Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Andrea Borghini | -- | 3126 | 2024-03-04 10:43:49 | | | |
| 2 | Lindsay Dong | + 1 word(s) | 3127 | 2024-03-05 01:53:20 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Borghini, A.; Labate, L.; Piccinini, S.; Panaino, C.M.V.; Andreassi, M.G.; Gizzi, L.A. FLASH Radiotherapy. Encyclopedia. Available online: https://encyclopedia.pub/entry/55815 (accessed on 06 February 2026).
Borghini A, Labate L, Piccinini S, Panaino CMV, Andreassi MG, Gizzi LA. FLASH Radiotherapy. Encyclopedia. Available at: https://encyclopedia.pub/entry/55815. Accessed February 06, 2026.
Borghini, Andrea, Luca Labate, Simona Piccinini, Costanza Maria Vittoria Panaino, Maria Grazia Andreassi, Leonida Antonio Gizzi. "FLASH Radiotherapy" Encyclopedia, https://encyclopedia.pub/entry/55815 (accessed February 06, 2026).
Borghini, A., Labate, L., Piccinini, S., Panaino, C.M.V., Andreassi, M.G., & Gizzi, L.A. (2024, March 04). FLASH Radiotherapy. In Encyclopedia. https://encyclopedia.pub/entry/55815
Borghini, Andrea, et al. "FLASH Radiotherapy." Encyclopedia. Web. 04 March, 2024.
Copy Citation
FLASH radiotherapy (RT) is considered one of the most promising revolutions in radiation oncology, placing itself at the intersection of technology, physics, and biology. The unique healthy tissue-sparing effect and, at the same time, the equivalent tumor response have already been identified in vivo for multiple organ systems, such as the lung, brain, skin, intestine, and blood, and even in the first human patient.
FLASH radiotherapy
FLASH effect
ultra-high dose rate
1. Introduction
The prevention or mitigation of radiation-induced damage to normal tissues has always been a theme of interest in radiotherapy research. Ongoing studies are focusing on developing new treatment modalities aiming to reduce the risk of complications arising from radiation treatments. FLASH radiotherapy (FLASH RT) is one of the most promising approaches based on the normal tissue-sparing effects of ultra-high dose rate (UHDR) irradiations [1].
FLASH RT is based on the delivery of UHDR radiation several orders of magnitude higher than what is presently used in clinical conventional radiotherapy (CONV RT) (≥40 Gy/s vs. ≤0.03 Gy/s) [2][3]. Even though FLASH RT has been defined using its mean dose rate, the complete definition requires other physical parameters, such as the repetition rate, number of pulses, and the total duration of irradiation. Moreover, the FLASH effect is most thoroughly characterized by electron irradiations, but proton and X-ray UHDR irradiations have been shown to reduce toxicity in healthy tissues, maintaining a similar tumor control compared to CONV RT [4][5][6].
FLASH RT has potential benefits corroborated by a growing body of preclinical data [7][8][9]. Once the potential of FLASH RT will be confirmed in clinical trials, this novel technology may revolutionize the field of radiation oncology, becoming the principal modality of radiotherapy for certain tumors [10]. In view of this exciting perspective, more research is needed to better understand the conditions inducing the FLASH effect.
2. FLASH Radiotherapy: Tumor and Normal Tissue Responses
2.1. Lung Tissue
In 2014, a well-established mouse model of lung fibrosis was presented as the first proof-of-principle study [1]. A significant reduction in normal tissue injury was identified with electron FLASH RT [1][11], while the overall treatment efficacy did not appear to differ at similar doses compared to CONV RT. FLASH irradiation showed a protective effect against pneumonia and fibrosis at a dose of 17 Gy compared to conventional dose rate irradiation. However, at a higher dose of 30 Gy, mice subjected to FLASH irradiation began to develop pneumonia and fibrosis [12].
The potential benefits of UHDRs from proton beams have been also investigated in a mouse model of non-small-cell lung cancers, receiving thoracic radiation therapy using CONV RT (<0.05 Gy/s) and FLASH RT (>60 Gy/s) [13]. FLASH dose rate proton delivery was shown to modulate the immune system, improving tumor control. In particular, proton FLASH RT was more efficient compared to CONV RT in increasing the infiltration of T-lymphocytes inside the tumor, simultaneously reducing the percentage of immunosuppressive regulatory T-cells. Moreover, FLASH RT was more effective in reducing pro-tumorigenic M2-like macrophages and the expression of checkpoint inhibitors in the tumor, indicating a decreased immune tolerance [13].
2.2. Brain Tissue
The most extensive data about FLASH RT for the central nervous system arises from Montay-Gruel and colleagues. In 2017, they first performed preclinical studies on the brain tissues of mice, demonstrating that spatial memory was significantly protected with an average dose rate of radiation >100 Gy/s. Even 2 months after irradiation, the ability of mice to recognize objects was significantly better after electron FLASH RT compared to CONV RT. Interestingly, the protective effect of FLASH RT on nerve regeneration depended on the protective effect of neural stem cells [14].
Further research in experimental models with intracranial tumors was performed in the succeeding years. Altogether, these studies concluded that FLASH RT had a more considerable protective effect on healthy brain tissue than conventional dose rates [15][16][17][18][19].
