The hunt for renewable and alternative fuels has driven research towards the biological conversion of lignocellulosic biomass (LCB) into biofuels, including bioethanol and biohydrogen. Among the natural biomass utilization systems (NBUS), termites represent a unique and easy-to-access model system to study host–microbe interactions towards lignocellulose bioconversion/valorization. Termites have gained significant interest due to their highly efficient lignocellulolytic systems. The wood-feeding termites apply a unique and stepwise process for the hydrolysis of lignin, hemicellulose, and cellulose via biocatalytic processes; therefore, mimicking their digestive metabolism and physiochemical gut environments might lay the foundation for an innovative design of nature-inspired biotechnology.
- lignocellulose biorefinery
- termites
- gut system
- gut microbiota
1. Introduction
2. Termite Gut as Unique Reservoir of Lignocellulolytic Microorganisms
Termites share an intricate relationship with gut microbial symbionts, particularly for the digestion and assimilation of lignocellulose into energy and nutritional resources. The gut microbiota not only help the host to digest lignocellulose but also contribute enzymes and nutrients deficient in the hosts. Keeping in view the functions that symbionts contribute to the host, the gut microbiota in termites can be considered an integrated organ [10][42]. Cellulose being a macromolecule is cleaved by the bacterial enzymes into short-chain fatty acids in the gut system of termites [11][43]. These short chain fatty acids are further broken down and metabolized by the termites. Most of the nitrogen economy in termites is attributed to nitrogen fixation from symbiotic bacteria. Many other bacteria are reportedly involved in the synthesis of amino acids and production of cofactors [12][13][14][44,45,46]. Termites possess a battery of enzymes required for the degradation of LCB into fermentable products of hydrogen and energy. These enzymes are majorly contributed by the gut inhabitants involving flagellates, bacteria, and fungi. Not all termites contain flagellates, as higher termites are devoid of it, while bacteria are reserved by all termite species studied to date. Moreover, a tremendous diversity of bacteria has been represented in termite guts, reporting more than 200 species of bacterial genes. Termites achieve this lignocellulolytic expertise by collaborating with over 200 species of microbes that reside in their gut systems. The endosymbionts, such as “Candidatus Endomicrobium trichonymphae” and “Candidatus Azobacteroides pseudotrichonymphae”, that live within the unicellular flagellates produce their energy from the fermentation of carbohydrates to acetate [13][15][45,47]. These bacteria are substrate specific, where the former uses glucose-6-phosphate and later degrades glucose, xylose, or hexuronates only. It is likely due to the availability of these substrates within the termite gut system derived from the cellulose and hemicellulose digestion.2.1. Termite Gut Bacteria
The termite gut system is a “gold mine” of symbiotic microorganisms, including bacteria, fungi, actinomycetes, and others. The total number of bacteria ranges from 107–1011 mL−1 in the hindgut of termites [16][48]. Despite the small size of the termite gut, it offers a unique reservoir of novel microbes particularly bacteria that are found nowhere else in nature [17][49]. For the digestion of recalcitrant lignocellulose, wood-eating termites maintain a variety of unusual microbial symbionts, reaching densities of up to 1011 cells mL−1 (Table 1). Termites shelter a diversity of lignocellulose-hydrolyzing bacteria that has been reported by many authors [4][18][19][4,27,38]. Bacteria belonging to four major phyla, such as Elusimicrobia, Bacteroidetes, Proteobacteria, and Actinobacteria, are found as endosymbionts in protist cells within the termite gut [20][21][22][50,51,52]. To date, several bacteria have been isolated and identified from the termite gut systems, including Acetonema longum and Clostridium mayombei from Macrotermes gilvus [23][53]. Spirochaetes are by far the most prevalent and species-rich bacteria in wood-feeding termites. Owing to their