| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yahima Frión-Herrera | + 1171 word(s) | 1171 | 2020-04-10 06:09:06 | | | |
| 2 | Camila Xu | + 368 word(s) | 1539 | 2020-04-14 04:44:22 | | | | |
| 3 | Camila Xu | Meta information modification | 1539 | 2020-04-14 04:55:50 | | | | |
| 4 | Camila Xu | + 1 word(s) | 1540 | 2020-04-14 04:59:31 | | | | |
| 5 | Camila Xu | + 1 word(s) | 1540 | 2020-04-14 05:15:58 | | | | |
| 6 | Camila Xu | -13 word(s) | 1527 | 2020-11-01 10:48:20 | | |
Video Upload Options
The majority of deaths related to colorectal cancer (CRC) are associated with the metastatic process. Alternative therapeutic strategies, such as traditional folk remedies, deserve attention for their potential ability to attenuate the invasiveness of CRC cells. The aim of this study is to investigate the biological activity of brown Cuban propolis (CP) and its main component nemorosone (NEM) and to describe the molecular mechanism(s) by which they inhibit proliferation and metastatic potential of 2 CRC cell lines. CP and NEM significantly decreased cell viability and inhibited clonogenic capacity of CRC cells in a dose and time-dependent manner, by arresting the cell cycle in the G0/G1 phase and inducing apoptosis. Furthermore, CP and NEM downregulated BCL2 gene expression and upregulated the expression of the proapoptotic genes TP53 and BAX, with a consequent activation of caspase 3/7. They also attenuated cell migration and invasion by inhibiting MMP9 activity, increasing E-cadherin and decreasing β-catenin and vimentin expression, proteins involved in the epithelial–mesenchymal transition (EMT). NEM, besides displaying antiproliferative activity on CRC cells, is able to decrease their metastatic potential by modulating EMT-related molecules. These findings provide new insights about the antitumoral properties of CP, due to NEM content.
1. Introduction
Colorectal cancer (CRC), one of the most common oncological disease worldwide, is an aggressive cancer with metastatic behavior [1][2][3]. Approximately 40%–60% of CRC patients develop metastases in other organs, in particular liver and lungs, which represent the main sites of CRC metastasis [4]. Although surgical resection of metastasis can here be often performed, metastatic CRC remains one of the principal causes of cancer-related morbidity and mortality [5][6].
Tumor cells acquire the capacity to infiltrate blood or lymphatic vessels through an epithelial to mesenchymal transition (EMT) process, which increases cell migration and invasion, intravasation and extravasation. Taken together, these processes result in increased tumor aggressiveness and metastasis [7][8]. Therefore, the proteins associated to cell migration and invasion, as well as the mechanisms involved in EMT, represent a pharmacological target for the treatment of CRC patients [9][10].
The pharmacological therapy of CRC involves biological or synthetic drugs able to suppress or prevent cancer progression [11][11]. In this context natural products, widely studied by the scientific community, are considered an interesting option, especially when they are safe and inexpensive [12][13]. Several clinical studies have shown that plant-based products exert anticancer effects by numerous mechanisms, i.e., the induction of cell cycle arrest and cell death by apoptosis or necrosis, the activation of DNA repair systems, the regulation of signaling pathways involved in cancer progression, and the inhibition of cancer invasion and metastasis [14][15][16].
It is known that many bee products, such as royal jelly, honey, pollen, propolis and bee venom, display antitumor and chemo-preventive activity and can be evaluated as alternative strategies for cancer treatment [17][18][19]. Propolis, a natural resinous bee product, has been used since ancient times as a traditional remedy for the treatment of various diseases, and in recent years its anticancer potential has been demonstrated [20][21][22][23]. The therapeutic effects of propolis depend on its chemical composition, which is related to the geographical area of origin and the environmental conditions of its production [24][25][26]. Several studies have shown that brown Cuban propolis (CP) exerts antiproliferative and cytotoxic properties on different cancer cell lines [27][28][29]. The chemical profiling of this type of propolis indicated that nemorosone (NEM), a prenylated benzophenone, is the main component of brown CP [30][31][32] to which the biological properties of this propolis can be attributed [33][34][35].