To approximate clinical treatment scenarios, hypofractionated electron FLASH RT has been proposed as an effective treatment against glioblastoma. Mice that received FLASH RT, either as a 10 Gy single dose or hypo-fractionated regimens (2 × 7 Gy and 3 × 10 Gy), exhibit neurocognitive sparing, maintaining the same efficiency as CONV-RT in delaying tumor growth [16].
2.3. Skin Tissue
Numerous preclinical studies investigated the FLASH effect in terms of reduced skin toxicity in mice using UHDR proton and electron irradiations [20][21][22][23]. Notably, Zhang et al. investigated the protective role of FLASH proton irradiation (130 Gy/s) on the skin varying the oxygen concentration. FLASH proton irradiation decreased skin contraction, epidermis thickness, and collagen deposition compared to conventional irradiations. Interestingly, this effect was controlled by changing oxygen concentration, highlighting the role of oxygen in the FLASH tissue-sparing effect. In fact, FLASH tissue sparing was not observed for mice breathing pure oxygen for 6 min pre-irradiation until after the irradiation was completed. Hypoxic skin also did not result in a difference in outcome between FLASH and conventional dose rate irradiations [23]. To prompt the clinical transfer, Vozenin and colleagues assessed the FLASH effect in higher mammals, including in minipigs and cats [24]. Using the radiation-induced depilation and skin fibrosis as acute and late endpoints, respectively, a protective effect of FLASH-RT was observed in minipigs and cats [24]. Pig skin was irradiated to single-fraction radiation doses of 28, 31, or 34 Gy using either CONV (0.083 Gy/s) or UHDR electron irradiation (300 Gy/s). The presence of late effects, such as fibronecrosis, collagen deposition, and skin contracture, was greater in animals irradiated with CONV dose rates [24]. These preclinical results are consistent with results obtained in a veterinarian clinical trial conducted in cat patients with squamous cell carcinoma of the nose. Cats were treated using single fractions from 25 to 41 Gy at ultra-high dose rates. No dose-limiting toxicity and relatively mild long-term toxicity were found. FLASH-RT treatment yielded a favorable outcome with complete response at 3 months for all cat patients and a free survival rate of 84% at 16 months [24].
2.4. Intestine Tissue
Several studies have investigated the FLASH effect on the intestine [25][26][27][28][29][30]. Electron FLASH has been shown to reduce changes in microbiota with UHDR of about 280 Gy/s at doses of 7–12 Gy [26]. Moreover, both proton and X-ray FLASH irradiation spare mouse intestinal crypts [27][29]. Interestingly, Diffenderfer et al. designed and dosimetrically validated a proton FLASH RT system with accurate control of beam flux on a millisecond timescale and online monitoring of the integral and dose delivery time structure. Utilizing this system, the authors first demonstrated that whole abdominal proton FLASH RT (78 ± 9 Gy/s) reduced acute cell loss and late fibrosis following both whole-abdomen and focal intestinal treatments while maintaining comparable tumor growth inhibition between the two modalities [30]. Proton beams were also found not to induce the sparing effect [25]. Partial abdominal FLASH irradiation (~120 Gy/s) delivered to C57BL/6j and immunodeficient Rag1-/-/C57 mice has been shown not to spare intestinal tissue or circulating blood lymphocytes [25]. There was no difference in the number of lymphocytes between FLASH and CONV RT; a similar number of proliferating crypt cells and thickness of the muscularis externa were found [25].
2.5. Blood Tissue
Most of the experimental studies were performed using models of whole organ irradiation; inversely, the impact on blood tissue at the UHDR has been only recently investigated [31]. Using a prototype 6 MeV electron beam linear accelerator, the effect of FLASH total body irradiation was analyzed on humanized models of T-cell acute lymphoblastic leukemia. In particular, three T-ALL patient-derived xenografts and hematopoietic stem and CD34+ cells isolated from umbilical cord blood were transplanted into immunocompromised mice. Mice were irradiated with 4 Gy FLASH and CONV, and tumor growth and normal hematopoiesis were assessed. Interestingly, FLASH RT reduced functional damage to human blood stem cells and presented a therapeutic effect on human leukemia [31].
Recently, the effects of FLASH RT on blood lymphocytes in humans and small animals were analyzed using a mathematical model [32]. This model has been developed to depict the survival level of lymphocytes in the bloodstream following FLASH RT and lower dose rates of partial-body irradiation. This model is expressed through analytic formulae, incorporating several parameters, such as physiological factors (blood flow characteristics), biophysical factors (lymphocyte radiosensitivity), and physical parameters related to irradiation. It has been observed that FLASH irradiation in humans, administered at doses ranging from 10 to 40 Gy and exposure durations significantly shorter (<1 s) than the blood circulation time (∼60 s), results in maximal blood lymphocyte sparing.
2.6. Zebrafish as an Emerging Model System
Zebrafish is emerging as an intriguing model to investigate the FLASH effect. The feasibility of electron FLASH RT has been tested with positive results in zebrafish embryos. In fact, electron beams showed fewer morphological alterations than CONV RT at doses above 10 Gy [3]. No significant impact of high proton dose rates was shown for embryonic survival, and the rate of spinal curvature was one type of developmental abnormality. For the rate of pericardial edema as an acute radiation effect, a significant reduction after proton FLASH RT (100 Gy/s) was also observed [33].
