
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Igor Sirak | -- | 2206 | 2024-03-04 09:36:37 | | | |
| 2 | Sirius Huang | Meta information modification | 2206 | 2024-03-05 01:36:53 | | |
Video Upload Options
The role of postmastectomy radiotherapy and regional nodal irradiation after radical mastectomy is defined in high-risk patients with locally advanced tumors, positive margins, and unfavorable biology. The benefit of postmastectomy radiotherapy in intermediate-risk patients (T3N0 tumors) remains a matter of controversy. It has been demonstrated that radiotherapy after breast-conserving surgery lowers the locoregional recurrence rate compared with surgery alone and improves the overall survival rate. In patients with four or more positive lymph nodes or extracapsular extension, regional lymph node irradiation is indicated regardless of the surgery type (breast-conserving surgery or mastectomy). Despite the consensus that patients with more than three positive lymph nodes should be treated with radiotherapy, there is controversy regarding the recommendations for patients with one to three involved lymph nodes. In patients with N0 disease with negative findings on axillary surgery, there is a trend to administer regional lymph node irradiation in patients with a high risk of recurrence. In patients treated with neoadjuvant systemic therapy and mastectomy, adjuvant radiotherapy should be administered in cases of clinical stage III and/or ≥ypN1. In patients treated with neoadjuvant systemic therapy and breast-conserving surgery, postoperative radiotherapy is indicated irrespective of pathological response.
1. Introduction
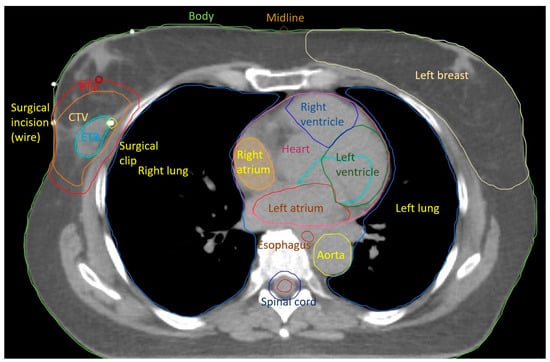
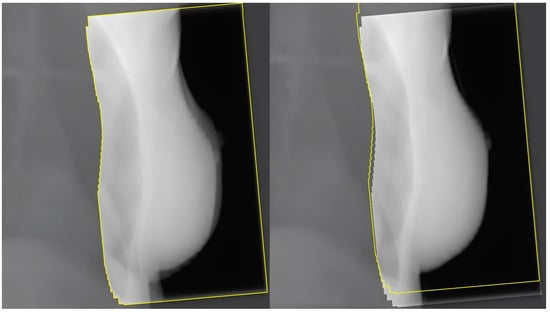
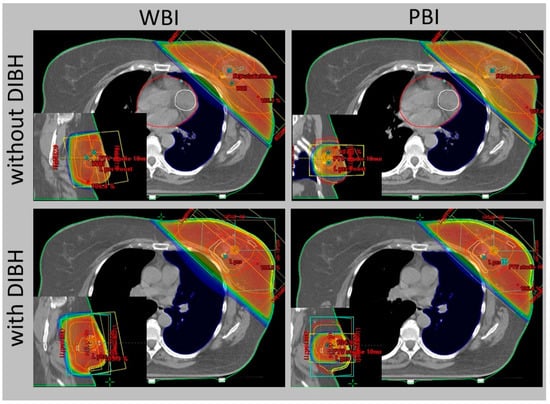
2. Radiotherapy after Radical Mastectomy
3. Radiotherapy after Breast-Conserving Surgery
4. Comparison of the Results of Breast-Conserving Surgery and Radical Mastectomy
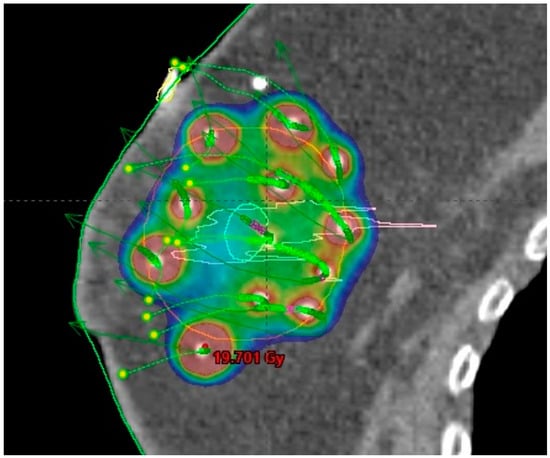
5. Radiotherapy after Neoadjuvant Chemotherapy
| RT after preoperative therapy and breast-conserving surgery | |
|---|---|
| cN0 → ypN0 (SLNB) | WBI + boost to the tumor bed |
| cN+ → ypN0 | WBI + boost to the tumor bed + comprehensive RNI (ongoing NSABP B-51 trial assessing the benefit of RNI) |
| cN+→ ypN+ (SLNB) | ALND followed by comprehensive RNI (ongoing A11202 trial assessing the benefit of ALND) |
| RT after preoperative therapy and mastectomy | |
| cN0 → ypN0 (SLNB) | Consider RT to the chest wall without RNI |
| cN+ → ypN0 (SLNB) | RT to the chest wall and comprehensive RNI |
| cN+ → ypN+ | ALND followed by comprehensive RNI |
| ypN | Tx: Level I/II | RT: Level III/IV ± IM | |
|---|---|---|---|
| cN0 → ycN0 → SLNB | ypN0 | No local Tx | No local Tx |
| ypN+ | ALDR or Axillary RT | RT if risk factors * | |
| cN+ → ycN0 → TAD | ypN0 | Axillary RT | RT if risk factors * |
| ypN+ | ALND or Axillary RT | RT | |
| cN+→ ycN+ → ALND | ypN0 | No local Tx | RT if risk factors * |
| ypN+ | RT (only forgotten nodes) | RT |
References
- Clarke, M.; Collins, R.; Darby, S.; Davies, C.; Elphinstone, P.; Evans, V.; Godwin, J.; Gray, R.; Hicks, C.; James, S.; et al. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomized trials. Lancet 2005, 366, 2087–2106.
