
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mudasir A. Dar | -- | 3573 | 2024-03-04 11:27:54 | | | |
| 2 | Catherine Yang | Meta information modification | 3573 | 2024-03-05 02:44:14 | | |
Video Upload Options
The hunt for renewable and alternative fuels has driven research towards the biological conversion of lignocellulosic biomass (LCB) into biofuels, including bioethanol and biohydrogen. Among the natural biomass utilization systems (NBUS), termites represent a unique and easy-to-access model system to study host–microbe interactions towards lignocellulose bioconversion/valorization. Termites have gained significant interest due to their highly efficient lignocellulolytic systems. The wood-feeding termites apply a unique and stepwise process for the hydrolysis of lignin, hemicellulose, and cellulose via biocatalytic processes; therefore, mimicking their digestive metabolism and physiochemical gut environments might lay the foundation for an innovative design of nature-inspired biotechnology.
1. Introduction
2. Termite Gut as Unique Reservoir of Lignocellulolytic Microorganisms
2.1. Termite Gut Bacteria
| Bacterial Genera | Prokaryote Type | Termite Species | Gut-Region | References |
|---|---|---|---|---|
| Streptomyces naraensis | Actinomycete | Coptotermes formosanus | Whole gut | [29] |
| S. filamentosus | Odontotermes formosanus | Whole gut | [30] | |
| Clostridium mayombei, Sporomusa termitida, Klebsiella variicola, Acetonema longum K. pneumoniae, M. cuticularis, M. curvans, M. filiformis |
Bacteria | Nasutitermes nigriceps | [31] | |
| Pterotermes occidentis | ||||
| O. formosanus | ||||
| Archaea | Reticulitermes speratus | Whole gut | [32] | |
| Cubitermes ugandensis | Whole gut | [33] | ||
| Treponema isoptericolens, Spirochaeta coccoides |
Spirochete | Incisitermes tabogae | Whole gut | [34] |
| Acinetobacter seifertii, Enterobacter asburiae, E. cloacae, Lysinibacillus macrolides, | Bacteria | C. formosanus | Foregut | [4] |
| S. marcescens, P. stutzeri, S. hominis, B. cereus, K. aerogenes, E. hormaechei | Bacteria | C. formosanus | Foregut Midgut Hindgut | [6] |
| E. cancerogenes, E. ludwigii, L. boronitolerans, Lysinibacillus sp., P. fluorescens, P. plecoglossicida, P. putida | Bacteria | C. formosanus | Midgut | [3] |
| A. calcoaceticus, B. simplex, Dietza sp., E. mori, L. fusiformis, P. nitroreducens | Bacteria | C. formosanus | Hindgut | [3] |
| Bacillus spp. | Bacteria | Psammotermes hypostoma | Whole gut | [35] |
| Paenibacillus lactis AFC1 | Bacteria | |||
| L. fusiformis AFC2 | ||||
| Stenotrophomonas maltophilia AFC3 |
||||
| L. macrolides AFC4 | ||||
| Bacillus cereus AFC5 | ||||
| Bacillus spp., Paenibacillus spp. | Bacteria | R. lucifugus | Whole gut | [36] |
| Cellulomonas/Oerskovia, Microbacterium and Kocuria |
Actinomycete | Z. angusticollis | Whole gut | [32] |
| Bacillus, Brevibacillus, Paenibacillus, fipia, Agrobacterium/Rhizobium, Brucella/Ochrobactrum, Pseudomonas and Sphingomonas/Zymomonas |
Bacteria | Z. angusticollis | ||
| Citrobacter farmeri | C. formosanus | Whole gut | [37] | |
| Bacillus spp. | O. formosanus | |||
| Bacteria | Mastotermes darwiniensis, Cryptotermes primus, N. arborum, Thoracotermes macrothorax, Anoplotermes pacificus |
Whole gut | [38] | |
| Isoptericola variabilis | Mastotermes darwiniensis | hindgut | [39][40] | |
| Sporomusa aerivorans | Thoracotermes macrothorax | Whole gut | [41] | |
| Candidatus Vestibaculum illigatum | Neotermes cubanus | Whole gut | [21] | |
| T. azotonutricium | Spirochaetes | Zootermopsis angusticollis | Whole gut | [42] |
| T. primitia | ||||
| Candidatus Endomicrobium trichonymphae, | R. santonensis | Whole gut | [21] |
2.2. Symbiotic Flagellates
| Termite Host | Protist/Flagellate | References | |||
|---|---|---|---|---|---|