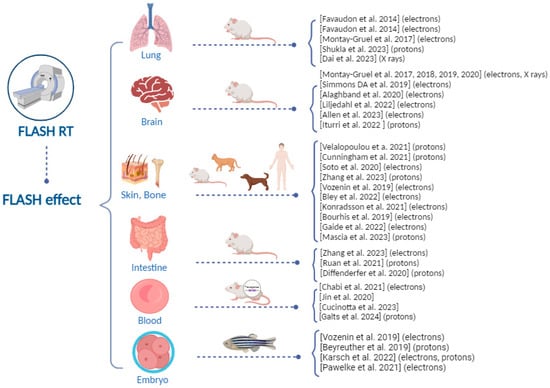
3. Treatment of Human Patients and First Clinical Trials
The first human patient with refractory cutaneous lymphoma was treated with electron FLASH RT in 2018 [34]. FLASH RT treatment was given with a 5.6-MeV linac (Oriatron, PMB Alcen, France) and resulted in being practicable with a positive outcome on the tumor and normal skin [34]. Regarding the skin surrounding the tumor, there was no decrease in the epidermal thickness and disruption at the basal membrane. An asymptomatic mild epithelitis and a grade 1 edema were found at 3 weeks. However, for this patient, when compared to previous skin reactions after exposure to 20 Gy in ten fractions or 21 Gy in six fractions, the FLASH RT adverse effects were minimal and disappeared in a much shorter time. Similarly, the tumor response was durable, with a follow-up of 5 months. Of interest, this patient was subsequently treated for two additional tumors with FLASH and conventional dose rates (166 Gy/s vs. 0.08 Gy/s). At the dose level of 15 Gy, ultra-high and conventional dose rates had similar tumor control along with similar acute and late toxic effects [35].
The protocol for the first-in-human clinical investigation of proton FLASH RT has been recently described by Daugherty et al. [36]. FLASH radiotherapy for the treatment of symptomatic bone metastases (FAST-01) is a prospective, single-center trial (NTC04592887) designed to evaluate the efficacy and toxicity of palliative FLASH treatment of bone metastases [36]. The study demonstrates that proton FLASH treatment (dose rate = 51–61 Gy/s, single dose = 8 Gy) was clinically practicable in the treatment of bone metastases, with the efficacy and the presence of adverse effects being analogous to CONV RT [37]. The main results of the trial indicated that eight out of the twelve treated sites experienced complete or partial pain following FLASH treatment.
Figure 1 summarizes the preclinical and first-in-human clinical evidence about the FLASH effect. These studies show that, to date, the capability of FLASH RT to spare healthy tissues has been investigated in several tissues using preclinical models of different genetic backgrounds. Further data at different experimental conditions (e.g., dose, dose rate, oxygen tension) are necessary to confirm the protection of normal tissue under FLASH RT.

Figure 1. Overview of the preclinical and first-in-human clinical evidence about the ultra-high dose rate FLASH effect [1][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][30][31][32][33][34][35][37][38][39][40][41][42][43][44][45][46].
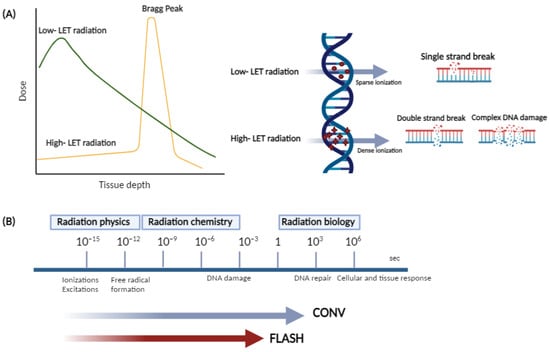
4. Biological Mechanisms behind the FLASH Effect: The Role of DNA Damage
Non-mutually exclusive hypotheses regarding the mechanism underlying the FLASH effect have been proposed, such as the rapid oxygen depletion and reactive oxygen species (ROS) production, DNA damage, and the immune and inflammatory processes [6][7][8]. However, even though one of the most widely considered hypotheses is that the effect is related to substantial oxygen depletion upon FLASH, recent observations showed that oxygen depletion during pulse irradiation at an ultra-high dose rate is marginal and cannot entirely account for the FLASH effect in healthy normoxic tissues [47][48][49].
It is well recognized that nuclear DNA is the primary target and the most crucial molecule in the response to radiotherapy [50][51]. The subsequent cascade of DNA damage response (DDR) and signaling pathways are essential in determining the fate of cancer cells, such as death or survival [50][51]. Thus, elucidating DNA damage associated with UHDR irradiation is the most crucial radiobiological mechanism in order to fully define the benefits associated with FLASH RT.
The extent of ionizing radiation-induced DNA damage alterations primarily depends on the density dose, dose rate, and linear energy transfer (LET), which is a measure of locally absorbed energy (kiloelectron volts, keV) per unit length (micrometer, µm). Low LET photon (X-ray or γ rays) irradiation implies a homogenous deposition of energy throughout the tissue volume, whereas high LET radiation (protons, alpha particles, and heavy ions), decelerates faster than photons, leading to the formation of a rapid Bragg peak [50][51], with penetration depth in tissues increasing with the beam energy (Figure 2).