- Nielsen, H.M.; Overgaard, M.; Grau, C.; Jensen, A.R.; Overgaard, J. Study of failure pattern among high-risk breast cancer patients with or without postmastectomy radiotherapy in addition to adjuvant systemic therapy: Long-term results from the Danish Breast Cancer Cooperative Group DBCG 82 b and c randomized studies. J. Clin. Oncol. 2006, 24, 2268–2275.
- Taghian, A. Adjuvant Radiation Therapy for Women with Newly Diagnosed, Non-Metastatic Breast Cancer. Introduction. In UpToDate, Post TW (Ed), UpToDate, Waltham, MA, USA. Available online: https://www.uptodate.com/contents/adjuvant-radiation-therapy-for-women-with-newly-diagnosed-non-metastatic-breast-cancer (accessed on 22 March 2022).
- Harris, J.R. Fifty years of progress in radiation therapy for breast cancer. Am. Soc. Clin. Oncol. Educ. Book 2014, 34, 21–25.
- Haussmann, J.; Corradini, S.; Nestle-Kraemling, C.; Bölke, E.; Njanang, F.J.D.; Tamaskovics, B.; Orth, K.; Ruckhaeberle, E.; Fehm, T.; Mohrmann, S.; et al. Recent advances in radiotherapy of breast cancer. Radiat. Oncol. 2020, 15, 71.
- Meattini, I.; Becherini, C.; Boersma, L.; Kaidar-Person, O.; Marta, G.N.; Montero, A.; Offersen, B.V.; Aznar, M.C.; Belka, C.; Brunt, A.M.; et al. European Society for Radiotherapy and Oncology Advisory Committee in Radiation Oncology Practice consensus recommendations on patient selection and dose and fractionation for external beam radiotherapy in early breast cancer. Lancet Oncol. 2022, 23, e21–e31.
- Remick, J.; Amin, N.P. Postmastectomy Breast Cancer Radiation Therapy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA; Available online: https://europepmc.org/article/NBK/nbk519034 (accessed on 8 September 2023).
- Taghian, A.G.; Jeong, J.H.; Mamounas, E.P.; Parda, D.S.; Deutsch, M.; Costantino, J.P.; Wolmark, N. Low locoregional recurrence rate among node-negative breast cancer patients with tumors 5 cm or larger treated by mastectomy, with or without adjuvant systemic therapy and without radiotherapy: Results from five national surgical adjuvant breast and bowel project randomized clinical trials. J. Clin. Oncol. 2006, 24, 3927–3932.
- Floyd, S.R.; Buchholz, T.A.; Haffty, B.G.; Goldberg, S.; Niemierko, A.; Raad, R.A.; Oswald, M.J.; Sullivan, T.; Strom, E.A.; Powell, S.N.; et al. Low local recurrence rate without postmastectomy radiation in node-negative breast cancer patients with tumors 5 cm and larger. Int. J. Radiat. Oncol. Biol. Phys. 2006, 66, 358–364.
- Johnson, M.E.; Handorf, E.A.; Martin, J.M.; Hayes, S.B. Postmastectomy radiation therapy for T3N0: A SEER analysis. Cancer 2014, 120, 3569–3574.
- EBCTCG (Early Breast Cancer Trialists’ Collaborative Group); McGale, P.; Taylor, C.; Correa, C.; Cutter, D.; Duane, F.; Ewertz, M.; Gray, R.; Mannu, G.; Peto, R.; et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: Meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 2014, 383, 2127–2135.
