Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nicolas Inguimbert | -- | 5750 | 2024-03-05 10:53:47 | | | |
| 2 | Camila Xu | Meta information modification | 5750 | 2024-03-06 02:47:22 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ratibou, Z.; Inguimbert, N.; Dutertre, S. Predatory and Defensive Strategies in Cone Snails. Encyclopedia. Available online: https://encyclopedia.pub/entry/55866 (accessed on 08 February 2026).
Ratibou Z, Inguimbert N, Dutertre S. Predatory and Defensive Strategies in Cone Snails. Encyclopedia. Available at: https://encyclopedia.pub/entry/55866. Accessed February 08, 2026.
Ratibou, Zahrmina, Nicolas Inguimbert, Sébastien Dutertre. "Predatory and Defensive Strategies in Cone Snails" Encyclopedia, https://encyclopedia.pub/entry/55866 (accessed February 08, 2026).
Ratibou, Z., Inguimbert, N., & Dutertre, S. (2024, March 05). Predatory and Defensive Strategies in Cone Snails. In Encyclopedia. https://encyclopedia.pub/entry/55866
Ratibou, Zahrmina, et al. "Predatory and Defensive Strategies in Cone Snails." Encyclopedia. Web. 05 March, 2024.
Copy Citation
Cone snails are specialized carnivorous marine mollusks that can be found in coral reef areas, from shallow intertidal to deeper waters, and spread across the tropical Indian, Pacific, and Atlantic Oceans. They are classified as gastropods within the Conidae family, which feature hollow radular teeth and venom glands. They use a complex venom mixture to paralyze and hunt fish, mollusks, and worms.
Conus species
conotoxins
“milked” venom
predatory and defensive venom
1. Introduction
Cone snails are specialized carnivorous marine mollusks that can be found in coral reef areas, from shallow intertidal to deeper waters, and spread across the tropical Indian, Pacific, and Atlantic Oceans [1]. They are classified as gastropods within the Conidae family, which feature hollow radular teeth and venom glands [2]. They use a complex venom mixture to paralyze and hunt fish, mollusks, and worms [3]. This venom is secreted through epithelial cells lining the cone’s venom gland, which is a long and thin tubular duct [4]. A singular radular tooth, analogous to a hypodermic needle, is then moved into the proboscis through which the rapid-acting venom is injected. The venom is acknowledged as a rich source of potent pharmacological components, raising high interest in the drug development field [5].
This venom consists primarily of biologically active peptides, generally characterized as conotoxins or conopeptides. They can be classified into two groups: conotoxins, which are cysteine-rich conopeptides consisting of 10 to 30 amino acids, while conopeptides are cysteine-poor, meaning 1 or no disulfide bond [4][5][6]. Moreover, conotoxins are highly structured and often show high affinity and selectivity toward membrane receptors, ion channels, and other transmembrane proteins of the nervous and non-nervous systems [4]. Conopeptides include several types of cysteine-poor peptides, such as contulakins, conantokins, conorfamides, conolysins, conophans, conomarphins, contryphans, conopressins, and more recently, hormone-like conopeptides, such as elevenins or prohormones [5][7]. Conopeptides are usually minor in comparison to conotoxins in the venom mixture and each presents a selective type of target [7]. These small peptides can work as ligands, which induce a physiological reaction by interacting with a given receptor [4]. Conotoxins and conopeptides are secreted as peptide precursors, which can be portioned into three characteristic sections: a highly conserved signal peptide, representative of the gene superfamily from which it was translated, a pro-peptide section, and a highly diversified mature peptide (Figure 1). The mature peptide is the active sequence portion, which is enzymatically cleaved and then modified into a highly stable structure within the injected venom [8].

Figure 1. Conotoxin precursors. An alignment of six conotoxins belonging to the same gene superfamily (O1). The signal region (framed in blue) presents a sequence of highly conserved residues, mainly hydrophobic, while the mature region (framed in purple) presents more diversity of sequence and a greater number of cysteine residues. Conotoxin precursors: ω-GVIA (Gastridium geographus), ω-SVIA (Pionoconus striatus), ω-CVID (Pionoconus catus), ω-MVIIA (Pionoconus magus), δ-PVIA (Chelyconus purpurascens), and δ-TxVIA (Cylinder textile). The conotoxin precursors were aligned, amino acid residues were highlighted (in purple) according to the conservation, and disulfide bonds are represented with black lines.
The cysteine pattern within the conotoxin sequence is designated with roman numerals and it directs the tridimensional structure, which in turn also influences their biological activity. So far, although only few conotoxins have been fully characterized pharmacologically, more than 20 pharmacological targets have been identified. Some of the biological targets involve, for the most part, ion channels, but also some G-protein-coupled receptors and transporters [3]. Conotoxins are classified according to their targets into pharmacological families, defined by Greek letters, such as α, δ, μ, ω, κ, γ, etc. (Figure 2) [3]. For instance, ω-conotoxins are antagonists of voltage-gated calcium channels, and some are effective against neuropathic pain [3]. Such activity was the basis for the development of the first marine-based drug isolated from a cone snail, known as Prialt®. This drug is a synthetic version of the ω-conotoxin MVIIA isolated from the piscivorous species, Pionoconus magus [9]. Likewise, some α-conotoxins have been characterized as nicotinic acetylcholine receptors (nAChRs) antagonists, with some of them having potential in the treatment of pain, cognitive, cardiovascular, and other disorders [9]. For the past three decades, research in the field has been mainly focused on finding new ligands for known targets, with a strong emphasis on modulators of pain receptors [9].
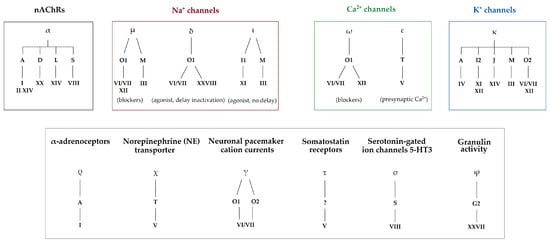
Figure 2. Pharmacological classification of conotoxins according to their gene superfamilies and cysteine framework. Pharmacological families are defined by Greek letters (α, μ, δ, ι, ω, ε, κ, ρ, χ, γ, τ, σ, and φ), gene superfamilies by Arabic capital letters (A, B, C, D, E, F, G, H, I, J, etc.), and cysteine frameworks by roman numbers (I, II, III, IV, V, VI, etc.). Identified biological targets may be linked to one or several pharmacological families (i.e., voltage-gated Na+ channels are targeted by µ-, δ-, and ι-conotoxins) [3][5][10].
The ~800 species of cone snails can be categorized into three main groups according to their diet. Piscivorous species hunt fish, molluscivorous species prey upon mollusks, and vermivorous species feed upon worms (Figure 3). The type of radula tooth seems to be directly correlated to the diet, and this criterion has been used to support the classification of species [11]. Based on molecular phylogenetic studies, cone snails have been classified into a single large family, Conidae, which can then be divided into four genera: Conus, Conasprella, Profundiconus, and Californiconus [2]. The genus Conus constitutes more than 85% of all cone snail species, which can then be further classified into 57 subgenera or ‘clades’ of Conus species, which represent a clear subgrouping within the genera [2]. These classifications can provide a better understanding of the “biotic interactions” within Conus species [4]. Unfortunately, rather than being tested on biologically relevant animal models, cone snail venoms have almost exclusively been investigated using mammalian bioassays. As a result, the conclusions drawn from these assays should be interpreted with caution when extrapolated to the biology of cone snails.
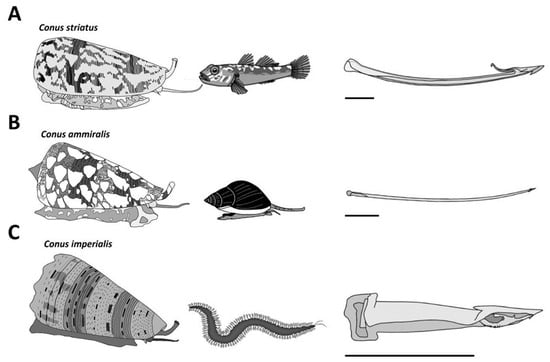
Figure 3. Major diet types observed in cone snails. (A) The piscivorous diet is represented here with a Pionoconus striatus specimen, which uses a “taser-and-tether” strategy to subdue its fish prey. The radula tooth is modified into a mini-harpoon. (B) Cylinder ammiralis is a molluscivorous species that injects thick venom multiple times through fine and long arrow-like radula teeth to incapacitate its gastropod prey. (C) Stephanoconus imperialis, which preys exclusively on amphinomid worms, uses a short and stout radula tooth to forcefully inject its greenish venom in large quantities. Horizontal bars indicate 1 mm.
1.1. Envenomation Strategies in Cone Snails
Piscivorous cone snails exhibit varying types of hunting behaviors. For instance, upon the detection of a prey, first through chemosensory cues [12], some cone snails extend their proboscis in order to inject a paralytic venom (Figure 4A). The venom is injected via a radula tooth that is comparable to a miniature harpoon that the cone snail uses to sting and tether the prey to avoid its escape [4][13]. Upon the strike, the prey often displays an immediate tetanic paralysis. The cone snail then retracts its proboscis to drag its victim toward its enlarged rostrum to engulf it [13]. The archetype of this behavior is the ‘taser-and-tether’ strategy employed by the majority of piscivorous species from the Pionoconus, Textilia, and Chelyconus clades, where injection of venom first produces an immediate paralysis (“taser”), followed by the reeling back of the tethered fish into the rostrum via the contraction of the proboscis, which is still tightly grasping the base of the radula tooth [4].