Figure 2. (A) Schematic illustration of depth dose distribution and DNA damage induction patterns for low and high LET beams; (B) timescales of physical, chemical, and biological phases of conventional and FLASH radiotherapy.
Both low and high LET radiation act directly or indirectly on the DNA target. The direct effects are induced by ionizations and excitations of DNA molecules directly, disrupting the molecular structure. The indirect effects are mediated by water radiolysis, and free radicals are produced, which act as intermediaries causing DNA damage.
Typically, radiolytic events occur in three main stages taking place on different typical timescales. During the first or “physical” stage, which takes place within 10−15−10−12 s, extremely reactive free radicals (e.g., aqueous or hydrated electrons and other reactive oxygen species, such as H2O2, O2−, or OH−) are produced and undergo fast reorganization in the chemical stage (10−12–10−6 s), leading to the formation of an array of reactive products, which, in turn, can break the chemical bonds and produce DNA damage and possible repair processes in the cell over a wide timescale (“biological” stage). FLASH irradiation is around 1000 times faster than conventional irradiation, and this might interfere with the radiation–chemical reactions, and, consequently, with the biological processes in response to irradiation.
High LET radiation is more lethal than similar doses of low LET radiation types, which is probably a result of the condensed energy deposition pattern and a very dense ionization pattern, which induces highly condensed DNA damage and is considered highly complex damage that is more difficult to repair. Ultra-high dose rate irradiation may have a significant impact on the DNA damage and the DNA response compared to conventional ion beam effects due to both spatial and temporal differences in their delivery (Figure 2).
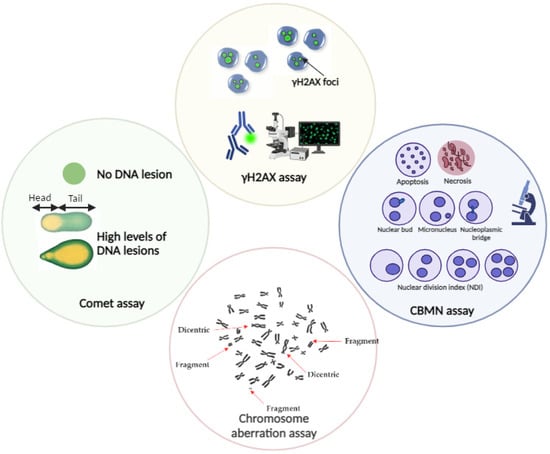
Knowing how cells respond to DNA damage is critical for understanding the FLASH effect, and suitable in vitro studies are required to fully evaluate this damage. Several in vitro assays can be employed to quantify ionizing radiation-induced DNA damage from different radiation beams. Two of the most commonly used tests are the comet assay and the analysis of the phosphorylated histone variant (γH2AX) (Figure 3).

Figure 3. Schematic representation of the in vitro tests for ionizing radiation-induced DNA damage quantification.
The comet assay can detect DSBs using neutral single-cell gel electrophoresis, whereas the alkaline single-cell gel electrophoresis is more sensitive for the detection of SSBs [52]. Even though the comet assay is a fast and easy method to evaluate the degree of DNA damage, it has limitations regarding specificity and sensitivity, such as a limited dynamic range [52]. γ-H2AX is a protein marker that is quickly phosphorylated at sites of DSB and, therefore, can be microscopically visualized as nuclear foci by immunofluorescence [53]. The analysis of γH2AX foci allows DSB detection even in the very low dose range, going down to a single cell [53]. The main disadvantage of this analysis is the highly dynamic change in γH2AX foci early after irradiation. Additionally, the loss of γ-H2AX foci is a reasonable indicator of the timescale of rejoining DSB induced by low LET radiation but is less appropriate for those induced by high LET radiation [54]. Cytogenetic tests are the golden standard in radiobiology to quantify radiation-induced DNA damage [55]. These tests include the chromosomal aberration analysis, especially dicentric chromosome formation and the cytokinesis block micronucleus assay (CBMN), which are considered the most sensitive and reliable DNA biomarkers (Figure 3). The gold standard technique is the dicentric chromosome assay due to its high specificity for radiation [55], but CBMN often remains the preferred approach as it has the important advantage of allowing an economical, easy, and quick analysis of chromosomal damage (chromosome fragments or whole chromosomes) [55].
5. Technologies for FLASH Radiation Beams
The characterization of beam parameters and dosimetry to produce the FLASH effect is fundamental for the clinical translation of the UHDR RT. FLASH RT relies on a combination of dose, dose rate, and irradiation time that falls outside the operational range of existing conventional clinical linear accelerators. These accelerators deliver doses through beams of X-ray photons with a broad energy spectrum produced by the bremsstrahlung of primary electrons, typically with energies ranging from 6 to 20 MeV. Unfortunately, the conversion of electrons into photons is highly inefficient, significantly limiting the maximum dose rate achievable at the treatment crosshair. Achieving the required dose rate for FLASH RT would necessitate a power increase of a thousand times or more in the existing clinical linear accelerators. These circumstances are motivating major efforts in the scientific and technological development of accelerators, including upgrades to existing experimental devices or the design and construction of entirely new systems based on advanced and disruptive concepts.