high surface-to-volume ratio and free-swimming nature, spirochaetes found in the hindgut of termites’ can circumvent the restrictions of metabolic diffusion in microoxic/anoxic habitats [24][54]. While most of the bacteria in lower termites reside in the cytoplasm or attach externally to the flagellate cells [17][49], certain tiny bacteria adhere to the cuticle or filamentous microorganisms, which are in turn affixed with the wall of the hindgut [25][55]. However, the diversity of bacterial species varies with respect to the termite species; for example, Coptotermes species are dominated by the members of Bacteroidetes [20][50], whereas Candidatus (Elusimicrobia: Endomicrobia) are predominant in Reticulitermes spp. [18][21][26][27,51,56]. Like Coptotermes termites, Odontotermes and Macrotermes species are also dominated by the Bacteroidetes and Firmicutes [27][57]. The Nasutitermitinae and Termitinae workers contain a significant amount of fibrobacteres belonging to the phylum TG3 [28][58]. These evolutionary changes in the bacterial diversity allowed for the termites to adapt to diverse habitats and diets while restricting the lower termites to wood-feeding habits [18][27]. Termites harbor a diverse array of microbiota, the majority of which are unculturable, with many taxa still unknown. Consequently, the mystery of termite–microbe symbiosis is still at its nascent stage. However, a genome-based analysis of the unculturable bacteria appears as a strong approach to address this issue.| Bacterial Genera | Prokaryote Type | Termite Species | Gut-Region | References |
|---|---|---|---|---|
| Streptomyces naraensis | Actinomycete | Coptotermes formosanus | Whole gut | [29][59] |
| S. filamentosus | Odontotermes formosanus | Whole gut | [30][60] | |
| Clostridium mayombei, Sporomusa termitida, Klebsiella variicola, Acetonema longum K. pneumoniae, M. cuticularis, M. curvans, M. filiformis |
| Termite Host | Protist/Flagellate | References | |||||
|---|---|---|---|---|---|---|---|
| Class | Order | Family | Genus | ||||
| Incisitermes minor | Trichomonadea | Trichomonadida | Devescovinidae | Metadevescovina cuspidata | [46][50][ | ||
| Bacteria | |||||||
| Nasutitermes nigriceps | [ | 31 | ][61] | ||||
| Pterotermes occidentis | |||||||
| 75 | , | 79 | ] | ||||
| Coptotermes spp. | Parabasalia | Spirotrichonymphida | Holomastigotoididae | ||||
| Holomastigotoides | [ | 53 | ] | [ | 54][55][82,83,84] | O. formosanus | |
| ] | Archaea | Reticulitermes speratus | Whole gut | [32][62] | |||
| Trichonymphida | Trichomonadidae | Trichomonas | Cubitermes ugandensis | Whole gut | [33 | ||
| Teranymphidae | Teranympha | ][63] | |||||
| Treponema isoptericolens, Spirochaeta coccoides |
Spirochete | Incisitermes tabogae | Whole gut | [34][64] | |||
| Trichonymphidae | Spirotrichonympha | Acinetobacter seifertii | |||||
| Trichonympha | |||||||
| Tritrichomonadida | Monocercomonadidae | Monocercomonas | |||||
| Preaxostyla | Oxymonadida | Pyrsonymphidae | Dinenympha | ||||
| [ | |||||||
| 38 | |||||||
| ] | |||||||
| [ | |||||||
| 68 | |||||||
| ] | |||||||
| Isoptericola variabilis | |||||||
| Mastotermes darwiniensis | hindgut | [ | 39][40][69,70] | ||||
| Sporomusa aerivorans | Thoracotermes macrothorax | Whole gut | [41 | ||||
| Trichonymphida | Teranymphidae | Pseudotrichonympha | ] | [ | |||
| Trichonymphidae | Spirotrichonympha | ||||||
| Cononympha | |||||||
| Reticulitermes spp. | Parabasalia | Spirotrichonymphida | Holomastigotoididae | Holomastigotoides | [56] | 71] | |
| [ | 57 | , Enterobacter asburiae, E. cloacae, Lysinibacillus macrolides, | Bacteria | C. formosanus | Foregut | [4] | |
| S. marcescens, P. stutzeri, S. hominis, B. cereus, K. aerogenes, E. hormaechei | Bacteria | C. formosanus | Foregut Midgut Hindgut | [6] | |||
| E. cancerogenes, E. ludwigii, L. boronitolerans, Lysinibacillus sp., P. fluorescens, P. plecoglossicida, P. putida | Bacteria | C. formosanus | Midgut | [3] | |||
| A. calcoaceticus, B. simplex, Dietza sp., E. mori, L. fusiformis, P. nitroreducens | Bacteria | C. formosanus | Hindgut | [3] | |||
| Pyrsonympha | Bacillus spp. | Bacteria | Psammotermes hypostoma | Whole gut | |||
| Rhinotermes spp. | [ | 35 | ] | [65] | |||