2. Results and Discussion
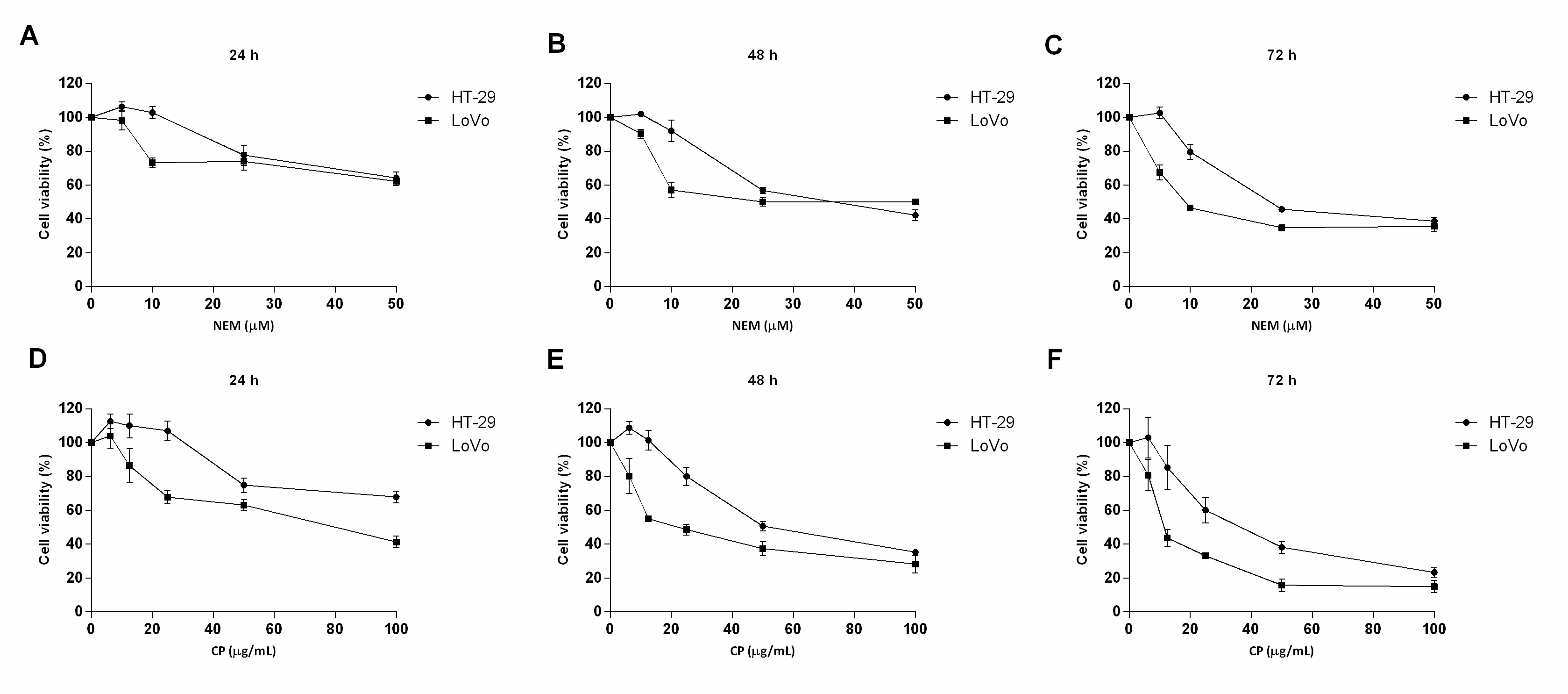
This study clearly showed that brown Cuban propolis and NEM displayed a pronounced inhibitory effect on the proliferation of HT-29 and LoVo cells. Furthermore, according to others reports [29][33][35], considering the NEM content of CP and the IC50 values obtained in this study, we further confirmed that the biological activity of CP is strictly dependent on its nemorosone content. (Figure 1)
Figure 1. Effect of NEM and CP on CRC cell viability. HT-29 and LoVo cells were exposed to increasing concentrations of NEM (A–C) and CP (D–F) for 24, 48 and 72 h. Data are presented as mean ± S.D. of three independent experiments.
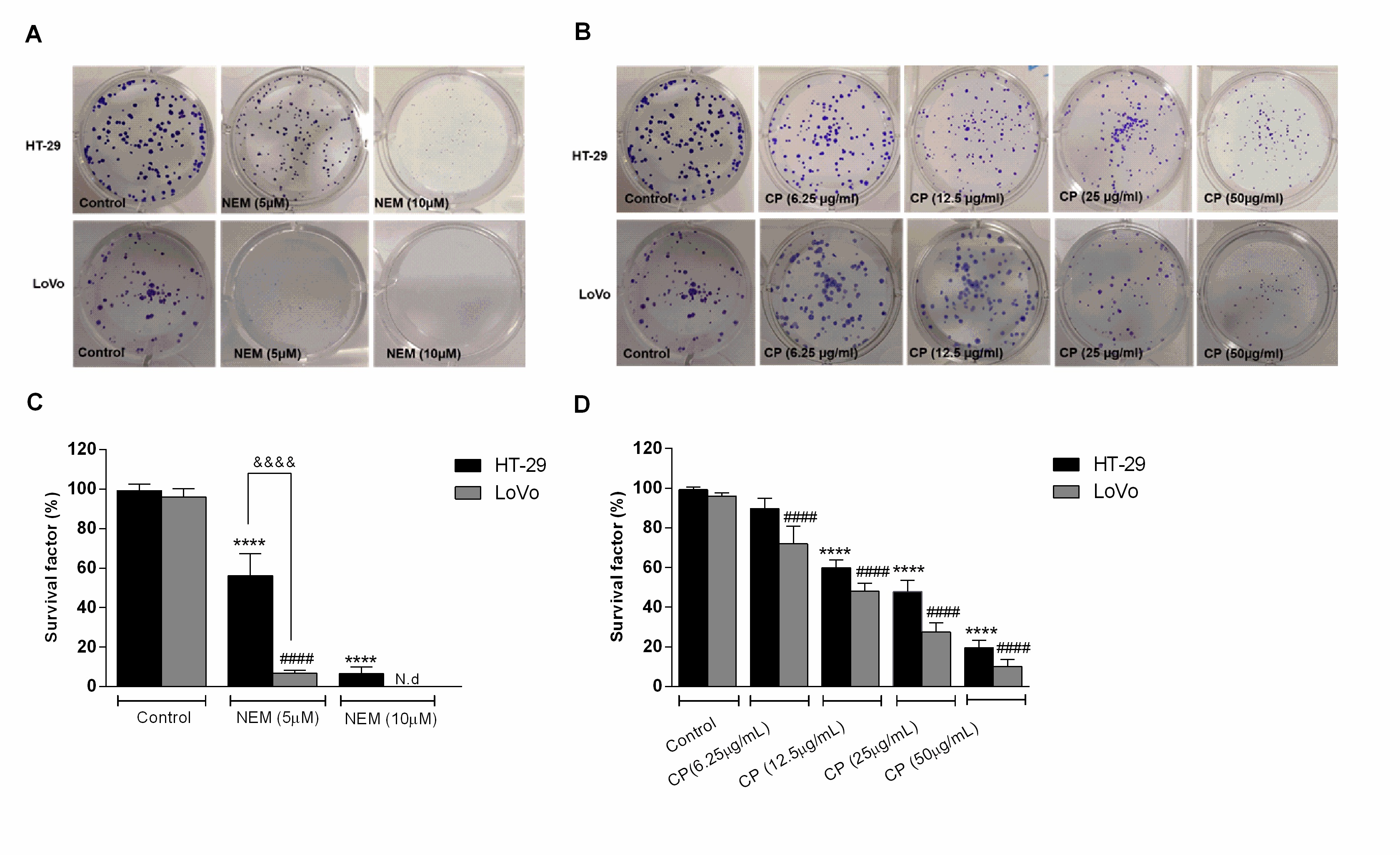
Their effect on HT-29 and LoVo viability was supported by the results of the clonogenic assay, which showed a significant decrease of the colony-forming capacity of CP- and NEM-treated cells compared to untreated cells, further confirming their antiproliferative effect. We have previously reported that CP and NEM have an inhibitory effect on LoVo cell viability [29], thus the results of the present study confirmed this finding and extended it to another CRC cell line. (Figure 2)