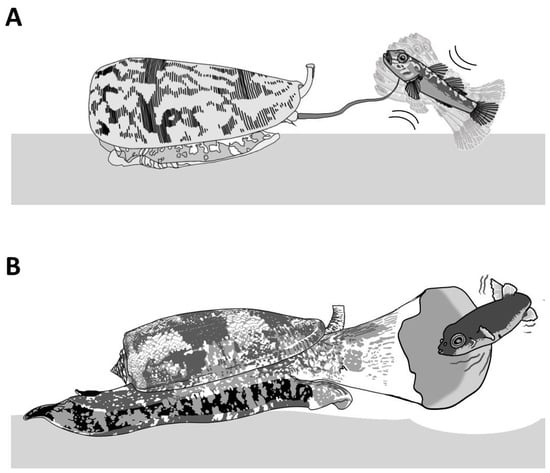
Figure 4. Piscivorous “taser-and-tether” and “net-hunting” strategies. (A) Pionoconus striatus is the prototypical species that uses a “taser-and-tether” strategy. The extended proboscis is reminiscent of a fish line and the radula tooth modified into a mini-harpoon to tether a prey. (B) The net-hunting strategy of a Gastridium geographus implies the extension of its rostrum in order to engulf a school of fish, which are already dazed by the hypothetical release of sedative compounds in the water.
Remarkably, some other cone snails have been observed to catch their prey without prior sting. In this case, the cone snail is hypothesized to release a set of toxins in the water, which places the prey into a sedative-sleepy state (Figure 4B). The cone snail then opens its rostrum to engulf it and may proceed to envenomate and predigest the prey [13]. Thus, cone snails that use this strategy, named as ‘net-hunting’, would supposedly release venom components in the water and inject paralytic peptides, which induces an irreversible neuromuscular paralysis of the captured prey. Lastly, the “strike-and-stalk” envenomation strategy is a variation of the taser-and-tether strategy, where the cone snail strikes a prey without tethering it and engulfs it after immobilization has occurred. The latter strategy remains less studied in terms of the neurobiological mechanism involved [13].
In non-piscivorous cone snails, the hunting behaviors have been much less investigated. For most molluscivorous species observed in captivity or in the wild, the predatory strategy involves actively chasing the prey and injecting, multiple times, fine, arrow-like radula teeth into the foot of the prey [14]. The firing of the radula tooth is usually accompanied by vigorous pumping of copious amount of venom, which can be seen, when injected in excess, as a whitish cloud escaping out the tip of the proboscis and/or out of the base of the tooth from back pressure [15]. In the case of the mass spectrometry (MS) analysis of successive stings by Cylinder textile, modest variations in the venom composition were described [16]. The first injection usually stops or slows down the prey but does not completely incapacitate it; therefore, it was suggested that a second, third, or more injections, possibly with different peptides, were needed to eventually overcome the prey.
Hunting behaviors for vermivorous species are even more elusive, except for only a few species. Both Stephanoconus imperialis and Stephanoconus regius prey almost exclusively on amphinomid worms (“fireworms”). These two species use a prey capture strategy reminiscent of the “taser-and-tether” strategy employed by many piscivorous species. Indeed, the targeted worm is first detected by the chemosensory organs, inducing the extension of a reddish proboscis. The short radula tooth (1–1.5 mm) is then fired and embedded into the worm’s body, forcefully pushing through a remarkable quantity of a greening venom (Figure 3C) [9]. As described for the fish-hunters, the envenomated prey shows immediate involuntary contractions, leading to incapacitation, and is reeled back into the rostrum. Our personal observations on other vermivorous species often reveal, surprisingly, an apparent venom-less strategy, where the snail directly attempts to swallow the worm through its extended rostrum without prior stinging via the proboscis. One of the most mysterious prey strategies relates to the vermivorous species hunting tube worms, as there is no description in the literature.
1.2. Reality Check on the Concept of Cabals
Early pharmacological characterization of conotoxins from venom gland extracts revealed a variety of targets and modes of action. From the pharmacological effects obtained mostly on mammals, extrapolations were made to explain the effects observed on prey, and this is how the concept of cabals was first crafted. The cabals are defined as a group of (artificially put together) conotoxins, which seem to modulate the same physiological target or may act synergistically. Thus, the “lightening-strike cabal” is defined as a set of κ- and δ-conotoxins, as well as conkunitzins, which would together elicit an excitatory state on the prey [17][18]. This reaction is due, respectively, to the inhibition of K+ channels, as well as a delayed inactivation of Na+ channels [4].
Meanwhile, the “nirvana cabal” is highly speculative, but could include the release in the surrounding water of a mixture of B1-conotoxins [19] and hormone-like peptides that would induce a “hypoactivity in sensory neuronal circuity” [4][20][21]. Although prey capture observations of net-hunting species seem to corroborate this hypothesis, there is currently no direct evidence to support any release of venom into the water. Lastly, an additional “motor cabal” was proposed to be responsible for the final flaccid paralysis that prevents the prey from recovering the initial excitatory shock. The latter involves α-, µ-, and ω-conotoxins that interfere with the neuromuscular junction [18].
Although these cabals were logically formulated, do they actually correspond to the reality of the predatory strategies employed by cone snails to defeat their prey? Nearly thirty years ago, an ingenious procedure, now commonly referred to as “milking”, was devised that allows for the collection of the injected venom, providing a direct means of interrogating the conotoxin cocktail used for prey capture [22]. Using a live prey to arouse the cone snail and trigger a predatory behavior, a microcentrifuge tube covered with parafilm, and a piece of the prey’s tissue, is presented to the tip of the extended proboscis. Sensory cilia at the tip of the proboscis identify the tissue as “prey” and instantaneously trigger the injection of venom through the radula tooth. Such recovered “milked venoms” can now be analyzed, and the composition revealed. Over the last two decades, the more milked venoms were investigated, the less obvious the role of the conotoxins described in these cabals was for prey capture [23].
Overall, in all cases investigated, milked venoms appear significantly less complex compared to dissected gland extracts. For instance, in Pionoconus species, the predatory venom is usually dominated by one class of conotoxins (sometimes the only conotoxins seemingly injected), the κA-conotoxins [24][25][26]. Therefore, it appears that κA-conotoxins are responsible for the immediate “taser” effect in this clade, not a combination of κ- and δ-conotoxins, as originally described for the lightning-strike cabal. Indeed, injection of κA-conotoxins alone into fish recapitulates the tetanic paralysis observed during prey capture [27]. However, it has to be noted that intraspecific variations in the injected venom can be dramatic and, occasionally, the paralytic peptides from the “motor cabal” are detected, suggesting that they could play a significant role in prey capture [23]. Although not fully explained at the time, one aspect of this diversification was later attributed, at least in part, to the unsuspected ability of some cone snails to produce two types of venoms [28].
1.3. Defensive Strategies
From the three dozen human deaths reported, it has long been known that cone snails can also inject their venom defensively [29]. In the literature, there is only anecdotal information on the natural predators of cone snails, but fish, mollusks (octopi), and some crustaceans are known to prey on them (Figure 5). For instance, a rare species of deep-water cone snail was first described only from a shell recovered from the stomach content of a large fish (personal communication). The defensive use of venom provides an obvious evolutionary advantage. Indeed, avoiding being eaten is one of the most important fitness-related criterion for the survival of a species, together with being able to feed and reproduce. In fact, some venomous animals only use their venom defensively (some hymenopterans, fish, etc.), whereas the reverse is not true, suggesting that the defensive use of venom may actually have a stronger evolutionary role than anticipated, possibly more than predation in some cases [30].
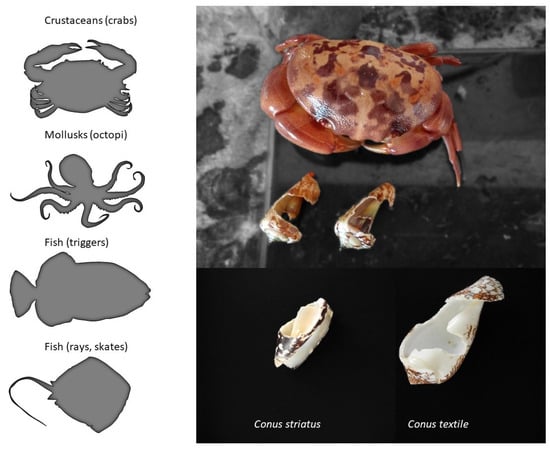
Figure 5. Natural predators of cone snails. The left panel shows the known predators of cone snails, whereas on the right is an example of the damages caused by a crab that was held in captivity together with various mollusks, including cone snails.