| Class | Order | Family | Genus | ||
| Incisitermes minor | Trichomonadea | Trichomonadida | Devescovinidae | Metadevescovina cuspidata | [46][50] |
| Coptotermes spp. | Parabasalia | Spirotrichonymphida | Holomastigotoididae | Holomastigotoides | [53][54][55] |
| Trichonymphida | Teranymphidae | Pseudotrichonympha | |||
| Trichonymphidae | Spirotrichonympha | ||||
| Cononympha | |||||
| Reticulitermes spp. | Parabasalia | Spirotrichonymphida | Holomastigotoididae | Holomastigotoides | [56][57] |
| Trichonymphida | Trichomonadidae | Trichomonas | |||
| Teranymphidae | Teranympha | ||||
| Trichonymphidae | Spirotrichonympha | ||||
| Trichonympha | |||||
| Tritrichomonadida | Monocercomonadidae | Monocercomonas | |||
| Preaxostyla | Oxymonadida | Pyrsonymphidae | Dinenympha | ||
| Pyrsonympha | |||||
| Rhinotermes spp. | Parabasalia | Cristamonadida | Lophomonadidae | Gigantomonas | [53][58] |
| Schedorhinotermes spp. | Parabasalia | Trichonymphida | Teranymphidae | Pseudotrichonympha | [59] |
| Cryptotermes spp. | Parabasalia | Cristamonadida | Lophomonadidae | Stephanonympha | [57][60] |
| Devescovina | |||||
| Epicalotermes spp. | Parabasalia | Trichonymphida | Staurojoeninidae | Staurojoenina | [61] |
| Glyptotermes spp. | Parabasalia | Cristamonadida | Lophomonadidae | Devescovina | [57] |
| Macrotrichomonas | |||||
| Incisitermes spp. | Parabasalia | Cristamonadida | Lophomonadidae | Coronympha | [55][57] |
| Trichonymphida | Staurojoeninidae | Staurojoenina | |||
| Trichonymphidae | Trichonympha | ||||
| Kalotermes spp. | Parabasalia | Cristamonadida | Lophomonadidae | Calonympha | [55] |
| Devescovina | |||||
| Joenia | |||||
| Stephanonympha | |||||
| Tritrichomonadida | Monocercomonadidae | Monocercomonas | |||
| Neotermes spp. | Parabasalia | Cristamonadida | Lophomonadidae | Devescovina | [62] |
| Foaina | |||||
| Preaxostyla | Oxymonadida | Oxymonadidae | Oxymonas | ||
| Archotermopsis spp. | Parabasalia | Honigbergiellida | Honigbergiellidae | Ditrichomonas | [63] |
| Trichonymphida | Teranymphidae | Pseudotrichonympha | |||
| Hodotermopsis spp. | Parabasalia | Spirotrichonymphida | Holomastigotoididae | Spirotrichonymphella | [56][64] |
| Trichomonadida | Trichomonadidae | Trichomonas | |||
| Trichonymphida | Hoplonymphidae | Hoplonympha | |||
| Teranymphidae | Eucomonympha | ||||
| Trichonymphidae | Spirotrichonympha | ||||
| Trichonympha | |||||
| Porotermes spp. | Parabasalia | Cristamonadida | Lophomonadidae | Joenina | [65][66] |
| Spirotrichonymphida | Holomastigotoididae | Spirotrichonymphella | |||
| Trichomonadida | Trichomonadidae | Pseudotrypanosoma | |||
| Trichonymphida | Teranymphidae | Pseudotrichonympha | |||
| Trichonymphidae | Trichonympha | ||||
| Zootermopsis spp. | Parabasalia | Hypotrichomonadida | Hypotrichomonadidae | Trichomitus | [60][67] |
| Trichonymphida | Trichonymphidae | Trichonympha | |||
| Preaxostyla | Oxymonadida | Streblomastigidae | Streblomastix | ||
| Hodotermes spp. | Parabasalia | Cristamonadida | Lophomonadidae | Devescovina Foaina Gigantomonas Stephanonympha |
[68][69] |
| Trichonymphida | Spirotrichosomidae | Leptospironympha | |||
| Mastotermes spp. | Parabasalia |
Cristamonadida Trichomonadida |
Lophomonadidae Trichomonadidae |
Deltotrichonympha Pentatrichomonoides |
[70][71][72] |
2.3. Symbiotic Fungi
3. Biorefinery Potential of Termite Symbiosis
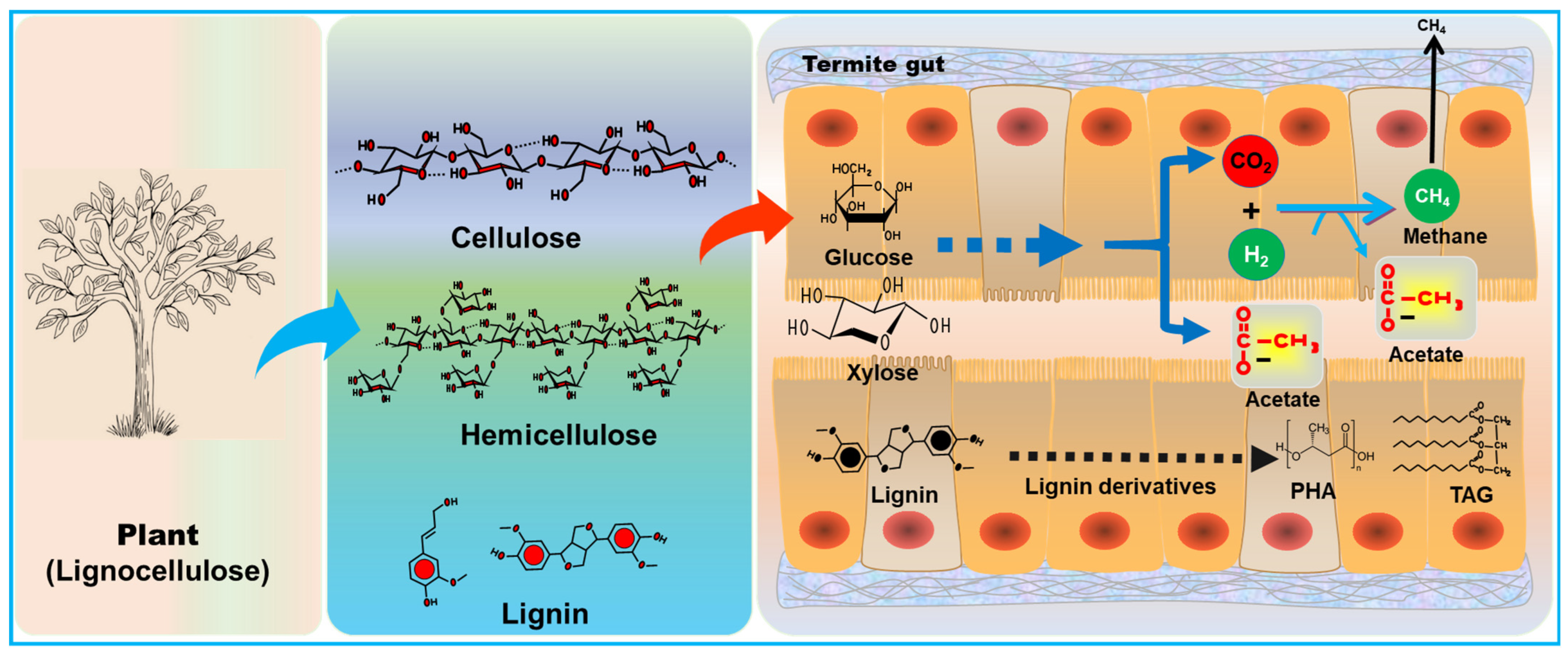
| Microorganism | Termite Host | Biofuel Type | Carbon Source | References |
|---|---|---|---|---|
| Bacteroides, Prevotella | N. ephratae | Biomethane | Wheat straw | [94] |
| Streptomyces sp. |
Microcerotermes species | Bioethanol | Wheat straw | [95] |
| Bacillus sp. BMP01, Ochrobactrum oryzae BMP03 | Cryptotermes brevis | Bioethanol | CMC, Xylan, Lignin | [96] |