- Hansen, E.; Roach, M., III (Eds.) Handbook of Evidence-Based Radiation Oncology; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; ISBN 978-3-31-962642-0, 978-3-31-962641-3.
- Tendulkar, R.D.; Rehman, S.; Shukla, M.E.; Reddy, C.A.; Moore, H.; Budd, G.T.; Dietz, J.; Crowe, J.P.; Macklis, R. Impact of postmastectomy radiation on locoregional recurrence in breast cancer patients with 1-3 positive lymph nodes treated with modern systemic therapy. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 577–581.
- Holland, R.; Veling, S.H.; Mravunac, M.; Hendriks, J.H. Histologic multifocality of Tis, T1-2 breast carcinomas. Implications for clinical trials of breast-conserving surgery. Cancer 1985, 56, 979–990.
- Fisher, B.; Anderson, S.; Bryant, J.; Margolese, R.G.; Deutsch, M.; Fisher, E.R.; Jeong, J.H.; Wolmark, N. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N. Engl. J. Med. 2002, 347, 1233–1241.
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG); Darby, S.; McGale, P.; Correa, C.; Taylor, C.; Arriagada, R.; Clarke, M.; Cutter, D.; Davies, C.; Ewertz, M.; et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011, 378, 1707–1716.
- Tendulkar, D.; Shah, C.H. Early breast cancer. In Essentials of Clinical Radiation Oncology, 1st ed.; Ward, M.C., Tendulkar, R.D., Videtic, G.M.M., Eds.; Demos Medical: New York, NY, USA, 2018; p. 158. ISBN 9780826168559.
- de Boniface, J.; Szulkin, R.; Johansson, A.L.V. Survival after Breast Conservation vs Mastectomy Adjusted for Comorbidity and Socioeconomic Status: A Swedish National 6-Year Follow-up of 48 986 Women. JAMA Surg. 2021, 156, 628–637.
- Correa, C.; Harris, E.E.; Leonardi, M.C.; Smith, B.D.; Taghian, A.G.; Thompson, A.M.; White, J.; Harris, J.R. Accelerated Partial Breast Irradiation: Executive summary for the update of an ASTRO Evidence-Based Consensus Statement. Pract. Radiat. Oncol. 2017, 7, 73–79.
- Swisher, S.K.; Vila, J.; Tucker, S.L.; Bedrosian, I.; Shaitelman, S.F.; Litton, J.K.; Smith, B.D.; Caudle, A.S.; Kuerer, H.M.; Mittendorf, E.A. Locoregional Control According to Breast Cancer Subtype and Response to Neoadjuvant Chemotherapy in Breast Cancer Patients Undergoing Breast-conserving Therapy. Ann. Surg. Oncol. 2016, 23, 749–756.
- Werutsky, G.; Untch, M.; Hanusch, C.; Fasching, P.A.; Blohmer, J.U.; Seiler, S.; Denkert, C.; Tesch, H.; Jackisch, C.; Gerber, B.; et al. Locoregional recurrence risk after neoadjuvant chemotherapy: A pooled analysis of nine prospective neoadjuvant breast cancer trials. Eur. J. Cancer. 2020, 130, 92–101.
- Ditsch, N.; Rubio, I.T.; Gasparri, M.L.; de Boniface, J.; Kuehn, T. Breast and axillary surgery in malignant breast disease: A review focused on literature of 2018 and 2019. Curr. Opin. Obstet. Gynecol. 2020, 32, 91–99.
- Polgár, C.; Kahán, Z.; Ivanov, O.; Chorváth, M.; Ligačová, A.; Csejtei, A.; Gábor, G.; Landherr, L.; Mangel, L.; Mayer, Á.; et al. Radiotherapy of Breast Cancer-Professional Guideline 1st Central-Eastern European Professional Consensus Statement on Breast Cancer. Pathol. Oncol. Res. 2022, 28, 1610378.
- Nikyar, N.; Tegnelius, E.; Valachis, A. Adjuvant locoregional radiation therapy in breast cancer patients with pathologic complete response after neoadjuvant chemotherapy: A systematic review and meta-analysis. Clin. Transl. Radiat. Oncol. 2022, 33, 45–52.
- Shah, C.; Al-Hilli, Z.; Vicini, F. Advances in Breast Cancer Radiotherapy: Implications for Current and Future Practice. JCO Oncol Pract. 2021, 17, 697–706.
- NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. Version 1. 2024. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (accessed on 6 February 2024).
- Kaidar-Person, O.; Pfob, A.; Gentilini, O.D.; Borisch, B.; Bosch, A.; Cardoso, M.J.; Curigliano, G.; De Boniface, J.; Denkert, C.; Hauser, N.; et al. The Lucerne Toolbox 2 to optimise axillary management for early breast cancer: A multidisciplinary expert consensus. EClinicalMedicine 2023, 61, 102085.