Figure 2. Effect of NEM (A,C) and CP (B,D) on the clonogenic capacity of colorectal cancer (CRC) cells. Magnification: 10×. Data are expressed as survival factor and represent the mean ± S.D. of three independent experiments. **** p < 0.0001 vs. untreated cells. LoVo cells: #### p < 0.0001 vs. untreated cells. &&&& p < 0.0001 vs the other cell line treated with the same conditions. N.d: not detected.
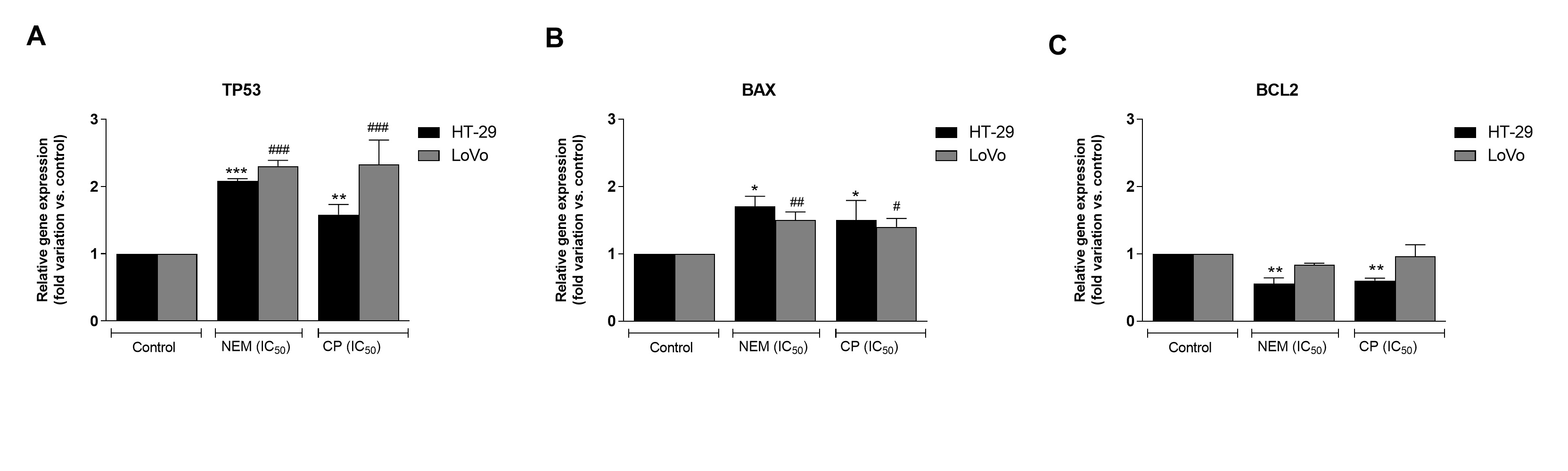
Cell cycle alteration is a critical step in the arrest of CRC progression [36]. In the present study, we observed that the antiproliferative effect of NEM and CP could be ascribable to the inhibition of cell cycle progression with an arrest in the G0/G1 phase and the induction of cell death by apoptosis. Both CP and NEM induced apoptosis in CRC cell lines in a time-dependent manner. In addition, the occurrence of apoptosis was associated with the upregulation of apoptosis-related genes TP53 and BAX, the downregulation of BCL2 and the activation of caspase-3. A previous study performed in our laboratory revealed that NEM and CP are able to induce mitochondrial dysfunction in LoVo cells [29]. The present findings were therefore in line with our previous results, thereby confirming that NEM and CP induced cell death via the mitochondrial apoptotic pathway in CRC. (Figure 3).