| Bacterial symbionts | Nasutitermes ephratae, Microcerotermes parvus, N. lujae, Termes hospes | Biomethane | Wheat straw | [97] |
| Actinobacteria | M. nervosus, Macrognathothermes sunteri, Tumulitermes pastinato | Hydrogen, Biomethane | Organic Carbon | [98] |
| Symbionts | Reticulitermes speratus | Hydrogen | Wood | [99] |
| Treponema primitia | Zootermopsis angusticollis | Acetate | Wood | [100] |
| Clostridium termitidis, Clostridium beijerinckii | Nasusitermes spp. | Hydrogen | Cellulose | [101] |
| Methylocella sp., and other symbionts | R. speratus, Z. nevadensis, Cubitermes orthognathus | Biomethane, Hydrogen | Wood | [93][102][103] |
| Enterococcus sp. | R. flavipes | Acetate | Lignocellulose | [104] |
| Sporomusa termitida, Sporomusa sp. strain TmAO3 | termites | Hydrogen | Lignocellulose | [41] |
| Microbiota | Cubitermes spp. | Biomethane | Lignocellulose | [105] |
| Sporomusa aerivorans | Thoracotermes macrothorax | Hydrogen | Lactate | [106] |
| Sporotalea propionica | T. macrothorax | Hydrogen | Glucose | [107] |
| Acetonema longum | Pterotermes occidentis | Hydrogen | Yeast extract, Rumen fluid, Resazurin | [86] |
| Enterobacter cloacae KBH3 | Globitermes sp. | Hydrogen | Glucose | [91] |
| Pseudotrichonympha grassii | C. formosanus | Hydrogen | Wood cellulose | [84] |
| Trichonympha sphaerica | Z. termites | Hydrogen | Lignocellulose | [108] |
| Trichomitopsis termopsidis, Hexamastix termopsidis and Tricercomitus termopsidis, Gut microbiota | R. santonensis, Z. nevadensis, Cryptotermes secundus | Hydrogen, Biomethane | CO2, Formate, Lactate | [103] |
| Trichomitopsis termopsidis, Trichonympha sphaerica | Z. termites | Hydrogen, Biomethane | Cellulose, Corncob, Cereal leaves | [109] |
References
- Dar, M.A.; Syed, R.; Pawar, K.D.; Dhole, N.P.; Xie, R.; Pandit, R.S.; Sun, J. Evaluation and characterization of the cellulolytic bacterium, Bacillus pumilus SL8 isolated from the gut of oriental leafworm, Spodoptera litura: An assessment of its potential value for lignocellulose bioconversion. Environ. Technol. Innov. 2022, 27, 102459.
- Dashtban, M.; Schraft, H.; Qin, W. Fungal bioconversion of lignocellulosic residues; opportunities and perspectives. Int. J. Boil. 2009, 5, 578–595.
- Patel, S.K.; Gupta, R.K.; Kim, I.; Lee, J. Coriolus versicolor laccase-based inorganic protein hybrid synthesis for application in biomass saccharification to enhance biological production of hydrogen and ethanol. Enzym. Microbial. Technol. 2023, 170, 110301.
- Dar, M.A.; Xie, R.; Pandit, R.S.; Danso, B.; Dong, C.C.; Sun, J. Exploring the region-wise diversity and functions of symbiotic bacteria in the gut-system of wood-feeding termite, Coptotermes formosanus, towards lignocellulose degradation. Insect Sci. 2022, 29, 1414–1432.
- Sun, J.Z.; Chen, C.R. Cellulolytic insects and their potentials for viable biofuels: A new frontier discipline in entomology and bioengineering. Chin. Bull. Entomol. 2010, 47, 1033–1042.
- Xie, R.; Dong, C.C.; Wang, S.; Danso, B.; Dar, M.A.; Pandit, R.S.; Pawar, K.D.; Geng, A.; Zhu, D.; Xia, L.; et al. Host-specific diversity of culturable bacteria in the gut systems of fungus-growing termites and their potential functions towards lignocellulose bioconversion. Insects 2023, 14, 403.
- Cragg, S.M.; Beckham, G.T.; Bruce, N.C.; Bugg, T.D.H.; Distel, D.L.; Dupree, P.; Etxabe, A.G.; Goodell, B.S.; Jellison, J.; McGeehan, J.E.; et al. Lignocellulose degradation mechanisms across the Tree of Life. Curr. Opin. Chem. Biol. 2015, 29, 108–119.
- Zabed, H.M.; Akhter, S.; Dar, M.A.; Tuly, J.A.; Awasthi, M.K.; Yun, J.; Li, J.; Qi, X. Enhanced fermentable sugar production in lignocellulosic biorefinery by exploring a novel corn stover and configuring high-solid pretreatment conditions. Biores. Technol. 2023, 386, 129498.
