Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Anthony Wong | -- | 3184 | 2024-03-01 14:57:18 | | | |
| 2 | Lindsay Dong | + 12 word(s) | 3196 | 2024-03-04 01:28:58 | | | | |
| 3 | Lindsay Dong | -1 word(s) | 3195 | 2024-03-05 09:59:37 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Wong, A.X.J.; Tang, D.H.; Kaliya-Perumal, A.; Oh, J.Y. Lateral Lumbar Interbody Fusion. Encyclopedia. Available online: https://encyclopedia.pub/entry/55773 (accessed on 07 February 2026).
Wong AXJ, Tang DH, Kaliya-Perumal A, Oh JY. Lateral Lumbar Interbody Fusion. Encyclopedia. Available at: https://encyclopedia.pub/entry/55773. Accessed February 07, 2026.
Wong, Anthony Xi Jie, Derek Haowen Tang, Arun-Kumar Kaliya-Perumal, Jacob Yoong-Leong Oh. "Lateral Lumbar Interbody Fusion" Encyclopedia, https://encyclopedia.pub/entry/55773 (accessed February 07, 2026).
Wong, A.X.J., Tang, D.H., Kaliya-Perumal, A., & Oh, J.Y. (2024, March 01). Lateral Lumbar Interbody Fusion. In Encyclopedia. https://encyclopedia.pub/entry/55773
Wong, Anthony Xi Jie, et al. "Lateral Lumbar Interbody Fusion." Encyclopedia. Web. 01 March, 2024.
Copy Citation
Lumbar interbody fusion procedures have seen a significant evolution over the years, with various approaches being developed to address spinal pathologies and instability, including posterior lumbar interbody fusion (PLIF), transforaminal lumbar interbody fusion (TLIF), anterior lumbar interbody fusion (ALIF), and lateral lumbar interbody fusion (LLIF). LLIF, a pivotal technique in the field, initially emerged as extreme/direct lateral interbody fusion (XLIF/DLIF) before the development of oblique lumbar interbody fusion (OLIF). To ensure comprehensive circumferential stability, LLIF procedures are often combined with posterior stabilization (PS) using pedicle screws.
lumber interbody fusion
robotic surgical procedures
spinal navigation
spine
spondylosis
1. Evolution of Lumbar Interbody Fusion
Spinal fusion dates back to early 20th century, when Hibbs and Albee used fragments from the spinous process, laminae, and tibia as bone grafts to achieve posterior fusion of the spine, primarily in patients with tuberculosis [1][2]. Over time, fusion techniques evolved, and lumbar interbody fusion (LIF), which involves the insertion of a cage along with bone graft into the intervertebral space, became popular as a procedure offering both stability and fusion [3][4]. Early LIF procedures that were developed include posterior lumbar interbody fusion (PLIF) by Cloward in 1943 [5], anterior lumbar interbody fusion (ALIF) by Lane and Moore in 1948 [6], and transforaminal lumbar interbody fusion (TLIF) by Harms and Rolinger in 1982 [7]. Brief descriptions of each of these procedures, as well as their advantages and disadvantages, are compiled in Table 1, as shown below. As highlighted, the LIF procedures are associated with certain advantages and disadvantages specific to each procedure. Posterior approaches, such as PLIF and TLIF, may affect posterior structures and the paraspinal musculature, and may cause retraction injury of the nerve roots and thecal sac [8][9][10]. While ALIF manages to avoid damaging the posterior structures, it may potentially damage intra-abdominal, intraperitoneal, and vascular structures [11][12][13][14][15].
| Procedure Description | Advantages | Disadvantages | |
|---|---|---|---|
| PLIF | Posterior midline incision in prone position; requires laminectomy and retraction of thecal sac and nerve roots to reach the intervertebral disc space |
|
|
| TLIF | Posterior incision with a more lateral trajectory; requires facetectomy to allow visualization of nerve roots and perform discectomy |
|
|
| ALIF | Longitudinal midline or paramedian incision to access retroperitoneal space in supine position |
|
|
2. A Safer Approach
2.1. The Extreme Lateral Interbody Fusion (XLIF) or Direct Lateral Interbody Fusion (DLIF)
Extreme lateral interbody fusion (XLIF), also known as direct lateral interbody fusion (DLIF), was developed by Pimenta in 2001 [22][23]. Instead of approaching the intervertebral disc anteriorly or posteriorly as in ALIF and PLIF, respectively, XLIF/DLIF accesses the intervertebral disc through a lateral retroperitoneal trans-psoas approach [24].