A combination of VHEEs and FLASH RT may offer a comprehensive solution for the clinical translation of this innovative approach. However, generating electron beams within the 150–250 MeV range with a compact footprint will demand advanced accelerator technology. Existing RF linac technology displays a low acceleration gradient, necessitating excessively large accelerator lengths. This results in clinical equipment of prohibitive size and cost, inevitably restricting access to future FLASH RT.
However, the energy required for VHEE RT, ranging between 100 and 250 MeV, might still be too high for a compact RF accelerator, even considering high gradient RF cavities. A disruptive approach based on laser plasma acceleration (LPA) is also being considered, which has no such limitations in terms of electron energy. LPA can easily provide VHEE beams [56] with a compact size and innovative setup based on optical technology rather than RF technology and can already deliver Gy doses per shot on a pencil beam-like configuration, with an instantaneous dose rate that can exceed by orders of magnitude the expected FLASH dose rates. Such pencil beams could be scanned to cover larger target volumes in a similar fashion as is currently performed with proton beams. Significant technological developments are still needed to reach the specifications of clinical FLASH-RT in terms of dose per fraction over a larger area for clinical treatment. The main approach here consists of increasing the average power and repetition rate of the driving laser power source from the current 10 W–10 Hz to 100 W–100 Hz that is currently being developed at an industrial level for this class of accelerators. The roadmap at CNR-INO for the development of a clinical VHEE device is indeed based on a laser plasma accelerator setup, exploiting proof of principle experimental demonstrations of VHEE beam generation and dosimetry [57] and building on fast-developing laser technology [58].
References
- Favaudon, V.; Caplier, L.; Monceau, V.; Pouzoulet, F.; Sayarath, M.; Fouillade, C.; Poupon, M.F.; Brito, I.; Hupé, P.; Bourhis, J.; et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci. Transl. Med. 2014, 6, 245ra93.
- Farr, J.B.; Parodi, K.; Carlson, D.J. FLASH: Current status and the transition to clinical use. Med. Phys. 2022, 49, 1972–1973.
- Vozenin, M.-C.; Hendry, J.H.; Limoli, C.L. Biological Benefits of Ultra-High Dose Rate FLASH Radiotherapy: Sleeping Beauty Awoken. Clin. Oncol. 2019, 31, 407–415.
- Limoli, C.L.; Vozenin, M.-C. Reinventing radiobiology in the light of FLASH radiotherapy. Annu. Rev. Cancer Biol. 2023, 7, 1–21.
- Friedl, A.A.; Prise, K.M.; Butterworth, K.T.; Montay-Gruel, P.; Favaudon, V. Radiobiology of the FLASH effect. Med. Phys. 2022, 49, 1993–2013.
- Kacem, H.; Almeida, A.; Cherbuin, N.; Vozenin, M.C. Understanding the FLASH effect to unravel the potential of ultra-high dose rate irradiation. Int. J/ Radiat. Biol. 2022, 98, 506–516.
- Bogaerts, E.; Macaeva, E.; Isebaert, S.; Haustermans, K. Potential Molecular Mechanisms behind the Ultra-High Dose Rate “FLASH” Effect. Int. J. Mol. Sci. 2022, 23, 12109.
- Hageman, E.; Pei-Pei, C.; Dahele, M.; Slotman, B.J.; Sminia, P. Radiobiological Aspects of FLASH Radiotherapy. Rev. Biomol. 2022, 12, 1376.
- Borghini, A.; Vecoli, C.; Labate, L.; Panetta, D.; Andreassi, M.G.; Gizzi, L.A. FLASH ultra-high dose rates in radiotherapy: Preclinical and radiobiological evidence. Int. J. Radiat. Biol. 2022, 98, 127–135.
- Vozenin, M.C.; Bourhis, J.; Durante, M. Towards clinical translation of FLASH radiotherapy. Nat. Rev. Clin. Oncol. 2022, 19, 791–803.
- Montay-Gruel, P.; Petersson, K.; Jaccard, M.; Boivin, G.; Germond, J.F.; Petit, B.; Doenlen, R.; Favaudon, V.; Bochud, F.; Bailat, C.; et al. Irradiation in a flash: Unique sparing of memory in mice after whole brain irradiation with dose rates above 100 Gy/s. Radiother. Oncol. 2017, 124, 365–369.
- Favaudon, V.; Fouillade, C.; Vozenin, M.C. Ultrahigh dose-rate, “flash” irradiation minimizes the side-effects of radiotherapy. Cancer Radiother. 2015, 19, 526–531.
- Shukla, S.; Saha, T.; Rama, T.; Acharya, A.; Le, T.; Bian, F.; Donovan, J.; Tan, L.A.; Vatner, R.; Kalinichenk, K.; et al. Ultra-high dose-rate proton FLASH improves tumor control. Radiother. Oncol. 2023, 186, 109741.
- Montay-Gruel, P.; Bouchet, A.; Jaccard, M.; Patin, D.; Serduc, R.; Aim, W.; Petersson, K.; Petit, B.; Bailat, C.; Bourhis, J.; et al. X-rays can trigger the FLASH effect: Ultra-high dose-rate synchrotron light source prevents normal brain injury after whole brain irradiation in mice. Radiother. Oncol. 2018, 129, 582–588.