- Mujtaba, M.; Fernandes, F.L.; Fazeli, M.; Mukherjee, S.; Savassa, S.M.; Araujo de Medeiros, G.; Do Espírito Santo Pereira, A.; Mancini, S.D.; Lipponen, J.; Vilaplana, F. Lignocellulosic biomass from agricultural waste to the circular economy: A review with focus on biofuels, biocomposites and bioplastics. J. Clean. Prod. 2023, 402, 136815.
- Engel, P.; Moran, N.A. The gut microbiota of insects diversity in structure and function. FEMS Microbiol. Rev. 2013, 37, 699–735.
- Tokuda, G.; Lo, N.; Watanabe, H. Marked variations in patterns of cellulase activity against crystalline vs. carboxymethylcellulose in the digestive systems of diverse, wood-feeding termites. Physiol. Entomol. 2005, 30, 372–380.
- Noda, S.; Kitade, O.; Inoue, T.; Kawai, M.; Hiroshima, K.; Hongoh, Y.; Constantino, R.; Uys, V.; Zhong, J.; Kudo, T.; et al. Cospeciation in the triplex symbiosis of termite gut protists (Pseudotrichonympha spp.), their hosts, and their bacterial endosymbionts. Mol. Ecol. 2007, 16, 1257–1266.
- Hongoh, Y.; Sharma, V.K.; Prakash, T.; Noda, S.; Toh, H.; Taylor, T.D.; Kudo, T.; Sakaki, Y.; Toyoda, A.; Hattori, M.; et al. Genome of an endosymbiont coupling N2 fixation to cellulolysis within protist cells in termite gut. Science 2008, 322, 1108–1109.
- Zheng, H.; Dietrich, C.; Radek, R.; Brune, A. Endomicrobium proavitum, the first isolate of Endomicrobia class. Nov. (phylum Elusimicrobia)–an ultramicrobacterium with an unusual cell cycle that fixes nitrogen with a Group IV nitrogenase. Environ. Microbiol. 2015, 18, 191–204.
- Hongoh, Y.; Sharma, V.K.; Prakash, T.; Noda, S.; Taylor, T.D.; Kudo, T.; Sakaki, Y.; Toyoda, A.; Hattori, M.; Ohkuma, M. Complete genome of the uncultured Termite Group 1 bacteria in a single host protist cell. Proc. Natl. Acad. Sci. USA 2008, 105, 5555–5560.
- König, H.; Fröhlich, J.; Hertel, H. Diversity and Lignocellulolytic Activities of Cultured Microorganisms. In Intestinal Microorganisms of Termites and Other Invertebrates; Springer: Berlin/Heidelberg, Germany, 2006; Volume 6, pp. 271–302.
- Ohkuma, M.; Brune, A. Diversity, Structure, and Evolution of the Termite Gut Microbial Community. In Biology of Termites: A Modern Synthesis, 2nd ed.; Bignell, D., Roisin, Y., Lo, N., Eds.; Springer: Dordrecht, The Netherlands; Singapore, 2010; Volume 1, pp. 413–438.
- Brune, A. Symbiotic digestion of lignocellulose in termite guts. Nat. Rev. Microbiol. 2014, 12, 168–180.
- Brune, A.; Dietrich, C. The Gut Microbiota of Termites: Digesting the Diversity in the Light of Ecology and Evolution. Annu. Rev. Microbiol. 2015, 69, 145–166.
- Noda, S.; Iida, T.; Kitade, O.; Nakajima, H.; Kudo, T.; Ohkuma, M. Endosymbiotic Bacteroidales bacteria of the flagellated protist Pseudotrichonympha grassii in the gut of the termite Coptotermes formosanus. Appl. Environ. Microbiol. 2005, 71, 8811–8817.
- Stingl, U.; Radek, R.; Yang, H.; Brune, A. “Endomicrobia”: Cytoplasmic symbionts of termite gut protozoa form a separate phylum of prokaryotes. Appl. Environ. Microbiol. 2005, 71, 1473–1479.
- Strassert, J.F.H.; Kohler, T.; Wienemann, T.H.G.; Ikeda-Ohtsubo, W.; Faivre, N.; Franckenberg; Plarre, R.; Radek, R.; Brune, A. Candidatus Ancillula trichonymphase, a novel lineage of endosymbiotic Actinobacteria in termite gut flagellates of the genus Trichonympha. Environ. Microbiol. 2012, 14, 3259–3270.
- Nakashima, K.; Watanabe, H.; Saitoh, H.; Tokuda, G.; Azuma, J.I. Dual cellulose digesting system of the wood-feeding termite, Coptotermes formosanus Shiraki. Insect Biochem. Mol. Biol. 2002, 32, 777–784.
- Leadbetter, J.R.; Breznak, J.A. Physiological ecology of Methanobrevibacter cuticularis sp. nov. and Methanobrevibacter curvatus sp. nov., isolated from the hindgut of the termite Reticulitermes flavipes. Appl. Environ. Microbiol. 1996, 62, 3620–3631.
- Tamschick, S.; Radek, R. Colonization of termite hindgut walls by oxymonad flagellates and prokaryotes in Incisitermes tabogae, I. marginipennis and Reticulitermes flavipes. Eur. J. Protistol. 2013, 49, 1–14.
- Hongoh, Y.; Ohkuma, M.; Kudo, T. Molecular analysis of bacterial microbiota in the gut of the termite Reticulitermes speratus (Isoptera; Rhinotermitidae). FEMS Microbiol. Ecol. 2003, 44, 231–242.