Thanks to their capacity to defend themselves, some species of cone snails have evolved some unique behaviors. However, for most species, the first line of defense is usually to retract deeply into the shell, which offers a strong and often inviolable fortress (Figure 6C). Others will respond aggressively to any threat by extending their proboscis (Figure 6D). If the threat intensifies, the cone snail will inject venom into the aggressor, but there are also reports of cone snails squirting venom (personal observations). Additional behavioral studies are needed to fully decipher the complex defensive responses displayed by cone snails.
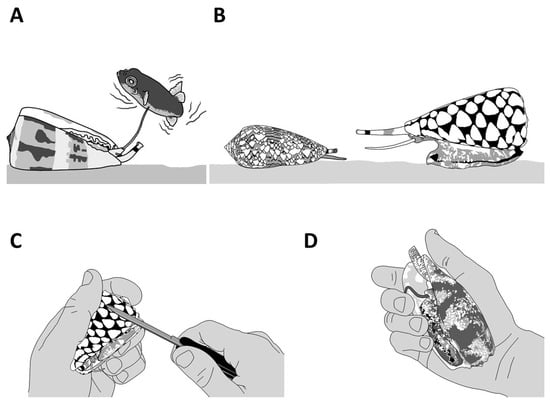
Figure 6. The defensive behaviors of cone snails. A defensive reaction can be triggered by different means, including using a natural predator (A,B) or aggravating the animal by directly interacting with it (C) or applying pressure to the shell (D). Live cone snails should not be handled.
The most dangerous species to humans, Gastridium geographus, displays an unusually aggressive behavior, and will readily use its venom defensively when handled. There seems to be a striking relationship between the fragility of the shell (as in the case of Gastridium geographus) and the propensity to use venom defensively. Typically, large vermivorous species will often be unfazed by any threat, being protected by heavily built shells and narrow apertures [28]. However, many species were reported to inflict injuries to humans, regardless of their diet, with varied degrees of consequences. From the known human Conus envenomation, various levels of severity were distinguished, from fatal to minor effects, comparable to bee stings, and the most adverse symptoms were attributed to piscivorous cone snails, especially Gastridium geographus [29].
The first investigation of a defense-evoked venom uncovered an unsuspected twist in cone snail biology [28]. Indeed, the defensive venom of Gastridium geographus was highly complex and contained massive amounts of paralytic conotoxins from the “motor cabal”, explaining the lethal symptoms in humans, as opposed to the predatory venom, which was devoid of these and instead contained prey-specific conotoxins with no activity on human receptors. Therefore, in this iconic species, paralytic conotoxins directed to the neuromuscular junction are essentially defensive weapons, not part of the prey capture strategy, a result in conflict with the cabal narrative. From this initial discovery, more data on different species were needed to evaluate how widespread this separate evolution of predatory and defensive venoms is among cone snail species. Triggering and collecting defensive venom can be achieved through different means, including using a natural predator (i.e., a molluscivorous species, such as Conus marmoreus or Cylinder textile), applying pressure to the shell, or pinching the foot of the cone (Figure 6D) [28].
Overall, the remarkable ability of cone snails to purposefully modify their venom composition upon different triggering stimuli (predatory or defensive) offers novel and unprecedented research opportunities. Indeed, separately collecting each venom type will allow unambiguous interpretation of the ecological and evolutionary roles of each conotoxin.
2. Piscivorous Cone Snails
2.1. Predatory Venom
Thus far, the Conus genus counts around 800 different species, representing about 70% of vermivorous, 20% piscivorous, and 10% of molluscivorous cone snails [31]. Over a hundred piscivorous cone snails have been classified into the following clades: Afonsoconus, Asprella, Chelyconus, Embrikena, Gastridium, Phasmoconus, Pionoconus, and Textilia, although the piscivorous diet requires confirmation for the Afonsoconus, Asprella, and Embrikena clades [13]. In comparison, the venoms of fish-hunting cone snails, such as Pionoconus striatus, Gastridium geographus, and Chelyconus purpurascens, have been extensively characterized against the prevailing vermivorous species (Figure 7) [28][32][33].
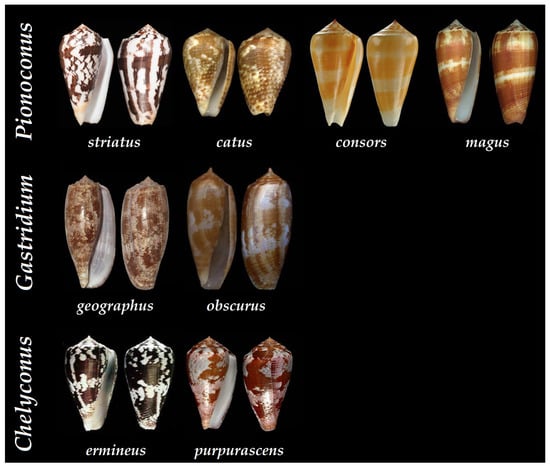
Figure 7. Shells of some of the piscivorous cone snails that have been characterized at the peptide level according to their clade. Pionoconus striatus, Pionoconus catus, Pionoconus consors, Pionoconus magus, Gastridium geographus, Gastridium obscurus, Chelyconus purpurascens, and Chelyconus ermineus [34].
In general, the predatory venoms of fish-hunting cone snails show major contributions of small disulfide-rich conotoxins over larger ones and cysteine-poor conopeptides, such as conophysins [28], conopressins [28][33], and contryphans [32][35] (Figure 8A). The conotoxins identified are scattered into a dozen gene superfamilies, dominated by A-, O1- and M-conotoxins. The rest are attributed to the I3, C, O2, T, and B1 superfamilies [23][29][33]. Most conotoxins that were identified in the predatory venoms of fish-hunting cone snails were previously biologically characterized from venom gland extracts. The α-, κA-, δ-, κ-, μ-, and ω-conotoxins constitute the major pharmacological families identified in the predatory venom (Figure 8B). κA-conotoxins are the most abundant (relative contribution to the injected venom) (Table 1), but α-conotoxins are the most prevalent (in terms of number of sequences identified) in the predatory venoms of fish-hunting cone snails (Table 2). These conotoxins are especially represented in the Pionoconus and Chelyconus clades, and less in the Gastridium clade.
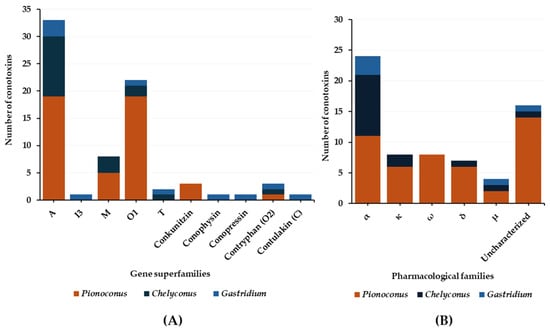
Figure 8. Gene superfamilies (A) and pharmacological families (B) identified within the predatory-evoked venoms of piscivorous cone snails.
Table 1. κ-Conotoxins identified from predatory and defense venoms of fish-hunting cone snails. Presented here are conotoxins found exclusively in the predation-evoked or in both venoms. Each conotoxin is characterized by its Conus clade, the Conus species in which it was detected, the given name, its sequence, its classification within the gene superfamilies, and the cysteine framework. Cysteine residues are highlighted in red.
| Clades | Conus Species | Conotoxins | Mature Sequence | Gene Superfamily |
Cysteine Framework | References |
|---|---|---|---|---|---|---|
| Pionoconus | striatus | κA-SIVA | ZKSLVP(gSr)VITTCCGYDOGTMCOOCRCTNSCX | A | IV | [32] |
| κA-SIVB | ZKELVP(gSr)VITTCCGYDOGTMCOOCRCTNSCOTKOKKOX | A | IV | [32] | ||
| κA-SIVC | AOAL(I)VVTATTNCCGYTGOACHOCL(I)CTQTC | IV | [36] | |||
| catus | C4.41 | QKELVPSTITTCCGHEPGTMCPKCMCDNTCPPQKEEKTRPQ | A | IV | [25] | |
| C1.5 | QKELVPSTITTCCGNGTGDNVDPKCMCDNTSSPKKKKRP | A | I | [25] | ||
| consors | κA-CcTx | AOWLVP(gSr)QITTCCGYNOGTMCOSCMCTNTC | A | IV | [24][37] | |
| Chelyconus | purpurascens | κA-PIVE | DCCGVKLEMCHPCLCDNSCKNYGKX | A | IV | [17][38] |
| κA-PIVF | DCCGVKLEMCHPCLCDNSCKKSGKX | A | IV | [17][38] |
Table 2. α-Conotoxins identified from predatory and defense venoms of fish-hunting cone snails. Presented here are conotoxins found exclusively in the predation-evoked, the defense-evoked, or in both venoms. Each conotoxin is characterized by its Conus clade, the Conus species in which it was detected, the given name, its sequence, its classification within the gene superfamilies, and the cysteine framework. Cysteine residues are highlighted in red.