Figure 3. Effect of NEM and CP (IC50/72 h) on apoptosis-related genes of CRC cell lines. The mRNA levels of P53 (A), BAX (B) and BCL2 (C) are expressed as fold of change vs. control. Data are expressed as mean ± S.D. of three independent experiments. HT-29 cells: *p < 0.05, ** p < 0.01, *** p < 0.001 vs. untreated cells. LoVo cells: # p < 0.05, ## p < 0.01, ### p < 0.001 vs. untreated cells.
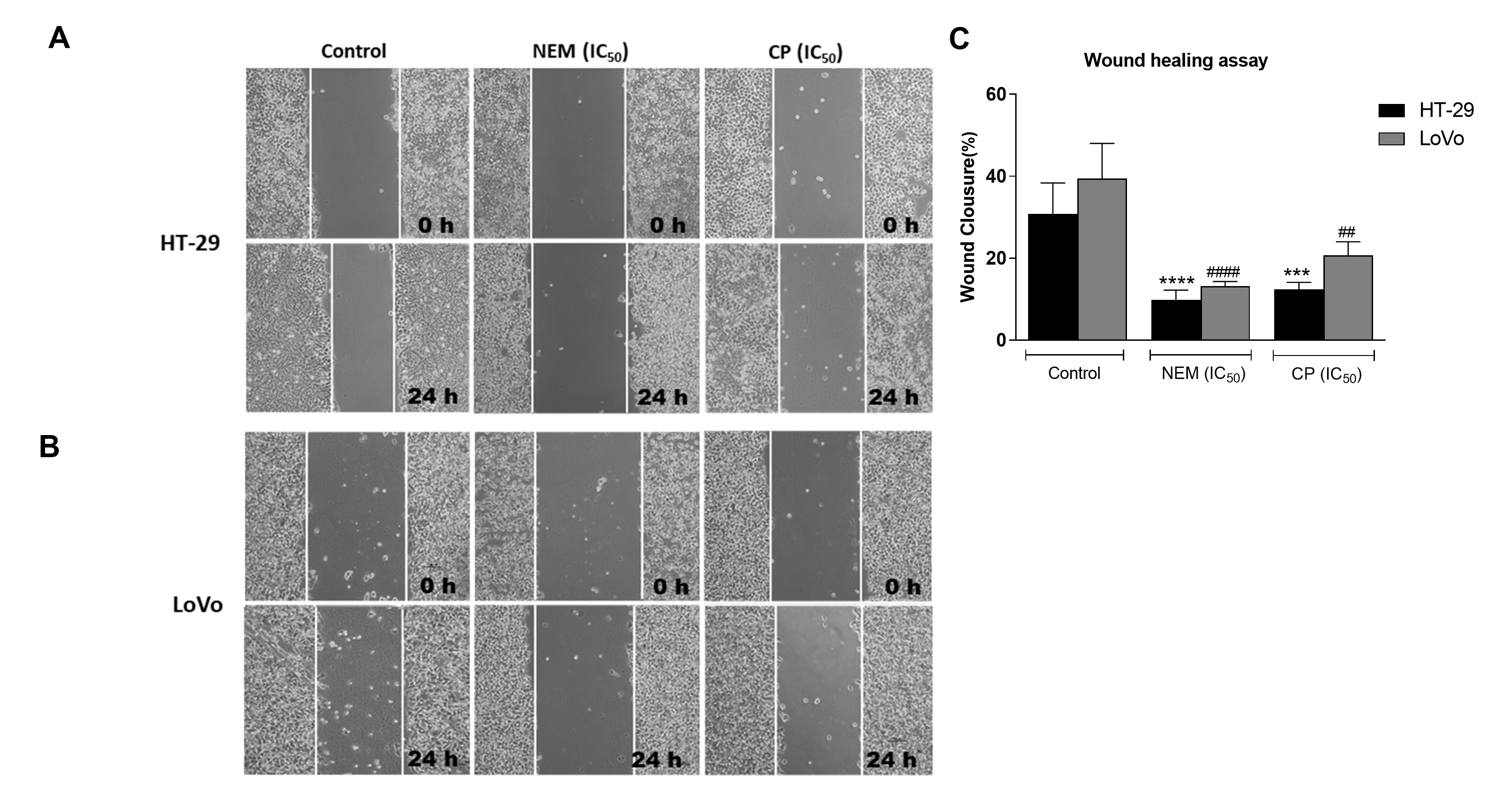
Migration and invasiveness of cancer cells are the main processes of tumor metastasis, which is the most frequent cause of colon cancer-associated death [6]. In order to evaluate the antimetastatic effect of NEM and CP on CRC cell lines, the wound healing and transwell migration/invasion assays were performed. Our results indicated that NEM and CP suppressed migration and invasion of both CRC cell lines. (Figure 4)
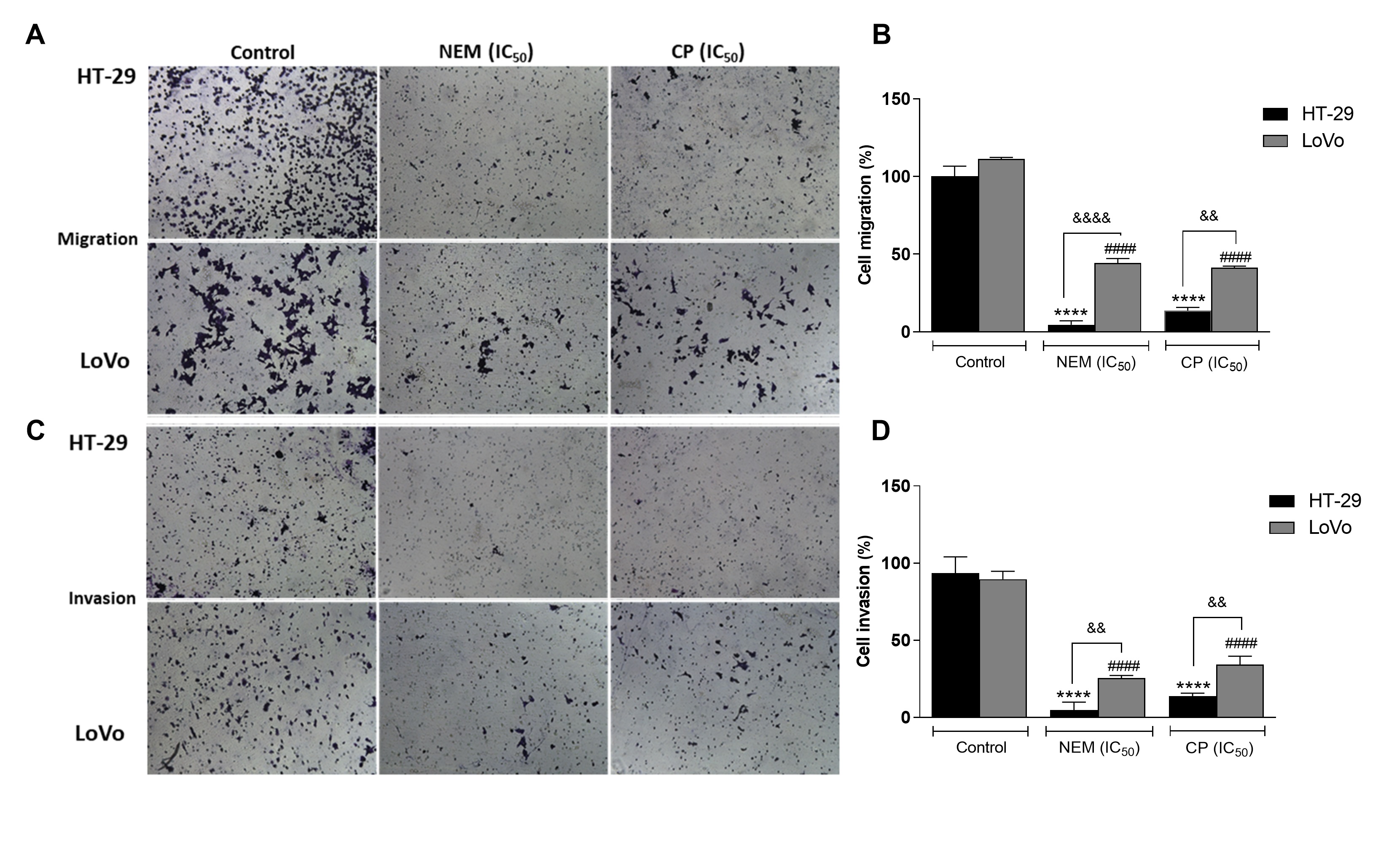
Figure 4. Wound healing assay in the CRC cell lines HT-29 (A) and LoVo (B; magnification 10×). Quantitative analysis of scratch wound healing assay after a 24 h-treatment with NEM or CP (IC50/72 h; C). Data are expressed as mean ± S.D. of three independent experiments. HT-29 cells: *** p < 0.001, **** p < 0.0001 vs. untreated cells. LoVo cells: ## p < 0.01, #### p < 0.0001 vs. control.