- Shinzato, N.; Muramatsu, M.; Matsui, T.; Watanabe, Y. Phylogenetic analysis of the gut bacterial microflora of the fungus growing termite Odontotermes formosanus. Biosci. Biotechnol. Biochem. 2007, 71, 906–915.
- Hongoh, Y.; Deevong, P.; Inoue, T.; Moriya, S.; Trakulnaleamsai, S.; Ohkuma, M.; Vongkaluang, C.; Noparatnaraporn, N.; Kudo, T. Intra- and interspecific comparisons of bacterial diversity and community structure support coevolution of gut microbiota and termite host. Appl. Environ. Microbiol. 2005, 71, 6590–6599.
- Bi, S.F.; Guo, Z.K.; Jiang, N.; Jiao, R.H. New alkaloid from Streptomyces koyangensis residing in Odontotermes formosanus. J. Asian Nat. Prod. Res. 2013, 15, 422–425.
- Harazono, K.; Yamashita, N.; Shinzato, N.; Watanabe, Y.; Fukatsu, T.; Kurane, R. Isolation and characterization of aromatics-degrading microorganisms from the gut of the lower termite Coptotermes formosanus. Biosci. Biotechnol. Biochem. 2003, 67, 889–892.
- Sun, L.Q.; Hse, C.Y.; Shupe, T.; Sun, M.J.; Wang, X.H.; Zhao, K. Isolation and characterization of an endophytic fungal strain with potent anti-microbial and termicidal activities from Port-Orford-Cedar. J. Econ. Entomol. 2015, 108, 962–968.
- Wenzel, M.; Schönig, I.; Berchtold, M.; Kämpfer, P.; König, H. Aerobic and facultatively anaerobic cellulolytic bacteria from the gut of the termite Zootermopsis angusticollis. J. Appl. Microbiol. 2002, 92, 32–40.
- Paul, K.; Nonoh, J.O.; Mikulski, L.; Brune, A. Methanoplasmatales, thermoplasmatales-related archaea in termite guts and other environments, are the seventh order of methanogens. Appl. Environ. Microbiol. 2012, 78, 8245–8253.
- Dröge, S.; Fröhlich, J.; Radek, R.; König, H. Spirochaeta coccoides sp. nov., a novel coccoid spirochete from the hindgut of the termite Neotermes castaneus. Appl. Environ. Microbiol. 2006, 72, 392–397.
- Ali, H.R.K.; Hemeda, N.F.; Abdelaliem, Y.F. Symbiotic cellulolytic bacteria from the gut of the subterranean termite Psammotermes hypostoma Desneux and their role in cellulose digestion. AMB Express 2019, 9, 111.
- Butera, G.; Ferraro, C.; Alonzo, G.; Colazza, S.; Quatrini, P. The gut microbiota of the wood-feeding termite Reticulitermes lucifugus (Isoptera; Rhinotermitidae). Ann. Microbiol. 2016, 66, 253–260.
- Adams, L.; Boopathy, R. Isolation and characterization of enteric bacteria from the hindgut of formosan termite. Biores. Technol. 2005, 96, 1592–1598.
- Brune, A.; Ohkuma, M. Role of termite gut microbiota in symbiotic digestion. In Biology of Termites: A Modern Synthesis; Bignell, D.E., Roisin, Y., Lo, N., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 439–475.
- Bakalidou, A.; Kampfer, P.; Berchtold, M.; Kuhnigk, T.; Wenzel, M.; Konig, H. Cellulosimicrobium variabile sp. nov., a cellulolytic bacterium from the hindgut of the termite Mastotermes darwiniensis. Int. J. Syst. Evol. Microbiol. 2002, 52, 1185–1192.
- Stackebrandt, E.; Schumann, P.; Cui, X.L. Reclassification of Cellulosimicrobium variabile Bakalidou et al. 2002 as Isoptericola variabilis gen. nov., comb. nov. Int. J. System. Evol. Microbiol. 2004, 54, 685–688.
- Boga, H.I.; Brune, A. Hydrogen-dependent oxygen reduction by homoacetogenic bacteria isolated from termite guts. Appl. Environ. Microbiol. 2003, 69, 779–786.
- Graber, J.R.; Leadbetter, J.R.; Breznak, J.A. Description of Treponema. Azotonutricium sp. nov. and Treponema Primitia sp. nov., the first spirochetes isolated from termite guts. Appl. Environ. Microbiol. 2004, 70, 1315–1320.
- Ikeda-Ohtsubo, W.; Brune, A. Cospeciation of termite gut flagellates and their bacterial endosymbionts: Trichonympha species and ‘Candidatus Endomicrobium trichonymphae. Mol. Ecol. 2009, 18, 332–342.
- Cleveland, L.R. The method by which Trichonympha campanula, a protozoön in the intestine of termites, ingests solid particles of wood for food. Biol. Bull. 1923, 48, 282–288.
- Arora, J.; Kinjo, Y.; Šobotník, J.; Buček, A.; Clitheroe, C.; Stiblik, P.; Roisin, Y.; Žifčáková, L.; Park, Y.C.; Kim, K.Y.; et al. The functional evolution of termite gut microbiota. Microbiome 2022, 10, 78.
- Todaka, N.; Moriya, S.; Saita, K.; Hondo, T.; Kiuchi, I.; Takasu, H.; Ohkuma, M.; Piero, C.; Hayashizaki, Y.; Kudo, T. Environmental cDNA analysis of the genes involved in lignocellulose digestion in the symbiotic protist community of Reticulitermes speratus. FEMS Microbiol. Ecol. 2007, 59, 592.
- Xie, L.; Zhang, L.; Zhong, Y.; Liu, N.; Long, Y.; Wang, S.; Zhou, X.; Zhou, Z.; Huang, Y.; Wang, Q. Profiling the metatranscriptome of the protistan community in Coptotermes formosanus with emphasis on the lignocellulolytic system. Genomics 2012, 99, 246.