| Clades | Conus Species | Conotoxins | Mature Sequence | Gene Superfamily |
Cysteine Framework | References |
|---|---|---|---|---|---|---|
| Pionoconus | striatus | α-SI | ICCNPACGPKYSCX | A | I | [23][32][39] |
| α-SIA | YCCHPACGKNFDCX | A | I | [23][32] | ||
| α-SII | GCCCNPACGPNYGCGTSCS | A | II | [23][32][39] | ||
| consors | α-CnIB | CCHPACGKYYSCX | A | I | [24][37] | |
| α-CnIA | GRCCHPACGKYYSCX | A | I | [24][37] | ||
| CnIG | CCHPACGKYFKCX | I | [37] | |||
| CnIJ | GRCCHPACGGKYFKCX | A | I | [37] | ||
| CnIH | NGRCCHPACGKHFSCX | A | I | [37] | ||
| CnIK | NGRCCHPACGKYYSCX | A | I | [37] | ||
| CnIL | DGRCCHPACGKYYSCX | A | I | [37] | ||
| catus | α-C4.3 | NGRCCHPACGKHFSC | A | I | [25] | |
| α-CIB | GCCSNPVCHLEHPNACX | A | I | [25] | ||
| α-C1.3 | GCCSNPVCHLEHSNLCX | A | I | [25] | ||
| magus | α-MI | GRCCHPACGKNYSCX | A | I | [40] | |
| α-MII | GCCSNPVCHLEHSNLCX | A | I | [40] | ||
| α-MIC | CCHPACGKNYSCX | A | I | [40] | ||
| Gastridium | geographus | α-GIC | GCCSHPACAGNNQHICX | A | I | [28][33] |
| α-GIA | ECCHPACGRHYSCGK | A | I | [28][33] | ||
| α-GII | ECCHPACGKHFSCX | A | I | [28][33] | ||
| α-GID | IRD(Gla)CCSNPACRVNNPHVC | A | I | [28][33] | ||
| obscurus | α-OIVA | CCGVONAACHOCVCKNTCX | A | IV | [28][41] | |
| α-OIVB | CCGVONAACPOCVCNKTCGX | A | IV | [28][42] | ||
| Chelyconus | purpurascens | α-PIB | ZSOGCCWNPACVKNRCX | A | I | [17][43] |
| α-PIC | SGCCKHOACGKNRC | A | I | [17][44] | ||
| α-PIVA | GCCGSYONAACHOCSCKDROSYCGQX | A | IV | [17] | ||
| α-PIIIE | HOOCCLYGKCRRYPGCSSASCCQRX | M | III | [17] | ||
| α-PIIIF | GOOCCLYGSCROFOGCYNALCCRKX | M | III | [17][45] | ||
| ermineus | α-EIVA | GCCGPYONAACHOCGCKVGROOYCDROSGGX | A | IV | [46][47] | |
| α-EIVB | GCCGKYONAACHOCGCTVGROOYCDROSGGX | A | IV | [46][47] | ||
| α-EIIA | ZTOGCCWNPACVKNRCX | A | I | [47][48] | ||
| α-EIIB | ZTOGCCWHPACGKNRCX | A | I | [47][48] | ||
| α-EI | RDOCCYHPTCNMSNPQICX | A | I | [47][49] |
As mentioned, the superfamily A is the most represented in the predatory venom thanks to κA-conotoxins and α-conotoxins. κA-conotoxins were first discovered in the predatory venom of Pionoconus striatus, with κA-SIVA and κA-SIVB [32], and their short and non-glycosylated equivalent κA-PIVE and κA-PIVF were identified in Chelyconus purpurascens (Table 1) [17][38]. Exhaustive investigation of the predatory venom of Pionoconus consors also revealed the importance of κA-conotoxins, with the abundant injection of κA-CcTx and the sequencing of a series of CcTx variants [24][37][50]. Although not confirmed, the major compounds found in the predatory venom of another Pionoconus species, Pionoconus magus, were determined within the mass range of κA-conotoxins [40]. More recently, these κA-conotoxins were identified abundantly in both predatory and defensive venom of Pionoconus striatus [32] and in the predatory venom of Pionoconus catus [25]. Interestingly, a recent study has identified a variant of the glycosylated κA-conotoxins (κA-SIVC) in the predatory venom of specimens of Pionoconus striatus from Mayotte (France), suggesting that geographical variations can be population-specific [36]. κA-conotoxins were initially characterized as excitatory peptides that block K+ channels, yet controversy remains over the molecular target since Na+ channels were also suggested as the targeted receptor [27]. Although they uphold the same IV cysteine framework as certain αA-conotoxins, such as α-OIVA (Table 2), their activities are different: the firsts are excitatory while the seconds are not [41]. Generally, these κA-conotoxins appear as the major and most abundant component in the predatory venom of Pionoconus species and are likely solely responsible for the rapid immobilization of prey.
Next, α-conotoxins (targets are the nAChRs) are the most prevalent pharmacological family in terms of number of sequences identified in the predatory venoms of fish-hunting cone snails. These α-conotoxins, although not systematically injected, are especially represented in the Pionoconus and Chelyconus clades, and less in the Gastridium clades [2]. For instance, some of the α-conotoxins identified in the Pionoconus clade include α-SI (Pionoconus striatus) [23][32][39], α-CnIB (Pionoconus consors) [37][51], α-MI (Pionoconus magus) [40], and α-CIB (Pionoconus catus) [25]. Similarly, α-PIB (Chelyconus purpurascens) and α-EIIA (Chelyconus ermineus) [52] are found in the Chelyconus clade. Finally, the Gastridium subgenus shows the least amount of identified α-conotoxins in their predatory venom, with only α-GIC (Gastridium geographus) [33] and α-OIVA (Gastridium obscurus) confirmed so far [41]. When injected into a prey, some of these α-conotoxins operate as slow (several minutes) paralytics analogous to the snake α-neurotoxins, as they selectively target and inhibit the muscle type of nAChRs (Figure 2) [10][53]. Interestingly, the subtle variations in length and amino acid residues determine the targeted site or nAChR subtype [10]. Usually, small 3/5 (3 and 5 correspond to the number of residues in inter-cysteine loops) α-conotoxins, such as α-SI, α-MI, and α-CnIB, selectively target muscle-type nAChRs [5], whereas larger 4/7 α-conotoxins, such as α-MII and α-GIC, target neuronal nAChRs, and their role in prey capture is less understood [43]. Considering their potentially useful paralytic capacities, and according to the “motor cabal” hypothesis, their presence in the predatory venoms would seem compulsory, but analysis of individual predatory venom rather than pool of collected venom suggests otherwise [10].
Exceptionally, δ-conotoxins were also detected in the predatory venom of some fish-hunters of the Pionoconus and Chelyconus clades (Table 3). This family of conotoxins was characterized as voltage-gated sodium channel (VGSC) modulators. Indeed, δ-conotoxins activate Nav channels via a delay in the inactivation mechanism (Figure 2) [54]. δ-Conotoxins, like the κ-conotoxins, are defined as excitatory peptides, which induce the rapid tetanic paralysis in their prey [32]. For example, the δ-PVIA isolated from Chelyconus purpurascens was characterized as the “lock-jaw peptide” because it causes a rigid paralysis of the prey, particularly visible around the mouth musculature [55]. Again, considering their critical role in the “lightning-strike cabal”, these conotoxins are expected to be always injected for prey capture, but it is almost never the case.
Table 3. δ-Conotoxins identified from predatory and defense venoms of fish-hunting cone snails. Presented here are conotoxins found exclusively in the predation-evoked venoms. Each conotoxin is characterized by its Conus clade, the Conus species in which it was detected, the given name, its sequence, its classification within the gene superfamilies, and the cysteine framework. Cysteine residues are highlighted in red.
| Clades | Conus Species | Conotoxins | Mature Sequence | Gene Superfamily |
Cysteine Framework | References |
|---|---|---|---|---|---|---|
| Pionoconus | consors | δ-CnVIA | YECYSTGTFCGINGGLCCSNLCLFFVCLTFS | O1 | VI/VII | [37] |
| δ-CnVIB | DECFSOGTFCGTKOGLCCSARCFSFFCISLEFX | O1 | VI/VII | [37] | ||
| δ-CnVIC | DECFSOGTFCGIKOGLCCSARCFSFFCISLEFX | O1 | VI/VII | [37] | ||
| striatus | δ-SVIE | DGCSSGGTFCGIHOGLCCSEFCFLWCITFID | O1 | VI/VII | [32] | |
| catus | δ-CVIE-2 | YGCSNAGAFCGIHOGLCCSELCLVWCT | O1 | VI/VII | [25] | |
| δ-C6.2 | DGCYNAGTFCGIROGLCCSEFCFLWCITFVDSX | O1 | VI/VII | [25] | ||
| Chelyconus | purpurascens | δ-PVIA | EACYAPGTFCGIKPGLCCSEFCLPGVCFGX | O1 | VI/VII | [55][56] |
Very few μ-conotoxins have been found in the predatory venom of three piscivorous clades: Pionoconus consors, Gastridium geographus, and Chelyconus purpurascens (Table 4). The molecular targets of μ-conotoxins are VGSCs, more precisely Nav channels, which play an important role in the central and peripheral nervous system (CNS and PNS) (Figure 2). However, contrary to δ-conotoxins, they act as blockers of the channel, instead of delaying its inactivation. The μ-conotoxin-GS is the only μ-conotoxin identified from Gastridium geographus predatory venom, and is highly potent on fish, but less on mammalian Nav channels [57]. Another example is the μ-CnIIIC, isolated from the venom of Pionoconus consors, from which a synthetic version was commercialized as a cosmetic to smoothen facial lines (XEPTM-018) [58]. The μ-CnIIIB was shown more specifically to block tetrodotoxin-resistant (TTX-R) Na channels, where the blockade was observed as “slow and reversible” [59]. Another type of μ-conotoxins, the μO-Conotoxins, restrict channel opening, which has been associated with many side effects upon intravenous application, such as paralysis and death of models in inflammatory and neuropathic pain, leading to a discouragement of further research as therapeutic agents [10].