| Parabasalia | Cristamonadida | Lophomonadidae | Gigantomonas | [ | 53][58][82,87] | Paenibacillus lactis AFC1 | Bacteria |
| Schedorhinotermes spp. | Parabasalia | Trichonymphida | Teranymphidae | Pseudotrichonympha | [59] | L. fusiformis AFC2 | |
| Stenotrophomonas maltophilia AFC3 |
|||||||
| L. macrolides AFC4 | |||||||
| [ | 88 | ] | Bacillus cereus AFC5 | ||||
| Bacillus spp., Paenibacillus spp. | Bacteria | R. lucifugus | Whole gut | [36][66] | |||
| Cellulomonas/Oerskovia, Microbacterium and Kocuria |
Actinomycete | Z. angusticollis | Whole gut | [32][62] | |||
| Bacillus, Brevibacillus, Paenibacillus, fipia, Agrobacterium/Rhizobium, Brucella/Ochrobactrum, Pseudomonas and Sphingomonas/Zymomonas |
Bacteria | Z. angusticollis | |||||
| Citrobacter farmeri | C. formosanus | Whole gut | [37][67] | ||||
| Bacillus spp. | O. formosanus | ||||||
| Bacteria | |||||||
| Cryptotermes spp. | Mastotermes darwiniensis, Cryptotermes primus, N. arborum, Thoracotermes macrothorax, Anoplotermes pacificus |
Whole gut | Candidatus Vestibaculum illigatum | Neotermes cubanus | Whole gut | [21][51] | |
| ] | [ | 85 | T. azotonutricium | Spirochaetes | Zootermopsis angusticollis | Whole gut | [42][72] |
| T. primitia | |||||||
| Candidatus Endomicrobium trichonymphae, | R. santonensis | Whole gut | [21][51] | ||||
2.2. Symbiotic Flagellates
The presence of flagellates is typically represented in the gut system of lower termites only, suggesting their evolutionary adaptations. In particular, the enlarged hindgut shelters a high number of protists that share symbiotic connection, offering two-way benefits to the host (Table 2). They are primarily responsible for the enzyme secretions to hydrolyze the cellulose and hemicellulose molecules. Second, they provide a large surface area for the colonization of ecto- as well as endo-symbiotic bacteria within the termite gut systems [43][73]. Due to their important support in the lignocellulose breakdown, protists are essential to the survival of the termite host. Cleveland in 1924 was the first to show that Reticulitermes flavipes could not consume cellulose and perished within 20 days after defaunation of intestinal protists [44][33]. To metabolize their cellulose-rich diet, protists secrete and express their own cellulases. This allows them to digest and break down the cellulose components and convert them into nutrients and energy [45][74]. The symbiotic protists in termites express several genes that encode cellulose- and hemicellulose-degrading enzymes belonging to different Glycoside Hydrolase Families (GHF). Several meta-transcriptomic investigations confirmed the involvement of these genes in lignocellulose bioconversion and metabolism [46][47][75,76]. Almost 1 in every 10 expressed genes of protists in termites are responsible for cellulose degradation. Moreover, the occurrence of the GH7 family cellulases in all the gut protists suggested them as “core enzyme set” in termites. Despite the uneven expression levels of glycosyl hydrolases, the GHF7 shows highest expression in termite guts. Among the expression of 1000 clones of an environmental expressed sequence tags (EST) reported in the R. flavipes protist community, 6.2% of the sequences corresponded to GHF7. This family contains cellobiohydrolase (CBH) and endoglucanase (EG) subtypes of cellulases. The GHF7 CBHs make up to 4.1% of all ESTs, whereas EGs occupy 2.1% only. The elevated expression of these enzymes in wood-feeding termites implies that they are crucial for the metabolism of cellulose. Additionally, GHF45-related protist cellulases have been discovered in Reticulitermes speratus and Mastotermes darwiniensis [| , | |||||
| 86 | |||||
| Parabasalia | |||||
| Cristamonadida | |||||
| Lophomonadidae | |||||
| Stephanonympha | |||||
| [ | |||||
| 57 | |||||
| ] | |||||
| [ | |||||
| 60 | |||||
| ] | [ | 86 | , | 89 | ] |
| Devescovina | |||||
| Epicalotermes spp. | Parabasalia | Trichonymphida | Staurojoeninidae | Staurojoenina | [61][90] |
| Glyptotermes spp. | Parabasalia | Cristamonadida | Lophomonadidae | Devescovina | [57][86] |
| Macrotrichomonas | |||||
| Incisitermes spp. | Parabasalia | Cristamonadida | Lophomonadidae | Coronympha | [55][57][84,86] |
| Trichonymphida | Staurojoeninidae | Staurojoenina | |||