- Montay-Gruel, P.; Acharya, M.M.; Petersson, K.; Alikhani, L.; Yakkala, C.; Allen, B.D.; Ollivier, J.; Petit, B.; Jorge, P.G.; Syage, A.R.; et al. Long-term neurocognitive benefits of FLASH radiotherapy driven by reduced reactive oxygen species. Proc. Natl. Acad. Sci. USA 2019, 116, 10943–10951.
- Montay-Gruel, P.; Acharya, M.M.; Gonçalves Jorge, P.; Petit, B.; Petridis, I.G.; Fuchs, P.; Leavitt, R.; Petersson, K.; Gondré, M.; Ollivier, J.; et al. Hypo-Fractionated FLASH-RT as an Effective Treatment Against Glioblastoma That Reduces Neurocognitive Side Effects in Mice. Clin. Cancer Res. 2020, 27, 3.
- Simmons, D.A.; Lartey, F.M.; Schüler, E.; Rafat, M.; King, G.; Kim, A.; Ko, R.; Semaan, S.; Gonzalez, S.; Jenkins, M.; et al. Reduced cognitive deficits after FLASH irradiation of whole mouse brain are associated with less hippocampal dendritic spine loss and neuroinflammation. Radiother. Oncol. 2019, 139, 4–10.
- Alaghband, Y.; Cheeks, S.N.; Allen, B.D.; Montay-Gruel, P.; Doan, N.L.; Petit, B.; Jorge, P.G.; Giedzinski, E.; Acharya, M.M.; Vozenin, M.C.; et al. Neuroprotection of radiosensitive juvenile mice by ultra-high dose rate FLASH irradiation. Cancers 2020, 12, 1671.
- Liljedahl, E.; Konradsson, E.; Gustafsson, E.; Jonsson, K.F.; Olofsson, J.K.; Ceberg, C.; Redebrandt, H.N. Long-term anti-tumor effects following both conventional radiotherapy and FLASH in fully immunocompetent animals with glioblastoma. Sci. Rep. 2022, 12, 12285.
- Velalopoulou, A.; Karagounis, I.V.; Cramer, G.M.; Kim, M.M.; Skoufos, G.G.; Goia, D.; Hagan, S.; Verginadis, I.I.; Shoniyozov, K.; Chiango, J.; et al. FLASH Proton Radiotherapy Spares Normal Epithelial and Mesenchymal Tissues While Preserving Sarcoma Response. Cancer Res. 2021, 81, 4808–4821.
- Cunningham, S.; McCauley, S.; Vairamani, K.; Speth, J.; Girdhani, S.; Abel, E.; Sharma, R.A.; Perentesi, J.P.; Wells, S.I.; Mascia, A.; et al. FLASH Proton Pencil Beam Scanning Irradiation Minimizes Radiation-Induced Leg Contracture and Skin Toxicity in Mice. Cancers 2021, 13, 1012.
- Soto, L.A.; Casey, K.M.; Wang, J.; Blaney, A.; Manjappa, R.; Breitkreutz, D.; Skinner, L.; Dutt, S.; Ko, R.B.; Bush, K.; et al. FLASH Irradiation Results in Reduced Severe Skin Toxicity Compared to Conventional-Dose-Rate Irradiation. Radiat. Res. 2020, 195, 618–624.
- Zhang, Q.; Gerweck, L.E.; Cascio, E.; Yang, Q.; Huang, P.; Niemierko, A.; Bertolet, A.; Nesteruk, K.P.; McNamara, A.; Schuemann, J. Proton FLASH effects on mouse skin at different oxygen tensions. Phys Med. Biol. 2023, 68, 5.
- Vozenin, M.C.; De Fornel, P.; Petersson, K.; Favaudon, V.; Jaccard, M.; Germond, J.F.; Petit, B.; Burki, M.; Ferrand, G.; Patin, D.; et al. The Advantage of FLASH Radiotherapy Confirmed in Mini-pig and Cat-cancer Patients. Clin. Cancer Res. 2019, 25, 35–42.
- Zhang, Q.; Gerweck, L.E.; Cascio, E.; Gu, L.; Yang, Q.; Dong, X.; Huang, P.; Bertolet, A.; Nesteruk, K.P.; Sung, W.; et al. Absence of Tissue-Sparing Effects in Partial Proton FLASH Irradiation in Murine Intestine. Cancers 2023, 15, 2269.
- Ruan, J.L.; Lee, C.; Wouters, S.; Tullis, I.D.C.; Verslegers, M.; Mysara, M.; Then, C.K.; Smart, S.C.; Hill, M.A.; Ruth, J.M.; et al. Irradiation at Ultra-High (FLASH) Dose Rates Reduces Acute Normal Tissue Toxicity in the Mouse Gastrointestinal System. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 1250–1261.
- Kim, M.M.; Verginadis, I.I.; Goia, D.; Haertter, A.; Shoniyozov, K.; Zou, W.; Maity, A.; Busch, T.M.; Metz, J.M.; Cengel, K.A.; et al. Comparison of FLASH Proton Entrance and the Spread-Out Bragg Peak Dose Regions in the Sparing of Mouse Intestinal Crypts and in a Pancreatic Tumor Model. Cancers 2021, 13, 4244.