Table 4. μ-Conotoxins identified from predatory and defense venoms of fish-hunting cone snails. Presented here are conotoxins found exclusively in the predation-evoked or the defense-evoked venoms. Each conotoxin is characterized by its Conus clade, the Conus species in which it was detected, the given name, its sequence, its classification within the gene superfamilies, and the cysteine framework. Cysteine residues are highlighted in red.
| Clades | Conus Species | Conotoxins | Mature Sequence | Gene Superfamily |
Cysteine Framework | References |
|---|---|---|---|---|---|---|
| Pionoconus | consors | μ-CnIIIB | ZGCCGEPNLCFTRWCRNNARCCRQQ | M | III | [24][37] |
| μ-CnIIIC | ZGCCNGPKGCSSKWCRDHARCCX | M | III | [24][37] | ||
| striatus | μ-S3-G02 | QKCCGEGSSCPKYFKNNFICGCC | M | III | [32] | |
| Chelyconus | purpurascens | μ-PIIIA | ZRLCCGFOKSCRSRQCKOHRCC | M | III | [56] |
| Gastridium | geographus | μ-Conotoxin-GS | ACSGRGSRCOOQCCMGLRCGRGNPQKCIGAH(Gla)DV | O1 | VI/VII | [28] |
| μ-GIIIA | RDCCTOOKKCKDRQCKOQRCCAX | M | III | [28][33] | ||
| μ-GIIIB | RDCCTOORKCKDRRCKOMKCCAX | M | III | [33] | ||
| μ-GIIIC | RDCCTOOKKCKDRRCKOLKCCA | M | III | [28][33] |
Trace amounts of ω-conotoxins known as “shaker” peptides have been detected in the predatory venoms of some fish-hunters [60]. ω-Conotoxins belong to the O1 gene superfamily with a VI/VII framework (Table 5). They are able to selectively block N-type Ca2+ channels (at the nerve terminals) and prevent the release of important neurotransmitters (such as glutamate, GABA, acetylcholine, dopamine, etc.) [61]. They are pore blockers, which means that they can physically block the influx of Ca2+ ions [62]. Upon intracerebral injection, they produce persistent shaking in mice [60]. ω-Conotoxins were extensively investigated for their potential in pain treatments, including ω-MVIIA, which is the only FDA-approved conotoxin drug (from Pionoconus magus, known as Ziconotide), as well as ω-CVID (Leconotide) isolated from Pionoconus catus, which is also under development for pain treatment [63]. Few ω-conotoxins have been detected in the predatory venom of Pionoconus cone snails, such as Pionoconus striatus (ω-SVIA and ω-SVIB) [39][64], Pionoconus consors (ω-CnVIIA) [37][51], and Pionoconus catus (ω-CVIA) [25][61].
Table 5. ω-Conotoxins identified from predatory and defense venoms of fish-hunting cone snails. Presented here are conotoxins found exclusively in the predation-evoked, the defense-evoked, or in both venoms. Each conotoxin is characterized by its Conus clade, the Conus species in which it was detected, the given name, its sequence, its classification within the gene superfamilies, and the cysteine framework. Cysteine residues are highlighted in red.
| Clades | Conus Species | Conotoxins | Mature Sequence | Gene Superfamily |
Cysteine Framework | References |
|---|---|---|---|---|---|---|
| Pionoconus | striatus | ω-SVIA | CRSSGSOCGVTSICCGRCYRGKCTX | O1 | VI/VII | [39][64] |
| ω-SVIB | CKLKGQSCRKTSYDCCSGSCGRSGKCX | O1 | VI/VII | [39][64] | ||
| SO4 | ATDCIEAGNYCGPTVMKICCGFCSPYSKICMNYPKN | O1 | VI/VII | [23][32] | ||
| SO5 | STSCMEAGSYCGSTTRICCGYCAYFGKKCIDYPSN | O1 | VI/VII | [23][32] | ||
| consors | ω-CnVIIA | CKGKGAOCTRL(Mox)YDCCHGSCSSSKGRCX | O1 | VI/VII | [24][37][51] | |
| magus | ω-MVIIA | CKGKGAKCSRLMYDCCTGSCRSGKCX | O1 | VI/VII | [40] | |
| ω-MVIIB | CKGKGASCHRTSYDCCTGSCNRGKCX | O1 | VI/VII | [40] | ||
| catus | ω-Catus-C2 | CQGRGASCRKTMYNCCSGSCNRGRC | O1 | VI/VII | [25] | |
| ω-CVIA | CKSTGASCRRTSYDCCTGSCRSGRCX | O1 | VI/VII | [25][61] | ||
| ω-CVID | CKSKGAKCSKLMYDCCSGSCSGTVGRCX | O1 | VI/VII | [25][61] | ||
| Gastridium | geographus | ω-GVIA | CKSOGSSCSOTSYNCCRSCNOYTKRCY | O1 | VI/VII | [28][33] |
| ω-GVIIA | CKSOGTOCSRGMRDCCTSCLLYSNKCRRY | O1 | VI/VII | [28][33] | ||
| ω-GVIIB | CKSOGTOCSRGMRDCCTSCLSYSNKCRRY | O1 | VI/VII | [28][33] |
Finally, some conopeptides, larger conotoxins, and proteins were also isolated from predatory venoms of fish-hunting cone snails (Table 6). These include contryphans, detected in Gastridium geographus, Chelyconus purpurascens, and Pionoconus striatus. Contryphans are known as Ca2+ channel modulators. For instance, contryphan-P (Chelyconus purpurascens) was associated with the “stiff-tail” syndrome when injected in mice [54]. Contryphan-S was found in the venom of Pionoconus striatus but was annotated as contryphan-G, as they present identical sequences [32]. Both contryphan-S and -G seem to be used for preying purposes by Pionoconus striatus and Gastridium geographus [28]. Moreover, Gastridium geographus injects non-paralytic compounds, including conopressin, conophysin, contulakin, and conantokin [28][33]. Conopressins and conophysins have been shown to have agonist/antagonist activity against vasopressin receptors [65], while conantokin-T was deemed responsible for the sleep-like state of the prey prior to being engulfed, especially for the “net-hunting” cone snails (Figure 4), which is caused by the inhibition of NMDA (N-methyl-D-aspartate) receptors [54][66]. Contulakin-G, on the other hand, is a glycopeptide identified as an agonist of neurotensin receptors, which has shown analgesic properties [67]. Interestingly, Pionoconus striatus also injects larger peptides, such as the conkunitzins, which have been characterized as voltage-gated K+ channel blockers, affiliated with the Shaker potassium channels [68]. Lastly, large polypeptides, such as p21a (Chelyconus purpurascens) [69], or con-ikot-ikot (Pionoconus striatus) [70] that inhibits AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid) receptors, as well as hyaluronidases (Pionoconus consors and Chelyconus purpurascens) [37][71], phospholipases A2 (Chelyconus purpurascens) [72], and proteases (Chelyconus purpurascens and Chelyconus ermineus) [73], have been identified in predatory venoms. More high-molecular-weight proteins, such as metalloproteases, are likely to be present, but their role in prey capture remains unclear [74].
Table 6. Conopeptides identified from predatory and defense venoms of fish-hunting cone snails. Presented here are conotoxins found exclusively in the predation-evoked, the defense-evoked, or in both venoms. Each conotoxin is characterized by its Conus clade, the Conus species in which it was detected, the given name, its sequence, its classification within the gene superfamilies, and the cysteine framework. Cysteine residues are highlighted in red.