It is reported that the activity of proteolytic enzymes, such as the metalloproteinase MMP9, plays an important role in metastasis, since it is associated with the degradation of the extracellular matrix (ECM), which is involved in invasion/metastatic process [37]. We demonstrated that MMP9 activity was significantly reduced by the treatment with NEM and CP. Since it is well known that the expression of MMP-9 is correlated with angiogenesis and metastasis of CRC [38], we hypothesized that NEM and CP could affect not only the MMP9 expression in colon cancer cells, but also the expression of EMT-related molecules such as E-cadherin, B -catenin and vimentin, all involved in the migration, invasion and angiogenesis of CRC cells.In this study, we observed that HT-29 and LoVo cell lines exhibited an increase in E-cadherin protein levels and a decrease of B-catenin and vimentin expression following treatment with NEM and CP. (Figure 5)
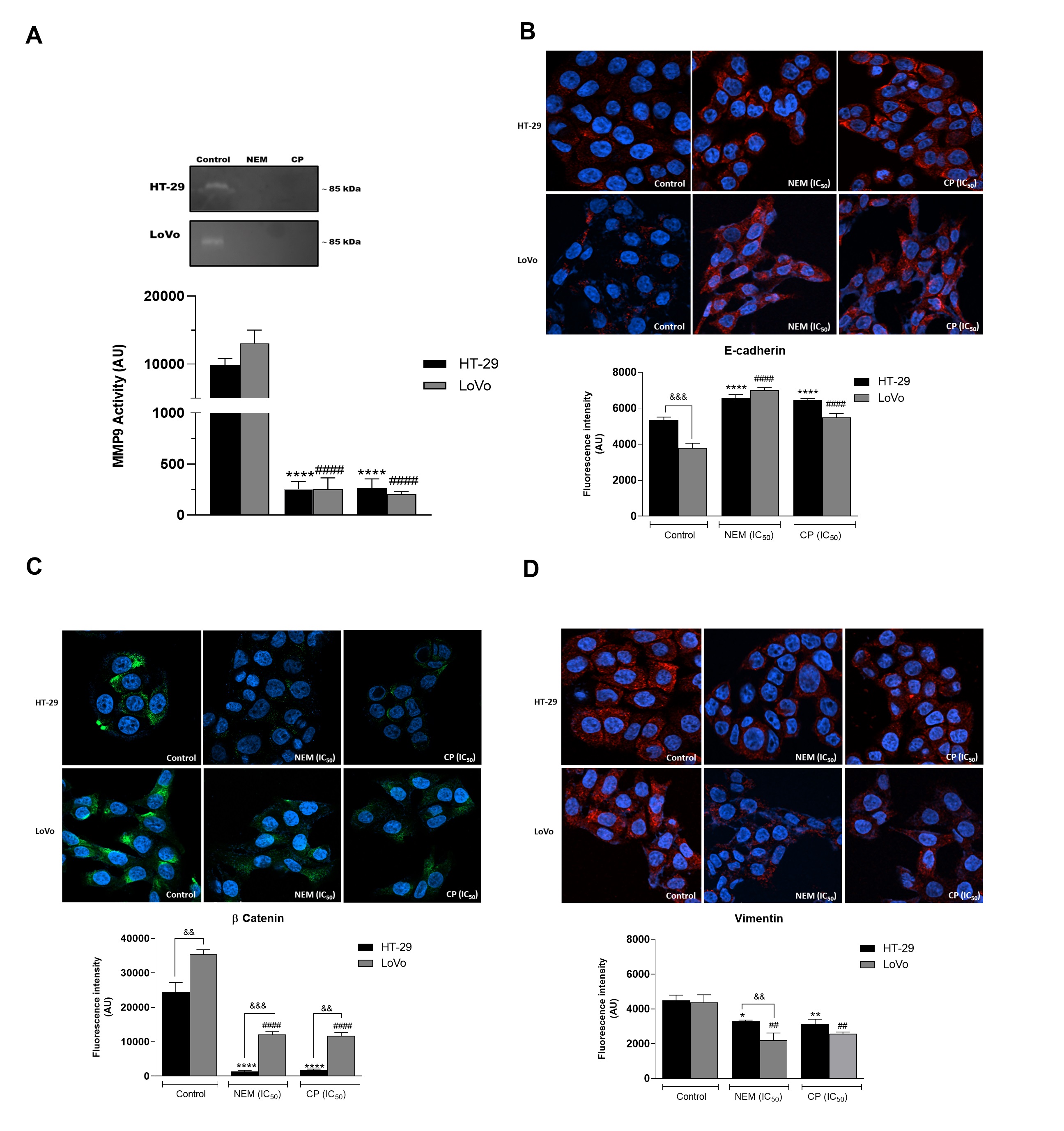
Figure 5. Effect of NEM and CP on proteolytic activity of MMP9 in CRC cells (A). Effect of NEM and CP (IC50/72 h) on the expression of epithelial–mesenchymal transition (EMT)-related markers E-cadherin (B), β-catenin (C) and vimentin (D) in CRC cells. In immunocytochemistry (ICC) images, E-cadherin and vimentin are in red and β-catenin is in green. Nuclei were stained with Hoechst (blue). Magnification: 40×. Data are reported as the mean ± SD of three independent experiments. ** p < 0.01, **** p < 0.0001 vs. untreated cells. LoVo cells: #### p < 0.0001 vs. untreated cells. && p < 0.01, &&& p < 0.001 vs. the other cell line treated with the same conditions. N.d: not detected.