- Watanabe, H.; Takase, A.; Tokuda, G.; Yamada, A.; Lo, N. Symbiotic "Archaezoa" of the primitive termite Mastotermes darwiniensis still play a role in cellulase production. Eukaryot Cell 2006, 5, 1571–1576.
- Ohtoko, K.; Ohkuma, M.; Moriya, S.; Inoue, T.; Usami, R.; Kudo, T. Diverse genes of cellulase homologues of glycosyl hydrolase family 45 from the symbiotic protists in the hindgut of the termite Reticulitermes speratus. Extremophiles 2000, 4, 343.
- Tartar, M.M.; Wheeler, X.; Zhou, M.R.; Coy, D.G.; Boucias; Scharf, M.E. Parallel metatranscriptome analyses of host and symbiont gene expression in the gut of the termite Reticulitermes flavipes. Biotechnol. Biofuels 2009, 2, 25.
- Ke, J.; Laskar, D.D.; Singh, D.; Chen, S. In situ lignocellulosic unlocking mechanism for carbohydrate hydrolysis in termites: Crucial lignin modification. Biotechnol. Biofuels 2011, 4, 17.
- Nishimura, Y.; Otagiri, M.; Yuki, M.; Shimizu, M.; Inoue, J.I.; Moriya, S.; Ohkuma, M. Division of functional roles for termite gut protists revealed by single-cell transcriptomes. ISME J. 2020, 14, 2449–2460.
- Gile, G.H. Protist symbionts of termites: Diversity, distribution, and coevolution. Biolog. Rev. 2024.
- Jasso-Selles, D.E.; Martini, F.D.; Velenovsky, J.F.; Mee, E.D.; Montoya, S.J.; Hileman, J.T.; Garcia, M.D.; Su, Y.; Chouvenc, T.; Gile, G.H. The complete protist symbiont communities of coptotermes formosanus and Coptotermes gestroi: Morphological and molecular characterization of five new species. J. Eukary. Microbiol. 2020, 67, 626–641.
- Ohkuma, M.; Sato, T.; Noda, S.; Ui, S.; Kudo, T.; Hongoh, Y. The candidate phylum ‘Termite Group 1′ of bacteria: Phylogenetic diversity, distribution, and endosymbiont members of various gut flagellated protists. FEMS Microbiol. Ecol. 2007, 60, 467–476.
- Noda, S.; Shimizu, D.; Yuki, M.; Kitade, O.; Ohkuma, M. Host-symbiont cospeciation of termite-gut cellulolytic protists of the genera Teranympha and Eucomonympha and their Treponema Endosymbionts. Microb. Environ. 2018, 33, 26–33.
- Ohkuma, M.; Ohtoko, K.; Iida, T.; Tokura, M.; Moriya, S.; Usami, R.; Horikoshi, K.; Kudo, T. Phylogenetic identification of Hypermastigotes, Pseudotrichonympha, Spirotrichonympha, Holomastigotoides, and Parabasalian symbionts in the hindgut of termites. J. Eukar. Microbiol. 2000, 47, 249–259.
- Del Campo, J.; James, E.R.; Hirakawa, Y.; Fiorito, R.; Kolisko, M.; Irwin, N.A.; Mathur, V.; Boscaro, V.; Hehenberger, E.; Karnkowska, A.; et al. Pseudotrichonympha leei, Pseudotrichonympha lifesoni, and Pseudotrichonympha pearti, new species of parabasalian flagellates and the description of a rotating subcellular structure. Sci. Rep. 2017, 7, 1–8.
- Kitade, O. Comparison of symbiotic flagellates faunae between termites and a wood-feeding cockroaches of the genus Cryptocercus. Microb. Environ. 2004, 19, 215–220. Available online: http://wwwsoc.nii.ac.jp/jsme2 (accessed on 10 September 2023).
- Nalepa, C.A. Origin of mutualism between termites and flagellated gut protists: Transition from horizontal to vertical transmission. Front. Ecol. Evol. 2020, 8, 507505.
- Gile, G.H.; Carpenter, K.J.; James, E.R.; Scheffrahn, R.H.; Keeling, P.J. Morphology and molecular phylogeny of Staurojoenina mulleri sp. Nov. (Trichonymphida, Parabasalia) from the hindgut of the kalotermitid Neotermes jouteli. J.Eukary. Microbiol. 2013, 60, 203–213.
- Dolan, M.F.; Wier, A.M.; Melnitsky, H.; Whiteside, J.H.; Margulis, L. Cysts and symbionts of Staurojoenina assimilis Kirby from Neotermes. Eur. J. Protist. 2004, 40, 257–264.
- Nalepa, C.A. What kills the hindgut flagellates of lower termites during the host molting cycle? Microorganisms 2017, 5, 82.
- Brugerolle, G.; Bordereau, C. The flagellates of the termite Hodotermopsis sjoestedti with special reference to Hoplonympha, Holomastigotes and Trichomonoides trypanoides n. Comb. Eur. J. Protist. 2004, 40, 163–174.
- Radek, R.; Meuser, K.; Altinay, S.; Lo, N.; Brune, A. Novel Lineages of Oxymonad Flagellates from the Termite Porotermes adamsoni (Stolotermitidae): The Genera Oxynympha and Termitimonas. Protist 2019, 170, 125683.
- Brugerolle, G.; Patterson, D.J. Ultrastructure of Joenina pulchella Grassi, 1917 (Protista, Parabasalia), a reassessment of evolutionary trends in the parabasalids, and a new order Cristamonadida for devescovinid, calonymphid and lophomonad flagellates. Org. Divers. Evol. 2000, 1, 147–160.