| Clades | Conus Species | Conotoxins | Mature Sequence | Gene Superfamily |
Cysteine Framework | References |
|---|---|---|---|---|---|---|
| Pionoconus | striatus | Conkunitzin S1 | KDRPSLCDLPADSGSGTKAEKRIYYNSARKQCLRFDYTGQGGNENNFRRTYDCQRTCLYT | Conkunitzin | XIV | [32] |
| Str54 | PSYCNLPADSGSGTKPEQRIYYNSAKKQCVTFTYNGKGGNGNNFSRTNDCRQTCQYPLYACISGCRCET | Conkunitzin | [32] | |||
| Con-ikot-ikot S | SGPADCCRMKECCTDRVNECLQRYSGREDKFVSFCYQEATVTCGSFNEIVGCCYGYQMCMIRVVKPNSLSGAHEACKTVSCGNPCA | Con-ikot-ikot | [32] | |||
| Conkunitzin S2 | ARPKDRPSYCNLPADSGSGTKPEQRIYYNSAKKQCVTFTYNGKGGNGNNFSRTNDCRQTCQYPVG | Conkunitzin | XIV | [32] | ||
| Chelyconus | purpurascens | Contryphan-P | GCVLLPWC | O2 | [35] | |
| Gastridium | geographus | Contryphan-G | GCPWEPWC | O2 | [32] | |
| Conopressin-G | CFIRNCPKGX | Conopressin | [28][33] | |||
| Contulakin-G | QSEEGGSNATKKPYIL | C | [33] | |||
| G5.1 | QGWCCKENIACCV | T | V | [28][33] | ||
| Scratcher peptide | KFLSGGFKIVCHRYCAKGIAKEFCNCPD | XIV | [28][33] | |||
| Conantokin-G | GE(Gla)(Gla)LQ(Gla)NQ(Gla)LIR(Gla)KSN | B1 | [28][33] | |||
| Conophysin-G | THPCMSCSFGQCVGPQICCGLGGCEMGTAEANKCIEEDDDQTPCQVLGDHCDLNNLDIEGHCVADGICCVDDTCAIHSSC | Conophysin | [28] |
2.2. Defensive Venom
The data on defensive venom are more scarce compared to predatory venoms. Indeed, so far only Pionoconus striatus and Gastridium geographus, as well as Gastridium obscurus, have been investigated regarding their defense strategies [32][33]. Six gene superfamilies and three conopeptide classes (Figure 9A) have been identified in the defense venoms. Most of them are A-, O1-, and M-conotoxins found in both Pionoconus and Gastridium clades. In addition, B1-, S-, and T-conotoxins are found exclusively in Gastridium cone snails, as well as conophysins. Moreover, Pionoconus striatus also injected conkunitzin and con-ikot-ikot (Table 6).
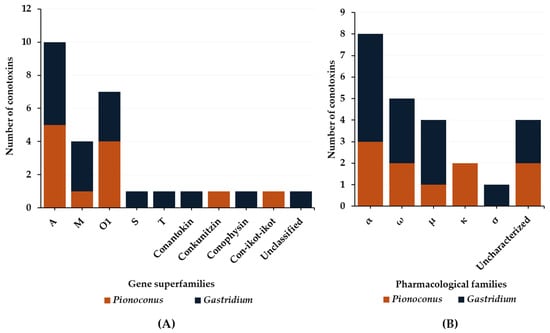
Figure 9. Gene superfamilies (A) and pharmacological families (B) identified within defense-evoked venoms of piscivorous cone snails. Cone snails include Pionoconus striatus, Gastridium geographus, and Gastridium obscurus.
Overall, five pharmacological families were characterized from the defensive venom of these cone snails: α, ω, μ, κ, and σ (Figure 9B). The α-conotoxins α-SI and α-SIA from Pionoconus striatus and α-OIVA and α-OIVB from Gastridium obscurus are used, both in the predatory and defense venoms. However, Gastridium geographus injects a specific set of α-conotoxins, including α-GIA, α-GII, and α-GID, all with a I cysteine framework [33]. Targeting neuronal rather than muscle-type nAChRs, α-GID has an unusual 4-residue N-terminal tail, which appears critical for activity at the α4β2 nAChR subtype but not the others (α3β2 or α7) [75]. In the defense venom of Pionoconus species, κA-conotoxins, such as κA-SIVA and κA-SIVB in Pionoconus striatus, are found abundantly [32].
In contrast to the predatory venom, Gastridium geographus uses large amounts of μ-conotoxins μ-GIIIA, μ-GIIIB, and μ-GIIIC [28][33] for defense purposes, but not the prey-specific μ-conotoxin-GS (Table 4). Moreover, Pionoconus striatus, which lacked μ-conotoxin in the predatory strategy, injects one in the defensive venom (μ-S3-G02) [32]. Defense venoms also include ω-conotoxins (Table 5). In addition to ω-SVIA and ω-SVIB, Pionoconus striatus injects two O1-conotoxins, SO4 and SO5, in rather high amounts [32]. Similarly, Gastridium geographus injects three potent and paralytic ω-conotoxins, ω-GVIA, ω-GVIIA, and ω-GVIIB [28][33].
An unusual conotoxin, σ-GVIIIA, was identified in the defense venom of Gastridium geographus (Table 7) [28][33]. This toxin is a σS-conotoxin presenting a VIII cysteine framework and has been characterized as a blocker of the 5-HT3 serotonin receptor (“involved in the inhibition of neurotransmitter release at motor and sensory synapses“) [76]. Additional conopeptides, such as conophysin-G [28], were also identified in the defensive strategies. Interestingly, Gastridium geographus also employs two unclassified conotoxins G5.1 and a so-called “scratcher peptide”, which was named from the “scratching” symptoms observed in mice [77]. No data are available for the defensive strategies of other piscivorous clades, including Chelyconus and Textilia.
Table 7. σ-Conotoxin identified from predatory venoms of a fish-hunting cone snail (Gastridium geographus). Presented here are conotoxins found exclusively in the defense-evoked venom. Each conotoxin is characterized by its Conus clade, the Conus species in which it was detected, the given name, its sequence, its classification within the gene superfamilies, and the cysteine framework. Cysteine residues are highlighted in red.
| Clades | Conus Species | Conotoxins | Mature Sequence | Gene Superfamily |
Cysteine Framework | References |
|---|---|---|---|---|---|---|
| Gastridium | geographus | σ-GVIIIA | GCTRTCGGOKCTGTCTCTNSSKCGCRYNVHPSG(Btr)GCGCACSX | S | VIII | [28][33] |
References
- Kohn, A.J. Microhabitats, Abundance and Food of Conus on Atoll Reefs in the Maldive and Chagos Islands. Ecology 1968, 49, 1046–1062.
- Puillandre, N.; Duda, T.F.; Meyer, C.; Olivera, B.M.; Bouchet, P. One, Four or 100 Genera? A New Classification of the Cone Snails. J. Molluscan Stud. 2015, 81, 1–23.
- Gao, B.; Peng, C.; Yang, J.; Yi, Y.; Zhang, J.; Shi, Q. Cone Snails: A Big Store of Conotoxins for Novel Drug Discovery. Toxins 2017, 9, 397.
- Olivera, B.M. “Conus” Venom Peptides: Reflections from the Biology of Clades and Species. Annu. Rev. Ecol. Syst. 2002, 33, 25–47.
- Akondi, K.B.; Muttenthaler, M.; Dutertre, S.; Kaas, Q.; Craik, D.J.; Lewis, R.J.; Alewood, P.F. Discovery, Synthesis, and Structure–Activity Relationships of Conotoxins. Chem. Rev. 2014, 114, 5815–5847.
- Puillandre, N.; Koua, D.; Favreau, P.; Olivera, B.M.; Stöcklin, R. Molecular Phylogeny, Classification and Evolution of Conopeptides. J. Mol. Evol. 2012, 74, 297–309.
- Lebbe, E.K.M.; Tytgat, J. In the Picture: Disulfide-Poor Conopeptides, a Class of Pharmacologically Interesting Compounds. J. Venom. Anim. Toxins Trop. Dis. 2016, 22, 30.
- Kaas, Q.; Westermann, J.-C.; Craik, D.J. Conopeptide Characterization and Classifications: An Analysis Using ConoServer. Toxicon 2010, 55, 1491–1509.
- Jin, A.-H.; Dutertre, S.; Dutt, M.; Lavergne, V.; Jones, A.; Lewis, R.; Alewood, P. Transcriptomic-Proteomic Correlation in the Predation-Evoked Venom of the Cone Snail, Conus Imperialis. Mar. Drugs 2019, 17, 177.
- Jin, A.-H.; Muttenthaler, M.; Dutertre, S.; Himaya, S.W.A.; Kaas, Q.; Craik, D.J.; Lewis, R.J.; Alewood, P.F. Conotoxins: Chemistry and Biology. Chem. Rev. 2019, 119, 11510–11549.
- Kohn, A.J.; Nishi, M.; Pernet, B. Snail Spears and Scimitars: A Character Analysis of Conus Radular Teeth. J. Molluscan Stud. 1999, 65, 461–481.
- Kohn, A.J. Piscivorous Gastropods of the Genus Conus. Proc. Natl. Acad. Sci. USA 1956, 42, 168–171.
- Olivera, B.M.; Seger, J.; Horvath, M.P.; Fedosov, A.E. Prey-Capture Strategies of Fish-Hunting Cone Snails: Behavior, Neurobiology and Evolution. Brain Behav. Evol. 2015, 86, 58–74.
- Kohn, M. Biology of Conus on Shores of the Dampier Archipelago, Northwestern Australia; University of Washington Seattle: Seattle, WA, USA, 2003.
- Salisbury, S.M.; Martin, G.G.; Kier, W.M.; Schulz, J.R. Venom Kinematics during Prey Capture in Conus: The Biomechanics of a Rapid Injection System. J. Exp. Biol. 2010, 213, 673–682.
- Prator, C.A.; Murayama, K.M.; Schulz, J.R. Venom Variation during Prey Capture by the Cone Snail, Conus textile. PLoS ONE 2014, 9, e98991.
- Himaya, S.W.A.; Marí, F.; Lewis, R.J. Accelerated Proteomic Visualization of Individual Predatory Venoms of Conus Purpurascens Reveals Separately Evolved Predation-Evoked Venom Cabals. Sci. Rep. 2018, 8, 330.