- Zhu, H.; Xie, D.; Yang, Y.; Huang, S.; Gao, X.; Peng, Y.; Wang, B.; Wang, J.; Xiao, D.; Wu, D.; et al. Radioprotective effect of X-ray abdominal FLASH irradiation: Adaptation to oxidative damage and inflammatory response may be benefiting factors. Med. Phys. 2022, 49, 4812–4822.
- Shi, X.; Yang, Y.; Zhang, W.; Wang, J.; Xiao, D.; Ren, H.; Wang, T.; Gao, F.; Liu, Z.; Zhou, K.; et al. FLASH X-ray spares intestinal crypts from pyroptosis initiated by cGAS-STING activation upon radioimmunotherapy. Proc. Natl. Acad. Sci. USA 2022, 119, e2208506119.
- Diffenderfer, E.S.; Verginadis, I.I.; Kim, M.M.; Shoniyozov, K.; Velalopoulou, A.; Goia, D.; Putt, M.; Hagan, S.; Avery, S.; Teo, K.; et al. Design, implementation, and in vivo validation of a novel proton FLASH radiation therapy system. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 440–448.
- Chabi, S.; To, T.H.; Leavitt, R.; Poglio, S.; Jorge, P.G.; Jaccard, M.; Petersson, K.; Petit, B.; Roméo, P.H.; Pflumio, F.; et al. Ultra-high-dose-rate FLASH and Conventional-Dose-Rate Irradiation Differentially Affect Human Acute Lymphoblastic Leukemia and Normal Hematopoiesis. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 819–829.
- Cucinotta, F.A.; Smirnova, E.A. Effects of Flash Radiotherapy on Blood Lymphocytes in Humans and Small Laboratory Animals. Radiat. Res. 2023, 199, 240–251.
- Beyreuther, E.; Brand, M.; Hans, S.; Hideghéty, K.; Karsch, L.; Leßmann, E.; Schürer, M.; Szabó, E.R.; Pawelke, J. Feasibility of proton FLASH effect tested by zebrafish embryo irradiation. Radiother. Oncol. 2019, 139, 46–50.
- Bourhis, J.; Sozzi, W.J.; Jorge, P.G.; Gaide, O.; Bailat, C.; Duclos, F.; Patin, D.; Ozsahin, M.; Bochud, F.; Germond, J.F.; et al. Treatment of a first patient with FLASH-radiotherapy. Radiother. Oncol. 2019, 139, 18–22.
- Gaide, O.; Herrera, F.; Sozzi, J.W.; Jorge, P.G.; Kinj, R.; Bailat, C.; Duclos, F.; Bochud, F.; Germond, J.F.; Gondré, M.; et al. Comparison of ultra-high versus conventional dose rate radiotherapy in a patient with cutaneous lymphoma. Radiother. Oncol. 2022, 174, 87–91.
- Daugherty, E.C.; Mascia, A.; Zhang, Y.; Lee, E.; Xiao, Z.; Sertorio, M.; Woo, J.; McCann, C.; Russell, K.; Levine, L.; et al. FLASH Radiotherapy for the Treatment of Symptomatic Bone Metastases (FAST-01): Protocol for the First Prospective Feasibility Study. JMIR. Res. Protoc. 2023, 12, e41812.
- Mascia, A.E.; Daugherty, E.C.; Zhang, Y.; Lee, E.; Xiao, Z.; Sertorio, M.; Woo, J.; Backus, L.R.; McDonald, J.M.; McCann, C.; et al. Proton FLASH Radiotherapy for the Treatment of Symptomatic Bone Metastases: The FAST-01 Nonrandomized Trial. JAMA Oncol. 2023, 9, 62–69.
- Dai, Y.; Liang, R.; Wang, J.; Zhang, J.; Wu, D.; Zhao, R.; Liu, Z.; Chen, F. Fractionated FLASH radiation in xenografted lung tumors induced FLASH effect at a split dose of 2 Gy. Int. J. Radiat. Biol. 2023, 99, 1542–1549.
- Allen, D.B.; Alaghband, Y.; Kramár, E.A.; Ru, N.; Petit, B.; Grilj, V.; Petronek, M.S.; Pulliam, C.F.; Kim, R.Y.; Doan, N.-L.; et al. Elucidating the neurological mechanism of the FLASH effect in juvenile mice exposed to hypofractionated radiotherapy. Neuro. Oncol. 2023, 25, 927–939.
- Iturri, L.; Bertho, A.; Lamirault, C.; Juchaux, M.; Gilbert, C.; Espenon, J.; Sebrie, C.; Jourdain, L.; Pouzoulet, F.; Verrelle, P.; et al. Proton FLASH Radiation Therapy and Immune Infiltration: Evaluation in an Orthotopic Glioma Rat Model. Int. J. Radiat. Oncol. Biol. Phys. 2023, 116, 655–665.
- Bley, C.R.; Wolf, F.; Jorge, P.G.; Grilj, V.; Petridis, I.; Petit, B.; Bohlen, T.T.; Moeckli, R.; Limoli, C.; Bourhis, J.; et al. Dose- and Volume-Limiting Late Toxicity of FLASH Radiotherapy in Cats with Squamous Cell Carcinoma of the Nasal Planum and in Mini Pigs. Clin. Cancer Res. 2022, 28, 3814–3823.