- Treitli, S.C.; Kolisko, M.; Husník, F.; Keeling, P.J.; Hampl, V. Revealing the metabolic capacity of Streblomastix strix and its bacterial symbionts using single-cell metagenomics. Proc. Natl. Acad. Sci. USA 2019, 116, 19675–19684.
- Noda, S.; Mantini, C.; Bordereau, C.; Kitade, O.; Dolan, M.F.; Viscogliosi, E.; Ohkuma, M. Molecular phylogeny of parabasalids with emphasis on the order Cristamonadida and its complex morphological evolution. Mol. Phylogen. Evol. 2009, 52, 217–224.
- Brugerolle, G. The symbiotic fauna of the African termite Hodotermes mossambicus identification of four flagellate species of the genera Spironympha, Trichomonoides and Retortamonas. Parasitol. Res. 2006, 98, 257–263.
- Inoue, T.; Kitade, O.; Yoshimura, T.; Yamaoka, I. Symbiotic Associations with Protists. In Termites: Evolution, Sociality, Symbioses, Ecology, 1st ed.; Abe, T., Bignell, D.E., Higashi, M., Eds.; Springer: Dordrecht, The Netherlands, 2000; Volume 1, pp. 275–288.
- Li, L.; Fröhlich, J.; Pfeiffer, P.; König, H. Termite Gut Symbiotic Archaezoa Are Becoming Living Metabolic Fossils. Eukaryot. Cell 2003, 2, 1091–1098.
- Berchtold, M.; Konig, H. Phylogenetic position of the two uncultivated trichomonads Pentatrichomonoides scroa Kirby and Metadevescovina extranea Kirby from the hindgut of the termite Mastotermes darwiniensis Froggatt. Syst. Appl. Microbiol. 1995, 18, 567–573.
- Bignell, D.E.; Eggleton, P. Termites: Evolution, Sociality, Symbioses, Ecology; Abe, T., Bignell, D.E., Higashi, M., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands; Singapore, 2000; pp. 1–363.
- da Costa, R.R.; Hu, H.; Li, H.; Poulsen, M. Symbiotic plant biomass decomposition in fungus-growing termites. Insects 2019, 10, 87.
- Hyodo, F.; Tayasu, I.; Inoue, T.; Azuma, T.I.; Kudo, T.; Abe, T. Differential role of symbiotic fungi in lignin degradation and food provision for fungus-growing termites (Macrotermitinae: Isoptera). Funct. Ecol. 2003, 17, 186–193.
- Johjima, T.; Taprab., Y.; Noparatnaraporn, N.; Kudo., T.; Ohkuma, M. Large-scale identification of transcripts expressed in a symbiotic fungus (Termitomyces) during plant biomass degradation. Appl. Microbiol. Biotechnol. 2006, 73, 195.
- Vikram, S.; Arneodo, J.D.; Calcagno, J.; Ortiz, M.; Mon, M.L.; Etcheverry, C.; Cowan, D.A.; Talia, P. Diversity structure of the microbial communities in the guts of four neotropical termite species. PeerJ 2021, 9, e10959.
- Sun, J.Z.; Ding, S.Y.; Doran-Peterson, J. Biological conversion of biomass for fuels and chemicals: Explorations from natural utilization systems. In Royal Society of Chemistry Energy and Environment Series 10; Royal Society of Chemistry: Cambridge, UK, 2014; pp. 1–380.
- Sun, J.Z.; Scharf, M.E. Exploring and integrating cellulolytic systems of insects to advance biofuel technology. Insect Sci. 2010, 17, 163.
- Peterson, B.F.; Stewart, H.L.; Scharf, M.E. Quantification of symbiotic contributions to lower termite lignocellulose digestion using antimicrobial treatments. Insect Biochem. Mol. Biol. 2015, 59, 80–88.
- Berlanga, M.; Paster, B.J.; Guerrero, R. Co-evolution of symbiotic spirochete diversity in lower termites. Int. Microbiol. 2007, 10, 133–139.
- Gomati, V.; Ramasamy, K.; Kumar, K.; Sivaramaiah, N.; Mula, R. Greenhouse gas emissions from termite ecosystem. Afr. J. Microbiol. Res. 2011, 5, 56–64.
- Bauer, S.; Tholen, A.; Overmann, J.; Brune, A. Characterization of abundance and diversity of lactic acid bacteria in the hindgut of wood- and soil-feeding termites by molecular and culture-dependent techniques. Arch. Microbiol. 2000, 173, 126–173.
- Inoue, J.; Saita, K.; Kudo, T.; Ui, S.; Ohkuma, M. Hydrogen production by termite gut protists: Characterization of iron hydrogenases of Parabasalian symbionts of the termite Coptotermes formosanus. Eukaryot. Cell 2007, 6, 1925–1932.
- Schmitt-Wagner, D.; Friedrich, M.W.; Wagner, B.; Brune, A. Phylogenetic diversity, abundance, and axial distribution of bacteria in the intestinal tract of two soil-feeding termites (Cubitermes spp.). Appl. Environ. Microbiol. 2003, 69, 6007–6017.
- Kane, M.D.; Breznak, J.A. Acetonema longum gen. nov. sp. Nov., an H2/CO2 acetogenic bacterium from the termite Pterotermes occidentis. Arch. Microbiol. 1991, 156, 91–98.
- Mathew, G.M.; Mathew, D.C.; Lo, S.C.; Alexios, G.M.; Yang, J.C.; Sashikumar, J.M.; Shaikh, T.M.; Huang, C.-C. Synergistic collaboration of gut symbionts in Odontotermes formosanus for lignocellulosic degradation and bio-hydrogen production. Bioresour. Technol. 2013, 145, 337–344.
- Cao, Y.Q.; Sun, J.Z.; Rodriguez, M.; Lee, K.C. Hydrogen emission by three wood-feeding subterranean termite species (Isoptera: Rhinotermitidae): Production and characteristics. Insect Sci. 2010, 17, 237.