| Trichonymphidae | Trichonympha | ||||
| Kalotermes spp. | Parabasalia | Cristamonadida | Lophomonadidae | Calonympha | [55][84] |
| Devescovina | |||||
| Joenia | |||||
| Stephanonympha | |||||
| Tritrichomonadida | Monocercomonadidae | Monocercomonas | |||
| Neotermes spp. | Parabasalia | Cristamonadida | Lophomonadidae | Devescovina | [62][91] |
| Foaina | |||||
| Preaxostyla | Oxymonadida | Oxymonadidae | Oxymonas | ||
| Archotermopsis spp. | Parabasalia | Honigbergiellida | Honigbergiellidae | Ditrichomonas | [63][92] |
| Trichonymphida | Teranymphidae | Pseudotrichonympha | |||
| Hodotermopsis spp. | Parabasalia | Spirotrichonymphida | Holomastigotoididae | Spirotrichonymphella | [56][64][85,93] |
| Trichomonadida | Trichomonadidae | Trichomonas | |||
| Trichonymphida | Hoplonymphidae | Hoplonympha | |||
| Teranymphidae | Eucomonympha | ||||
| Trichonymphidae | Spirotrichonympha | ||||
| Trichonympha | |||||
| Porotermes spp. | Parabasalia | Cristamonadida | Lophomonadidae | Joenina | [65][66][94,95] |
| Spirotrichonymphida | Holomastigotoididae | Spirotrichonymphella | |||
| Trichomonadida | Trichomonadidae | Pseudotrypanosoma | |||
| Trichonymphida | Teranymphidae | Pseudotrichonympha | |||
| Trichonymphidae | Trichonympha | ||||
| Zootermopsis spp. | Parabasalia | Hypotrichomonadida | Hypotrichomonadidae | Trichomitus | [60][67][89,96] |
| Trichonymphida | Trichonymphidae | Trichonympha | |||
| Preaxostyla | Oxymonadida | Streblomastigidae | Streblomastix | ||
| Hodotermes spp. | Parabasalia | Cristamonadida | Lophomonadidae | Devescovina Foaina Gigantomonas Stephanonympha |
[68][69][97,98] |
| Trichonymphida | Spirotrichosomidae | Leptospironympha | |||
| Mastotermes spp. | Parabasalia |
Cristamonadida Trichomonadida |
Lophomonadidae Trichomonadidae |
Deltotrichonympha Pentatrichomonoides |
[70][71][72][99,100,101] |
2.3. Symbiotic Fungi
Termites share an intricate relation with fungi. Hitherto, a number of fungal and yeast species have been observed in the gut systems of termites. Fungus-growing termites are prevalent in the tropical regions of Asia and Africa [73][41]. Higher termites from the subfamily Macrotermitine coexist with the fungus, Termitomyces spp. It is well acknowledged that the fungal symbionts play a significant role in the breakdown of lignocellulose, thus aiding the host termites. In fungus-growing termites, young workers ingest Termitomyces nodules along with LCB and excrete lignin-rich feces to build fresh fungus combs [74][102]. Within 40 days, Termitomyces converts the fresh comb into a well-decomposed mature comb (old comb), which is subsequently ingested by old worker termites. The enzymatic contribution from the termite host, endosymbiont (gut microbiota), and exosymbiont (Termitomyces) greatly facilitates the degradation of plant biomass in fungus-growing termites [6]. Further, the symbiosis of termites and fungi for lignocellulose bioconversion is confirmed in two ways. First, the biochemical detection reveals an apparent increase in the C-to-N ratio and higher nitrogen quality in certain fungus combs. Second, the identification and expression of laccase genes in the genome of symbiotic Termitomyces spp., found in fungus-growing termites, also demonstrates lignin the degradation capacity of the fungal symbionts [75][103]. The breakdown of the lignin barrier apparently allows access for glycosyl hydrolases to attack cellulose and hemicellulose, thereby increasing the overall degradation. Third, according to a subtractive EST study of the cultured Termitomyces spp. of Macrotermes gilvus [76][104], Termitomyces releases a variety of cellulolytic or hemicellulolytic enzymes to break down plant polysaccharides in the termite nests. The cDNA library of the termite revealed a high expression of genes encoding cellulose (EG and CBH), hemicellulose (endo-1, 4-b-xylanases, β-mannanase, etc.), and pectin (endo-polygalacturonase, exo-polygalacturonase) as well as pectate lyase (PL) and rhamnogalacturonan lyase, implicated in lignocellulose degradation. These fungal enzymes belong to the CAZy families, such as GH6, GH7, and GH61 (cellulases); GH11 (xylanase); and the pectinases of PL2 and PL4. The partially digested plant material generated from the fungal combs of worker termites is exposed to gut microbiota for further digestion. The members of the genera, such as Ascomycota, Byssochlamys, Spiromastox, and Malassezia, have been defined as core microbiota in Microceroterme strunckii, Nasutitermes corniger, and Termes riograndensis [77][105].3. Biorefinery Potential of Termite Symbiosis