- Konradsson, E.; Arendt, M.L.; Bastholm Jensen, K.; Børresen, B.; Hansen, A.E.; Bäck, S.; Kristensen, A.T.; Munck af Rosenschöld, P.; Ceberg, C.; Petersson, K. Establishment and Initial Experience of Clinical FLASH Radiotherapy in Canine Cancer Patients. Front. Oncol. 2021, 11, 658004.
- Jin, J.Y.; Gu, A.; Wang, W.; Oleinick, N.L.; Machtay, M.; Spring Kong, F.M. Ultra-high dose rate effect on circulating immune cells: A potential mechanism for FLASH effect? Radiother. Oncol. 2020, 149, 55–62.
- Galts, A.; Hammi, A. FLASH radiotherapy sparing effect on the circulating lymphocytes in pencil beam scanning proton therapy: Impact of hypofractionation and dose rate. Phys. Med. Biol. 2024, 69, 2.
- Karsch, L.; Pawelke, J.; Brand, M.; Hans, S.; Hideghéty, K.; Jansen, J.; Lessmann, E.; Löck, S.; Schürer, M.; Schurig, R.; et al. Beam Pulse Structure and Dose Rate as Determinants for the Flash Effect Observed in Zebrafish Embryo. Radiother. Oncol. 2022, 173, 49–54.
- Pawelke, J.; Brand, M.; Hans, S.; Hideghéty, K.; Karsch, L.; Lessmann, E.; Löck, S.; Schürer, M.; Szabó, E.R.; Beyreuther, E. Electron Dose Rate and Oxygen Depletion Protect Zebrafish Embryos from Radiation Damage. Radiother. Oncol. 2021, 158, 7–12.
- Cao, X.; Zhang, R.; Esipova, T.V.; Allu, S.R.; Ashraf, R.; Rahman, M.; Gunn, J.R.; Bruza, P.; Gladstone, D.J.; Williams, B.B.; et al. Quantification of Oxygen Depletion During FLASH Irradiation In Vitro and In Vivo. Int.J. Radiat. Oncol. Biol. Phys. 2021, 111, 240–248.
- Jansen, J.; Knoll, J.; Beyreuther, E.; Pawelke, J.; Skuza, R.; Hanley, R.; Brons, S.; Pagliari, F.; Seco, J. Does FLASH deplete oxygen? Experimental evaluation for photons, protons, and carbon ions. Med. Phys. 2021, 48, 3982–3990.
- El Khatib, M.; Van Slyke, A.L.; Velalopoulou, A.; Kim, M.M.; Shoniyozov, K.; Allu, S.R.; Diffenderfer, E.E.; Busch, T.M.; Wiersma, R.D.; Koch, C.J.; et al. Ultrafast Tracking of Oxygen Dynamics During Proton FLASH. Int. J. Radiat. Oncol. Biol. Phys. 2022, 113, 624–634.
- Joiner, M.C.; van der Kogel, A.J. Basic Clinical Radiobiology; CRC Press: Boca Raton, FL, USA, 2018.
- Lomax, M.E.; Folkes, L.K.; O’Neill, P. Biological consequences of radiation-induced DNA damage: Relevance to radiotherapy. Clin. Oncol. 2013, 25, 578–585.
- Kumaravel, T.S.; Jha, A.N. Reliable Comet assay measurements for detecting DNA damage induced by ionising radiation and chemicals. Mutat. Res. 2006, 605, 7–16.
- Celeste, A.; Fernandez-Capetillo, O.; Kruhlak, M.J.; Pilch, D.R.; Staudt, D.W.; Lee, A.; Bonner, R.F.; Bonner, W.M.; Nussenzweig, A. Histone H2AX phosphorylation is dispensable for the initial recognition of DNA breaks. Nat. Cell. Biol. 2003, 5, 675–679.
- Leatherbarrow, E.L.; Harper, J.V.; Cucinotta, F.A.; O’Neill, P. Induction and quantification of gamma-H2AX foci following low and high LET-irradiation. Int. J. Radiat. Biol. 2006, 82, 111–118.
- International Atomic Energy Agency. Cytogenetic Dosimetry: Applications in Preparedness for and Response to Radiation Emergencies; IAEA: Vienna, Austria, 2011.
- Faure, J.; Glinec, Y.; Pukhov, A.; Kiselev, S.; Gordienko, S.; Lefebvre, E.; Rousseau, J.P.; Burgy, F.; Malka, V. A laser-plasma accelerator producing monoenergetic electron beams. Nature 2004, 431, 541.
- Labate, L.; Palla, D.; Panetta, D.; Avella, F.; Baffigi, F.; Brandi, F.; Di Martino, F.; Fulgentini, L.; Giulietti, A.; Köster, P.; et al. Toward an effective use of laser-driven very high energy electrons for radiotherapy: Feasibility assessment of multi-field and intensity modulation irradiation schemes. Sci. Rep. 2020, 10, 17307.
- Gizzi, A.L.; Mathieu, F.; Mason, P.; Rajeev, P.P. Laser drivers for plasma accelerators. New J. Phys. 2021, 23, 031101.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
773
Revisions:
2 times
(View History)
Update Date:
05 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