3. Conclusion
Our study demonstrated that brown Cuban propolis and its main component nemorosone inhibited cell viability and clonogenic capacity of HT-29 and LoVo cell lines, since they induced apoptosis-mediated G0/G1 phase cell cycle arrest, upregulated TP53 and BAX gene expression and downregulated that of BCL2. In addition, we demonstrated that the apoptotic intrinsic pathway was involved. Furthermore, CP and NEM suppressed cell migration and invasion of CRC cells by inhibiting of EMT-related markers expression. In conclusion, we provided new insights into the anticancer mechanism of NEM, in particular about its antimetastatic e ect, thereby confirming its promising pharmacological features for CRC therapy. Further in vivo studies are needed to assess the translational relevance of our findings.
References
- Herb Brody; Colorectal cancer. Nature 2015, 521, S1-S1, 10.1038/521s1a.
- Rebecca L. Siegel; Kimberly D. Miller; Stacey A. Fedewa; Dennis J. Ahnen; Reinier G.S. Meester; Afsaneh Barzi; Ahmedin Jemal; Colorectal cancer statistics, 2017. CA: A Cancer Journal for Clinicians 2017, 67, 177-193, 10.3322/caac.21395.
- A. Vogel; R.D. Hofheinz; S. Kubicka; Dirk Arnold; Treatment decisions in metastatic colorectal cancer – Beyond first and second line combination therapies. Cancer Treatment Reviews 2017, 59, 54-60, 10.1016/j.ctrv.2017.04.007.