- Abalde, S.; Tenorio, M.J.; Afonso, C.M.L.; Zardoya, R. Conotoxin Diversity in Chelyconus ermineus (Born, 1778) and the Convergent Origin of Piscivory in the Atlantic and Indo-Pacific Cones. Genome Biol. Evol. 2018, 10, 2643–2662.
- Hu, H.; Bandyopadhyay, P.K.; Olivera, B.M.; Yandell, M. Elucidation of the Molecular Envenomation Strategy of the Cone Snail Conus Geographus through Transcriptome Sequencing of Its Venom Duct. BMC Genom. 2012, 13, 284.
- Safavi-Hemami, H.; Gajewiak, J.; Karanth, S.; Robinson, S.D.; Ueberheide, B.; Douglass, A.D.; Schlegel, A.; Imperial, J.S.; Watkins, M.; Bandyopadhyay, P.K.; et al. Specialized Insulin Is Used for Chemical Warfare by Fish-Hunting Cone Snails. Proc. Natl. Acad. Sci. USA 2015, 112, 1743–1748.
- Robinson, S.D.; Li, Q.; Bandyopadhyay, P.K.; Gajewiak, J.; Yandell, M.; Papenfuss, A.T.; Purcell, A.W.; Norton, R.S.; Safavi-Hemami, H. Hormone-like Peptides in the Venoms of Marine Cone Snails. Gen. Comp. Endocrinol. 2017, 244, 11–18.
- Hopkins, C.; Grilley, M.; Miller, C.; Shon, K.-J.; Cruz, L.J.; Gray, W.R.; Dykert, J.; Rivier, J.; Yoshikami, D.; Olivera, B.M. A New Family of Conus Peptides Targeted to the Nicotinic Acetylcholine Receptor (*). J. Biol. Chem. 1995, 270, 22361–22367.
- Jakubowski, J.A.; Kelley, W.P.; Sweedler, J.V.; Gilly, W.F.; Schulz, J.R. Intraspecific Variation of Venom Injected by Fish-Hunting Conus Snails. J. Exp. Biol. 2005, 208, 2873–2883.
- Dutertre, S.; Biass, D.; Stöcklin, R.; Favreau, P. Dramatic Intraspecimen Variations within the Injected Venom of Conus consors: An Unsuspected Contribution to Venom Diversity. Toxicon 2010, 55, 1453–1462.
- Himaya, S.W.A.; Jin, A.-H.; Dutertre, S.; Giacomotto, J.; Mohialdeen, H.; Vetter, I.; Alewood, P.F.; Lewis, R.J. Comparative Venomics Reveals the Complex Prey Capture Strategy of the Piscivorous Cone Snail Conus catus. J. Proteome Res. 2015, 14, 4372–4381.
- Kelley, W.P.; Schulz, J.R.; Jakubowski, J.A.; Gilly, W.F.; Sweedler, J.V. Two Toxins from Conus striatus That Individually Induce Tetanic Paralysis. Biochemistry 2006, 45, 14212–14222.
- Le Gall, F.; Favreau, P.; Benoit, E.; Mattei, C.; Bouet, F.; Menou, J.-L.; Ménez, A.; Letourneux, Y.; Molgó, J. A New Conotoxin Isolated from Conus Consors Venom Acting Selectively on Axons and Motor Nerve Terminals through a Na+-Dependent Mechanism. Eur. J. Neurosci. 1999, 11, 3134–3142.
- Dutertre, S.; Jin, A.-H.; Vetter, I.; Hamilton, B.; Sunagar, K.; Lavergne, V.; Dutertre, V.; Fry, B.G.; Antunes, A.; Venter, D.J.; et al. Evolution of Separate Predation- and Defence-Evoked Venoms in Carnivorous Cone Snails. Nat. Commun. 2014, 5, 3521.
- Kohn, A.J. Conus Envenomation of Humans: In Fact and Fiction. Toxins 2019, 11, 10.
- Casewell, N.R.; Wüster, W.; Vonk, F.J.; Harrison, R.A.; Fry, B.G. Complex Cocktails: The Evolutionary Novelty of Venoms. Trends Ecol. Evol. 2013, 28, 219–229.
- Zhao, Y.; Antunes, A. Biomedical Potential of the Neglected Molluscivorous and Vermivorous Conus Species. Mar. Drugs 2022, 20, 105.
- Himaya, S.W.A.; Jin, A.-H.; Hamilton, B.; Rai, S.K.; Alewood, P.; Lewis, R.J. Venom Duct Origins of Prey Capture and Defensive Conotoxins in Piscivorous Conus striatus. Sci. Rep. 2021, 11, 13282.
- Dutertre, S.; Jin, A.-H.; Alewood, P.F.; Lewis, R.J. Intraspecific Variations in Conus geographus Defence-Evoked Venom and Estimation of the Human Lethal Dose. Toxicon 2014, 91, 135–144.
- Shells For Sale|The Largest Official Seashells Website|Conchology. Available online: https://www.conchology.be/ (accessed on 18 July 2023).
- Gajewiak, J.; Azam, L.; Imperial, J.; Walewska, A.; Green, B.R.; Bandyopadhyay, P.K.; Raghuraman, S.; Ueberheide, B.; Bern, M.; Zhou, H.M.; et al. A Disulfide Tether Stabilizes the Block of Sodium Channels by the Conotoxin μO§-GVIIJ. Proc. Natl. Acad. Sci. USA 2014, 111, 2758–2763.
- Teichert, R.W.; Jacobsen, R.; Terlau, H.; Yoshikami, D.; Olivera, B.M. Discovery and Characterization of the Short κA-Conotoxins: A Novel Subfamily of Excitatory Conotoxins. Toxicon 2007, 49, 318–328.
- Biass, D.; Dutertre, S.; Gerbault, A.; Menou, J.-L.; Offord, R.; Favreau, P.; Stöcklin, R. Comparative Proteomic Study of the Venom of the Piscivorous Cone Snail Conus consors. J. Proteom. 2009, 72, 210–218.
- Violette, A.; Biass, D.; Dutertre, S.; Koua, D.; Piquemal, D.; Pierrat, F.; Stöcklin, R.; Favreau, P. Large-Scale Discovery of Conopeptides and Conoproteins in the Injectable Venom of a Fish-Hunting Cone Snail Using a Combined Proteomic and Transcriptomic Approach. J. Proteom. 2012, 75, 5215–5225.
- Kapono, C.A.; Thapa, P.; Cabalteja, C.C.; Guendisch, D.; Collier, A.C.; Bingham, J.-P. Conotoxin Truncation as a Post-Translational Modification to Increase the Pharmacological Diversity within the Milked Venom of Conus magus. Toxicon 2013, 70, 170–178.
- Saintmont, F.; Cazals, G.; Bich, C.; Dutertre, S. Proteomic Analysis of the Predatory Venom of Conus striatus Reveals Novel and Population-Specific κA-Conotoxin SIVC. Toxins 2022, 14, 799.
- Teichert, R.W.; Rivier, J.; Dykert, J.; Cervini, L.; Gulyas, J.; Bulaj, G.; Ellison, M.; Olivera, B.M. αA-Conotoxin OIVA Defines a New αA-Conotoxin Subfamily of Nicotinic Acetylcholine Receptor Inhibitors. Toxicon 2004, 44, 207–214.
- Ramilo, C.A.; Zafaralla, G.C.; Nadasdi, L.; Hammerland, L.G.; Yoshikami, D.; Gray, W.R.; Kristipati, R.; Ramachandran, J.; Miljanich, G. Novel Alpha- and Omega-Conotoxins and Conus striatus Venom. Biochemistry 1992, 31, 9919–9926.
- Biass, D.; Violette, A.; Hulo, N.; Lisacek, F.; Favreau, P.; Stöcklin, R. Uncovering Intense Protein Diversification in a Cone Snail Venom Gland Using an Integrative Venomics Approach. J. Proteome Res. 2015, 14, 628–638.
- Quinton, L.; Servent, D.; Girard, E.; Molgó, J.; Le Caer, J.-P.; Malosse, C.; Haidar, E.A.; Lecoq, A.; Gilles, N.; Chamot-Rooke, J. Identification and Functional Characterization of a Novel α-Conotoxin (EIIA) from Conus ermineus. Anal. Bioanal. Chem. 2013, 405, 5341–5351.
- Terlau, H.; Olivera, B.M. Conus Venoms: A Rich Source of Novel Ion Channel-Targeted Peptides. Physiol. Rev. 2004, 84, 41–68.
- López-Vera, E.; Jacobsen, R.B.; Ellison, M.; Olivera, B.M.; Teichert, R.W. A Novel Alpha Conotoxin (α-PIB) Isolated from C. purpurascens Is Selective for Skeletal Muscle Nicotinic Acetylcholine Receptors. Toxicon 2007, 49, 1193–1199.
- Teichert, R.W.; Rivier, J.; Torres, J.; Dykert, J.; Miller, C.; Olivera, B.M. A Uniquely Selective Inhibitor of the Mammalian Fetal Neuromuscular Nicotinic Acetylcholine Receptor. J. Neurosci. 2005, 25, 732–736.