Recently, termites have attracted a lot of attention from scientists and academicians due to their pest nature as well as symbiosis for lignocellulose digestion. Correspondingly, the termite research has produced a wealth of information with several biotechnological applications. The highly explored knowledge of lignocellulose digestion by termites along with their metabolic and physicochemical processes has provided a basis for the redesign of novel and efficient bioreactors for the fermentation of LCB into bioenergy. The wood-feeding termites apply a unique and stepwise process for the hydrolysis of lignin, hemicellulose, and cellulose via biocatalytic processes (Figure 15); therefore, mimicking their digestive metabolism and physiochemical gut environment will lay the foundation for a nature-inspired lignocellulose processing system. The underexplored biodiversity and biochemistry of the termite guts represent a promising resource of novel catalytic processes [78][26]. Investigating diverse termite lignocellulolytic systems will undoubtedly unveil numerous genes encoding novel biocatalysts along with their expression systems and associated mechanisms, providing valuable insights for the innovative design of nature-inspired technology. Despite a century-old research area, termite biology has recently evolved and developed into a multidisciplinary area that will pave the way for innovations and future breakthroughs in the bioconversion of lignocellulose for biofuels. Termite biotechnology involves biomimetics of the termite gut for future biorefinery apart from other industrial applications.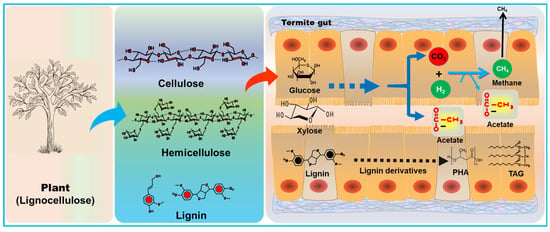
| Microorganism | Termite Host | Biofuel Type | Carbon Source | References |
|---|---|---|---|---|
| Bacteroides, Prevotella | N. ephratae | Biomethane | Wheat straw | [94][121] |
| Streptomyces sp. |
Microcerotermes species | Bioethanol | Wheat straw | [95][122] |
| Bacillus sp. BMP01, Ochrobactrum oryzae BMP03 | Cryptotermes brevis | Bioethanol | CMC, Xylan, Lignin | [96][123] |
| Bacterial symbionts | Nasutitermes ephratae, Microcerotermes parvus, N. lujae, Termes hospes | Biomethane | Wheat straw | [97][124] |
| Actinobacteria | M. nervosus, Macrognathothermes sunteri, Tumulitermes pastinato | Hydrogen, Biomethane | Organic Carbon | [98][125] |
| Symbionts | Reticulitermes speratus | Hydrogen | Wood | [99][126] |
| Treponema primitia | Zootermopsis angusticollis | Acetate | Wood | [100][127] |
| Clostridium termitidis, Clostridium beijerinckii | Nasusitermes spp. | Hydrogen | Cellulose | [101][128] |
| Methylocella sp., and other symbionts | R. speratus, Z. nevadensis, Cubitermes orthognathus | Biomethane, Hydrogen | Wood | [93][102][103][120,129,130] |
| Enterococcus sp. | R. flavipes | Acetate | Lignocellulose | [104][131] |
| Sporomusa termitida, Sporomusa sp. strain TmAO3 | termites | Hydrogen | Lignocellulose | [41][71] |
| Microbiota | Cubitermes spp. | Biomethane | Lignocellulose | [105][132] |
| Sporomusa aerivorans | Thoracotermes macrothorax | Hydrogen | Lactate | [106][133] |
| Sporotalea propionica | T. macrothorax | Hydrogen | Glucose | [107][134] |
| Acetonema longum | Pterotermes occidentis | Hydrogen | Yeast extract, Rumen fluid, Resazurin | [86][113] |
| Enterobacter cloacae KBH3 | Globitermes sp. | Hydrogen | Glucose | [91][118] |
| Pseudotrichonympha grassii | C. formosanus | Hydrogen | Wood cellulose | [84][111] |
| Trichonympha sphaerica | Z. termites | Hydrogen | Lignocellulose | [108][135] |
| Trichomitopsis termopsidis, Hexamastix termopsidis and Tricercomitus termopsidis, Gut microbiota | R. santonensis, Z. nevadensis, Cryptotermes secundus | Hydrogen, Biomethane | CO2, Formate, Lactate | [103][130] |
| Trichomitopsis termopsidis, Trichonympha sphaerica | Z. termites | Hydrogen, Biomethane | Cellulose, Corncob, Cereal leaves | [109][136] |