- Clarisse Dromain; C. Caramella; P. Dartigues; D. Goéré; M. Ducreux; F. Deschamps; Liver, lung and peritoneal metastases in colorectal cancers: Is the patient still curable? What should the radiologist know. Diagnostic and Interventional Imaging 2014, 95, 513-523, 10.1016/j.diii.2014.03.011.
- G Tsoulfas; Manousos Georgios Pramateftakis; Ioannis Kanellos; Surgical treatment of hepatic metastases from colorectal cancer. World Journal of Gastrointestinal Oncology 2011, 3, 1-9, 10.4251/wjgo.v3.i1.1.
- A. Andres; G. Mentha; R. Adam; E. Gerstel; O. G. Skipenko; Eduardo Barroso; S. Lopez-Ben; Catherine Hubert; P. E. Majno; C. Toso; et al. Surgical management of patients with colorectal cancer and simultaneous liver and lung metastases. British Journal of Surgery 2015, 102, 691-699, 10.1002/bjs.9783.
- Lance A. Liotta; Cancer Cell Invasion and Metastasis. Scientific American 1992, 266, 54-63, 10.1038/scientificamerican0292-54.
- Lubna H. Tahtamouni; Mamoun Ahram; Jennifer Koblinski; Christian D. Rolfo; Molecular Regulation of Cancer Cell Migration, Invasion, and Metastasis.. Analytical Cellular Pathology 2019, 2019, 1356508-2, 10.1155/2019/1356508.
- Minal Garg; Epithelial-mesenchymal transition - activating transcription factors - multifunctional regulators in cancer. World Journal of Stem Cells 2013, 5, 188-195, 10.4252/wjsc.v5.i4.188.
- Ievgenia Pastushenko; Cedric Blanpain; EMT Transition States during Tumor Progression and Metastasis. Trends in Cell Biology 2019, 29, 212-226, 10.1016/j.tcb.2018.12.001.
- Alessandro Buriani; Stefano Fortinguerra; Vincenzo Sorrenti; Stefano Dall Acqua; Gabbriella Innocenti; Monica Montopoli; Daniela Gabbia; Maria Carrara; Human Adenocarcinoma Cell Line Sensitivity to Essential Oil Phytocomplexes from Pistacia Species: a Multivariate Approach. Molecules 2017, 22, 1336, 10.3390/molecules22081336.
- J.M. Loree; Scott Kopetz; Recent developments in the treatment of metastatic colorectal cancer. Therapeutic Advances in Medical Oncology 2017, 9, 551-564, 10.1177/1758834017714997.
- Saúl Redondo-Blanco; Javier Fernández; Ignacio Gutiérrez-Del-Río; Claudio J. Villar; Felipe Lombó; New Insights toward Colorectal Cancer Chemotherapy Using Natural Bioactive Compounds. Frontiers in Pharmacology 2017, 8, 32089, 10.3389/fphar.2017.00109.
- Mark S. Butler; Natural products to drugs: natural product-derived compounds in clinical trials. Nat. Prod. Rep. 2008, 25, 475, 10.1039/b514294f.
- Marius Alexandru Moga; Dimienescu Oana; Cristian Andrei Arvatescu; Aurel Mironescu; Laura Dracea; Liana Ples; The Role of Natural Polyphenols in the Prevention and Treatment of Cervical Cancer—An Overview. Molecules 2016, 21, 1055, 10.3390/molecules21081055.
- Shahid Sarwar; Hongjie Zhang; Siu Wai Tsang; Perspectives of Plant Natural Products in Inhibition of Cancer Invasion and Metastasis by Regulating Multiple Signaling Pathways. Current Medicinal Chemistry 2019, 25, 5057-5087, 10.2174/0929867324666170918123413.
- Diana Sawicka; Halina Car; Maria Borawska; Jacek Nikliński; The anticancer activity of propolis. Folia Histochemica et Cytobiologica 2012, 50, 25-37, 10.5603/fhc.2012.0004.
- Mariateresa Badolato; Gabriele Carullo; Erika Cione; Francesca Aiello; Maria Cristina Caroleo; From the hive: Honey, a novel weapon against cancer. European Journal of Medicinal Chemistry 2017, 142, 290-299, 10.1016/j.ejmech.2017.07.064.
- Rim Wehbe; Jacinthe Frangieh; Mohamad Rima; Dany El Obeid; Jean-Marc Sabatier; Ziad Fajloun; Bee Venom: Overview of Main Compounds and Bioactivities for Therapeutic Interests.. Molecules 2019, 24, 2997, 10.3390/molecules24162997.
- G.A. Burdock; Review of the biological properties and toxicity of bee propolis (propolis). Food and Chemical Toxicology 1998, 36, 347-363, 10.1016/s0278-6915(97)00145-2.
- Nada Orsolic; A review of propolis antitumour action in vivo and in vitro. Journal of ApiProduct and ApiMedical Science 2010, 2, 1, 10.3896/ibra.4.02.1.01.
- Seema Patel; Emerging Adjuvant Therapy for Cancer: Propolis and its Constituents. Journal of Dietary Supplements 2015, 13, 245-268, 10.3109/19390211.2015.1008614.
- Nada Zabaiou; Allan Fouache; Amalia Trousson; Silvère Baron; Amar Zellagui; Mesbah Lahouel; Jean-Marc A. Lobaccaro; Biological properties of propolis extracts: Something new from an ancient product. Chemistry and Physics of Lipids 2017, 207, 214-222, 10.1016/j.chemphyslip.2017.04.005.