- Hoggard, M.F.; Rodriguez, A.M.; Cano, H.; Clark, E.; Tae, H.-S.; Adams, D.J.; Godenschwege, T.A.; Marí, F. In Vivo and In Vitro Testing of Native α-Conotoxins from the Injected Venom of Conus purpurascens. Neuropharmacology 2017, 127, 253–259.
- Van Wagoner, R.M.; Jacobsen, R.B.; Olivera, B.M.; Ireland, C.M. Characterization and Three-Dimensional Structure Determination of ψ-Conotoxin P IIIF, a Novel Noncompetitive Antagonist of Nicotinic Acetylcholine Receptors. Biochemistry 2003, 42, 6353–6362.
- Jacobsen, R.; Yoshikami, D.; Ellison, M.; Martinez, J.; Gray, W.R.; Cartier, G.E.; Shon, K.-J.; Groebe, D.R.; Abramson, S.N.; Olivera, B.M.; et al. Differential Targeting of Nicotinic Acetylcholine Receptors by Novel αA-Conotoxins. J. Biol. Chem. 1997, 272, 22531–22537.
- Rivera-Ortiz, J.A.; Cano, H.; Marí, F. Intraspecies Variability and Conopeptide Profiling of the Injected Venom of Conus ermineus. Peptides 2011, 32, 306–316.
- Echterbille, J.; Gilles, N.; Araóz, R.; Mourier, G.; Amar, M.; Servent, D.; De Pauw, E.; Quinton, L. Discovery and Characterization of EIIB, a New α-Conotoxin from Conus Ermineus Venom by nAChRs Affinity Capture Monitored by MALDI-TOF/TOF Mass Spectrometry. Toxicon 2017, 130, 1–10.
- Martinez, J.S.; Olivera, B.M.; Gray, W.R.; Craig, A.G.; Groebe, D.R.; Abramson, S.N.; McIntosh, J.M. Alpha-Conotoxin EI, A New Nicotinic Acetylcholine Receptor Antagonist with Novel Selectivity. Biochemistry 1995, 34, 14519–14526.
- Robinson, S.D.; Norton, R.S. Conotoxin Gene Superfamilies. Mar. Drugs 2014, 12, 6058–6101.
- Shon, K.-J.; Grilley, M.M.; Marsh, M.; Yoshikami, D.; Hall, A.R.; Kurz, B.; Gray, W.R.; Imperial, J.S.; Hillyard, D.R.; Olivera, B.M. Purification, Characterization, Synthesis, and Cloning of the Lockjaw Peptide from Conus Purpurascens Venom. Biochemistry 1995, 34, 4913–4918.
- Himaya, S.; Lewis, R. Venomics-Accelerated Cone Snail Venom Peptide Discovery. Int. J. Mol. Sci. 2018, 19, 788.
- Yanagawa, Y.; Abe, T.; Satake, M.; Odani, S.; Suzuki, J.; Ishikawa, K. A Novel Sodium Channel Inhibitor from Conus geographus: Purification, Structure, and Pharmacological Properties. Biochemistry 1988, 27, 6256–6262.
- Del Río-Sancho, S.; Cros, C.; Coutaz, B.; Cuendet, M.; Kalia, Y.N. Cutaneous Iontophoresis of μ-Conotoxin CnIIIC-A Potent NaV1.4 Antagonist with Analgesic, Anaesthetic and Myorelaxant Properties. Int. J. Pharm. 2017, 518, 59–65.
- Zhang, M.-M.; Fiedler, B.; Green, B.R.; Catlin, P.; Watkins, M.; Garrett, J.E.; Smith, B.J.; Yoshikami, D.; Olivera, B.M.; Bulaj, G. Structural and Functional Diversities among μ-Conotoxins Targeting TTX-Resistant Sodium Channels. Biochemistry 2006, 45, 3723–3732.
- Olivera, B.M.; McIntosh, J.M.; Curz, L.J.; Luque, F.A.; Gray, W.R. Purification and Sequence of a Presynaptic Peptide Toxin from Conus geographus Venom. Biochemistry 1984, 23, 5087–5090.
- Lewis, R.J.; Nielsen, K.J.; Craik, D.J.; Loughnan, M.L.; Adams, D.A.; Sharpe, I.A.; Luchian, T.; Adams, D.J.; Bond, T.; Thomas, L.; et al. Novel ω-Conotoxins from Conus Catus Discriminate among Neuronal Calcium Channel Subtypes. J. Biol. Chem. 2000, 275, 35335–35344.
- Zhou, K.; Luo, W.; Liu, T.; Ni, Y.; Qin, Z. Neurotoxins Acting at Synaptic Sites: A Brief Review on Mechanisms and Clinical Applications. Toxins 2023, 15, 18.
- Safavi-Hemami, H.; Brogan, S.E.; Olivera, B.M. Pain Therapeutics from Cone Snail Venoms: From Ziconotide to Novel Non-Opioid Pathways. J. Proteom. 2019, 190, 12–20.
- Zafaralla, G.C.; Ramilo, C.; Gray, W.R.; Karlstrom, R.; Olivera, B.M.; Cruz, L.J. Phylogenetic Specificity of Cholinergic Ligands:.Alpha.-Conotoxin SI. Biochemistry 1988, 27, 7102–7105.
- Dutertre, S.; Croker, D.; Daly, N.L.; Andersson, Å.; Muttenthaler, M.; Lumsden, N.G.; Craik, D.J.; Alewood, P.F.; Guillon, G.; Lewis, R.J. Conopressin-T from Conus tulipa Reveals an Antagonist Switch in Vasopressin-like Peptides. J. Biol. Chem. 2008, 283, 7100–7108.
- Haack, J.A.; Rivier, J.; Parks, T.N.; Mena, E.E.; Cruz, L.J.; Olivera, B.M. Conantokin-T. A Gamma-Carboxyglutamate Containing Peptide with N-Methyl-d-Aspartate Antagonist Activity. J. Biol. Chem. 1990, 265, 6025–6029.
- Craig, A.G.; Norberg, T.; Griffin, D.; Hoeger, C.; Akhtar, M.; Schmidt, K.; Low, W.; Dykert, J.; Richelson, E.; Navarro, V.; et al. Contulakin-G, an O-Glycosylated Invertebrate Neurotensin. J. Biol. Chem. 1999, 274, 13752–13759.
- Bayrhuber, M.; Vijayan, V.; Ferber, M.; Graf, R.; Korukottu, J.; Imperial, J.; Garrett, J.E.; Olivera, B.M.; Terlau, H.; Zweckstetter, M.; et al. Conkunitzin-S1 Is the First Member of a New Kunitz-Type Neurotoxin Family. J. Biol. Chem. 2005, 280, 23766–23770.
- Möller, C.; Marí, F. 9.3 KDa Components of the Injected Venom of Conus purpurascens Define a New Five-Disulfide Conotoxin Framework. Pept. Sci. 2011, 96, 158–165.
- Walker, C.S.; Jensen, S.; Ellison, M.; Matta, J.A.; Lee, W.Y.; Imperial, J.S.; Duclos, N.; Brockie, P.J.; Madsen, D.M.; Isaac, J.T.R.; et al. A Novel Conus Snail Polypeptide Causes Excitotoxicity by Blocking Desensitization of AMPA Receptors. Curr. Biol. 2009, 19, 900–908.
- Möller, C.; Clark, E.; Safavi-Hemami, H.; DeCaprio, A.; Marí, F. Isolation and Characterization of Conohyal-P1, a Hyaluronidase from the Injected Venom of Conus purpurascens. J. Proteom. 2017, 164, 73–84.
- Möller, C.; Davis, W.C.; Clark, E.; DeCaprio, A.; Marí, F. Conodipine-P1-3, the First Phospholipases A2 Characterized from Injected Cone Snail Venom. Mol. Cell. Proteom. MCP 2019, 18, 876–891.
- Möller, C.; Vanderweit, N.; Bubis, J.; Marí, F. Comparative Analysis of Proteases in the Injected and Dissected Venom of Cone Snail Species. Toxicon 2013, 65, 59–67.
- Safavi-Hemami, H.; Möller, C.; Marí, F.; Purcell, A.W. High Molecular Weight Components of the Injected Venom of Fish-Hunting Cone Snails Target the Vascular System. J. Proteom. 2013, 91, 97–105.
- Nicke, A.; Loughnan, M.L.; Millard, E.L.; Alewood, P.F.; Adams, D.J.; Daly, N.L.; Craik, D.J.; Lewis, R.J. Isolation, Structure, and Activity of GID, a Novel A4/7-Conotoxin with an Extended N-Terminal Sequence. J. Biol. Chem. 2003, 278, 3137–3144.
- England, L.J.; Imperial, J.; Jacobsen, R.; Craig, A.G.; Gulyas, J.; Akhtar, M.; Rivier, J.; Julius, D.; Olivera, B.M. Inactivation of a Serotonin-Gated Ion Channel by a Polypeptide Toxin from Marine Snails. Science 1998, 281, 575–578.
- Diversity of Conus Neuropeptides. Available online: https://www.science.org/doi/10.1126/science.2165278 (accessed on 14 December 2023).
More
Information
Subjects:
Marine & Freshwater Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
701
Revisions:
2 times
(View History)
Update Date:
06 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




