and characterization. J. Polym. Res. 2020, 27, 168.
- Hong, J.; Lee, M.; Lee, B.; Seo, D.H.; Park, C.B.; Kang, K. Biologically inspired pteridine redox centres for rechargeable batteries. Nat. Commun. 2014, 5, 5335.
- López-Herraiz, M.; Castillo-Martínez, E.; Carretero-González, J.; Carrasco, J.; Rojo, T.; Armand, M. Oligomeric-Schiff bases as negative electrodes for sodium ion batteries: Unveiling the nature of their active redox centers. Energy Environ. Sci. 2015, 8, 3233–3241.
- Chen, Y.Q.; Manzhos, S. Lithium and sodium storage on tetracyanoethylene (TCNE) and TCNE-(doped)-graphene complexes: A computational study. Mater. Chem. Phys. 2015, 156, 180–187.
- Chen, Y.; Manzhos, S. A comparative computational study of lithium and sodium insertion into van der Waals and covalent tetracyanoethylene (TCNE)-based crystals as promising materials for organic lithium and sodium ion batteries. Phys. Chem. Chem. Phys. 2016, 18, 8874–8880.
- Lei, Z.; Yang, Q.; Xu, Y.; Guo, S.; Sun, W.; Liu, H.; Lv, L.P.; Zhang, Y.; Wang, Y. Boosting lithium storage in covalent organic framework via activation of 14-electron redox chemistry. Nat. Commun. 2018, 9, 576.
- Wu, C.G.; Hu, M.J.; Yan, X.R.; Shan, G.C.; Liu, J.Z.; Yang, J. Azo-linked covalent triazine-based framework as organic cathodes for ultrastable capacitor-type lithium-ion batteries. Energy Storage Mater. 2021, 36, 347–354.
- Wu, Z.Z.; Liu, Q.R.; Yang, P.; Chen, H.; Zhang, Q.C.; Li, S.; Tang, Y.B.; Zhang, S.Q. Molecular and Morphological Engineering of Organic Electrode Materials for Electrochemical Energy Storage. Electrochem. Energy Rev. 2022, 5, 26.
- Peng, C.X.; Ning, G.H.; Su, J.; Zhong, G.M.; Tang, W.; Tian, B.B.; Su, C.L.; Yu, D.Y.; Zu, L.H.; Yang, J.H.; et al. Reversible multi-electron redox chemistry of π-conjugated N-containing heteroaromatic molecule-based organic cathodes. Nat. Energy 2017, 2, 17074.
- Xu, S.; Wang, G.; Biswal, B.P.; Addicoat, M.; Paasch, S.; Sheng, W.; Zhuang, X.; Brunner, E.; Heine, T.; Berger, R.; et al. A Nitrogen-Rich 2D sp(2) -Carbon-Linked Conjugated Polymer Framework as a High-Performance Cathode for Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2019, 58, 849–853.
- Wang, W.; Kale, V.S.; Cao, Z.; Lei, Y.; Kandambeth, S.; Zou, G.; Zhu, Y.; Abouhamad, E.; Shekhah, O.; Cavallo, L.; et al. Molecular Engineering of Covalent Organic Framework Cathodes for Enhanced Zinc-Ion Batteries. Adv. Mater. 2021, 33, e2103617.
- Wu, M.M.; Zhao, Y.; Sun, B.Q.; Sun, Z.H.; Li, C.X.; Han, Y.; Xu, L.Q.; Ge, Z.; Ren, Y.X.; Zhang, M.T.; et al. A 2D covalent organic framework as a high-performance cathode material for lithium-ion batteries. Nano Energy 2020, 70, 104498.
- Yang, X.; Gong, L.; Liu, X.; Zhang, P.; Li, B.; Qi, D.; Wang, K.; He, F.; Jiang, J. Mesoporous Polyimide-Linked Covalent Organic Framework with Multiple Redox-Active Sites for High-Performance Cathodic Li Storage. Angew. Chem. Int. Ed. 2022, 61, e202207043.
- Shehab, M.K.; Weeraratne, K.S.; Huang, T.; Lao, K.U.; El-Kaderi, H.M. Exceptional Sodium-Ion Storage by an Aza-Covalent Organic Framework for High Energy and Power Density Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 15083–15091.
- Huang, H.; Wang, K.Y. Conductive metal-covalent organic frameworks as novel catalytic platforms for reduction of nitrate to ammonia. Green. Chem. 2023, 25, 9167–9174.
- Li, S.W.; Liu, Y.Z.; Dai, L.; Li, S.; Wang, B.; Xie, J.; Li, P.F. A stable covalent organic framework cathode enables ultra-long cycle life for alkali and multivalent metal rechargeable batteries. Energy Storage Mater. 2022, 48, 439–446.
- Liu, X.; Jin, Y.; Wang, H.; Yang, X.; Zhang, P.; Wang, K.; Jiang, J. In Situ Growth of Covalent Organic Framework Nanosheets on Graphene as the Cathode for Long-Life High-Capacity Lithium-Ion Batteries. Adv. Mater. 2022, 34, e2203605.
- Chu, J.; Cheng, L.; Chen, L.; Wang, H.-g.; Cui, F.; Zhu, G. Integrating multiple redox-active sites and universal electrode-active features into covalent triazine frameworks for organic alkali metal-ion batteries. Chem. Eng. J. 2023, 451, 139016.
- Vitaku, E.; Gannett, C.N.; Carpenter, K.L.; Shen, L.; Abruna, H.D.; Dichtel, W.R. Phenazine-Based Covalent Organic Framework Cathode Materials with High Energy and Power Densities. J. Am. Chem. Soc. 2020, 142, 16–20.
- Wang, Y.Y.; Wang, X.L.; Tang, J.; Tang, W.H. A quinoxalinophenazinedione covalent triazine framework for boosted high-performance aqueous zinc-ion batteries. J. Mater. Chem. A 2022, 10, 13868–13875.
- Sun, R.; Hou, S.; Luo, C.; Ji, X.; Wang, L.; Mai, L.; Wang, C. A Covalent Organic Framework for Fast-Charge and Durable Rechargeable Mg Storage. Nano Lett. 2020, 20, 3880–3888.
- Wang, T.; Gaugler, J.A., 2nd; Li, M.; Thapaliya, B.P.; Fan, J.; Qiu, L.; Moitra, D.; Kobayashi, T.; Popovs, I.; Yang, Z.; et al. Construction of Fluorine- and Piperazine-Engineered Covalent Triazine Frameworks Towards Enhanced Dual-Ion Positive Electrode Performance. ChemSusChem 2023, 16, e202201219.