- Efstathia Giannopoulou; Chemical diversity of propolis and the problem of standardization. Journal of Ethnopharmacology 2005, 100, 114-117, 10.1016/j.jep.2005.05.004.
- Anna Lisa Piccinelli; Cinzia Lotti; Luca Campone; Osmany Cuesta-Rubio; Mercedes Campo Fernandez; Luca Rastrelli; Cuban and Brazilian Red Propolis: Botanical Origin and Comparative Analysis by High-Performance Liquid Chromatography–Photodiode Array Detection/Electrospray Ionization Tandem Mass Spectrometry. Journal of Agricultural and Food Chemistry 2011, 59, 6484-6491, 10.1021/jf201280z.
- Sudjaswadi Wiryowidag; Partomuan Simanjunta; Wan Lelly Heffen; Sudjaswadi Wiryowidagdo; Partomuan Simanjuntak; Chemical Composition of Propolis from Different Regions in Java and their Cytotoxic Activity. American Journal of Biochemistry and Biotechnology 2009, 5, 180-183, 10.3844/ajbbsp.2009.180.183.
- David Diaz-Carballo; Sascha Malak; Walter Bardenheuer; Michael Freistuehler; H. Peter Reusch; The contribution of plukenetione A to the anti-tumoral activity of Cuban propolis. Bioorganic & Medicinal Chemistry 2008, 16, 9635-9643, 10.1016/j.bmc.2008.10.019.
- Ada Popolo; Anna Lisa Piccinelli; Silvana Morello; Osmany Cuesta-Rubio; Rosalinda Sorrentino; Luca Rastrelli; Aldo Pinto; Antiproliferative activity of brown Cuban propolis extract on human breast cancer cells.. Natural Product Communications 2009, 4, 1711–1716, 10.1177/1934578x0900401221.
- Yahima Frión-Herrera; Daniela Gabbia; Alexis Díaz-García; Osmany Cuesta-Rubio; Maria Carrara; Chemosensitizing activity of Cuban propolis and nemorosone in doxorubicin resistant human colon carcinoma cells.. Fitoterapia 2019, 136, 104173, 10.1016/j.fitote.2019.104173.
- O. Cuesta Rubio; Cuellar; N. Rojas; Hermán Vélez Castro; Luca Rastrelli; Rita P. Aquino; A Polyisoprenylated Benzophenone from Cuban Propolis. Journal of Natural Products 1999, 62, 1013-1015, 10.1021/np980339n.
- Ingrid Márquez Hernández; Mercedes Campo Fernandez; Osmany Cuesta-Rubio; Anna Lisa Piccinelli; Luca Rastrelli; Polyprenylated Benzophenone Derivatives from Cuban Propolis. Journal of Natural Products 2005, 68, 931-934, 10.1021/np0495884.
- Osmany Cuesta-Rubio; Anna Lisa Piccinelli; Mercedes Campo Fernandez; Ingrid Márquez Hernández; Aristides Rosado; Luca Rastrelli; Chemical Characterization of Cuban Propolis by HPLC−PDA, HPLC−MS, and NMR: theBrown,Red, andYellowCuban Varieties of Propolis. Journal of Agricultural and Food Chemistry 2007, 55, 7502-7509, 10.1021/jf071296w.
- Osmany Cuesta-Rubio; Bernardo A. Frontana-Uribe; Teresa Ramírez-Apan; Jorge Cárdenas; Polyisoprenylated benzophenones in cuban propolis; biological activity of nemorosone.. Zeitschrift für Naturforschung C 2002, 57, 372-378, 10.1515/znc-2002-3-429.
- Lianet Monzote; Osmany Cuesta-Rubio; Mercedes Campo Fernandez; Ingrid Márquez Hernandez; Jorge Fraga; Kleich Pérez; Monique Kerstens; Louis Maes; Paul Cos; In vitro antimicrobial assessment of Cuban propolis extracts.. Memórias do Instituto Oswaldo Cruz 2012, 107, 978-984, 10.1590/s0074-02762012000800003.
- Gilberto L. Pardo Andreu; Felippe Henrique Zuccolotto- Dos- Reis; Felipe M. Dalalio; Yanier Núñez-Figueredo; Osmany Cuesta Rubio; Sérgio A. Uyemura; Carlos Curti; Luciane Carla Alberici; The cytotoxic effects of brown Cuban propolis depend on the nemorosone content and may be mediated by mitochondrial uncoupling. Chemico-Biological Interactions 2015, 228, 28-34, 10.1016/j.cbi.2015.01.010.
- Janet A. Houghton; F G Harwood; P J Houghton; Cell cycle control processes determine cytostasis or cytotoxicity in thymineless death of colon cancer cells.. Cancer Research 1994, 54, 4967-73, 1994 Sep 15;54(18).
- Peter Chen; William C. Parks; Role of matrix metalloproteinases in epithelial migration.. Journal of Cellular Biochemistry 2009, 108, 1233-43, 10.1002/jcb.22363.
- E. T. Waas; T. Wobbes; Roger Lomme; J. DeGroot; T. Ruers; Thijs Hendriks; Matrix metalloproteinase 2 and 9 activity in patients with colorectal cancer liver metastasis. British Journal of Surgery 2003, 90, 1556-1564, 10.1002/bjs.4346.