- Ramin, M.; Alimon, A.R.; Abdullah, N.; Panandam, J.M.; Sijam, K. Isolation and identification of three species of bacteria from the termite Coptotermes curvignathus (Holmgren) present in the vicinity of University Putra Malaysia. Res. J. Microbiol. 2008, 1, 288–292.
- Chang, J.J.; Lin, J.J.; Ho, C.Y.; Chin, W.C.; Huang, C.C. Establishment of rumenmimic bacterial consortia: A functional union for bio-hydrogen production from cellulosic bioresource. Int. J. Hydrog. Energy 2010, 35, 13399–13406.
- Harun, I.; Jahim, J.M.; Anuar, N.; Hassan, O. Hydrogen production performance by Enterobacter cloacae KBH3 isolated from termite guts. Int. J. Hydrog. Energy 2012, 37, 15052–15061.
- Ramachandran, U.; Wrana, N.; Cicek, N.; Sparling, R.; Levin, D.B. Hydrogen production and end-product synthesis patterns by Clostridium termitidis strain CT1112 in batch fermentation cultures with cellobiose or α-cellulose. Int. J. Hydrog. Energy 2008, 33, 7006–7012.
- Rajeswari, G.; Jacob, S.; Chandel, A.K.; Kumar, V. Unlocking the potential of insect and ruminant host symbionts for recycling of lignocellulosic carbon with a biorefinery approach: A review. Microb. Cell Fact. 2021, 20, 107.
- Lazuka, A.; Auer, L.; O’Donohue, M.; Hernandez-Raquet, G. Anaerobic lignocellulolytic microbial consortium derived from termite gut: Enrichment, lignocellulose degradation and community dynamics. Biotechnol. Biofuels 2018, 11, 284.
- Danso, B.; Ali, S.S.; Xie, R.; Sun, J. Valorisation of wheat straw and bioethanol production by a novel xylanase- and cellulase-producing Streptomyces strain isolated from the wood-feeding termite, Microcerotermes species. Fuel 2022, 310, 122333.
- Tsegaye, B.; Balomajumder, C.; Roy, P. Isolation and Characterization of Novel Lignolytic, Cellulolytic, and Hemicellulolytic Bacteria from Wood-Feeding Termite Cryptotermes brevis. Int. Microbiol. 2019, 22, 29–39.
- Auer, L.; Lazuka, A.; Sillam-Dussès, D.; Miambi, E.; Hernandez-Raquet, G. Uncovering the Potential of Termite Gut Microbiome for Lignocellulose Bioconversion in Anaerobic Batch Bioreactors. Front. Microbiol. 2017, 8, 2623.
- Chiri, E.; Nauer, P.A.; Lappan, R.; Jirapanjawat, T.; Waite, D.W.; Handley, K.M.; Hugenholtz, P.; Cook, P.L.; Arndt, S.K.; Greening, C. Termite gas emissions select for hydrogenotrophic microbial communities in termite mounds. PNAS 2021, 118, e2102625118.
- Konishi, T.; Yamamoto, D.; Umezawa, K.; Itakura, S. Hydrogen production by microorganisms in the hindgut of the termite Reticulitermes speratus under anaerobic and aerobic conditions. J. Environ. Entomol. Zool. 2020, 31, 51–55.
- Graber, J.R.; Breznak, J.A. Physiology and nutrition of Treponema primitia, an H2/CO2-acetogenic spirochete from termite hindguts. Appl. Environ. Microbiol. 2004, 70, 1307–1314.
- Gomez-Flores, M.; Nakhla, G.; Hafez, H. Hydrogen production and microbial kinetics of Clostridium termitidis in mono-culture and co-culture with Clostridium beijerinckii on cellulose. AMB Expr. 2017, 7, 84.
- Pester, M.; Tholen, A.; Friedrich, M.W.; Brune, A. Methane Oxidation in Termite Hindguts: Absence of Evidence and Evidence of Absence. Appl. Environ. Microbiol. 2007, 73, 2024–2028.
- Pester, M.; Brune, A. Hydrogen is the central free intermediate during lignocellulose degradation by termite gut symbionts. ISME J. 2007, 1, 551–565.
- Tholen, A.; Schink, B.; Brune, A. The gut microflora of Reticulitermes flavipes, its relation to oxygen, and evidence for oxygen-dependent acetogenesis by the most abundant Enterococcus sp. FEMS Microbiol. Ecology 1997, 24, 137–149.
- Schmitt-Wagner, D.; Brune, A. Hydrogen profiles and localization of methanogenic activities in the highly compartmentalized hindgut of soil-feeding higher termites (Cubitermes spp.). Appl. Environ. Microbiol. 1999, 65, 4490–4496.
- Boga, H.I.; Ludwig, W.; Brune, A. Sporomusa aerivorans sp. nov., an oxygen-reducing homoacetogenic bacterium from the gut of a soil-feeding termite. Int. J. Syst. Evol. Microbiol. 2003, 53, 1397–1404.
- Boga, H.I.; Ji, R.; Ludwig, W.; Brune, A. Sporotalea propionica gen. nov. sp. nov., a hydrogen-oxidizing, oxygen-reducing, propionigenic firmicute from the intestinal tract of a soil-feeding termite. Arch. Microbiol. 2007, 187, 15–27.
- Yamin, M.A. Cellulose metabolism by the flagellate trichonympha from a termite is independent of endosymbiotic bacteria. Science 1981, 211, 58–59.
- Odelson, D.A.; Breznak, J.A. Nutrition and Growth Characteristics of Trichomitopsis termopsidis, a Cellulolytic Protozoan from Termites. Appl. Environ. Microbiol. 1985, 49, 614–621.