2.2. The Procedure of XLIF/DLIF
The patient is generally placed in a right lateral decubitus position with the left side up. Strapping of the pelvis and chest wall is carried out to prevent changes in position, and adequate cushioning is provided at bony prominences. The operating table may be flexed to increase the distance between the iliac crest and rib cage. For a single-level exposure, a small incision is made on the lateral side over the affected disc space, utilizing X-ray guidance. A dilator is inserted through the incision, guided by the surgeon’s finger, to reach the psoas muscle while ensuring the protection of the peritoneum and abdominal contents. The psoas muscle is carefully parted between the middle and anterior third using blunt dissection, keeping the nerves posteriorly and great vessels anteriorly. The dilator is advanced through the psoas muscle, monitoring electromyography (EMG) responses to ensure safe passage protecting the lumbar plexus.
2.3. Benefits of XLIF over Other LIF Procedures
By utilizing the lateral retroperitoneal trans-psoas approach, XLIF avoids the risks of damaging the paraspinal muscles and the bony posterior elements as compared to PLIF and TLIF [25]. Unlike ALIF, XLIF does not require great vessel mobilization, and peritoneal structures are less likely to be injured [26]. In addition, preservation of the anterior and posterior longitudinal ligaments ensures stability of the treated levels [27]. Over the years, numerous studies have highlighted the effectiveness of XLIF in improving pain and disability scores, such as the Visual Analog Scale (VAS) and the Oswestry Disability Index (ODI), in addition to providing fusion and stability [27][28][29][30].
Cage subsidence is a common complication following LIF procedures, where the implanted cage sinks into the adjacent endplates, potentially compromising fusion [31]. If severe, it may also cause neural foraminal narrowing leading to nerve root compression, exacerbating pain and function [32][33]. While low bone mineral density and inappropriate cage positioning play crucial roles as risk factors in contributing to this problem, the intrinsic differences in cages used in PLIF or TLIF procedures also contribute significantly to a higher risk of cage subsidence compared to XLIF/OLIF cages [4][34].
Cages commonly used in TLIF and PLIF are the banana and bullet cages, respectively. These cages are smaller in size and, hence, the surface area that is in contact with the endplates is significantly less when compared to XLIF cages. There is also a reported increase in the risk of posterior cage migration when using smaller cages [35][36][37]. Given the smaller surface area in contact with the vertebral endplate, pressure dynamics lead to an increased force directly affecting the unsupported areas of the endplates, reducing overall stability [38].
On the other hand, while performing XLIF/DLIF, a wider (up to 26 mm) and longer (up to 60 mm) cage can be utilized, thereby improving endplate coverage and reducing subsidence risk [36][39][40]. This also allows sufficient distraction of the disc space and generates tension in the conserved ligaments, further enhancing stability.
2.4. Limitations of Trans-Psoas Approach
Despite its advantages, XLIF does also have its own limitations. Its main drawback is that it is commonly associated with postoperative hip flexion weakness (psoas weakness) due to the blunt dissection of the psoas muscle [41][42][43][44][45][46][47]. Most of these cases are transient and usually resolve within 2 weeks [48][49]. Despite neuromonitoring, the lumbar plexus may also be damaged, leading to lower limb weakness and paresthesia [50][51]. The lumbar plexus tends to adopt a more anterior location at lower spinal levels; hence, it is more prone to injury. Nevertheless, new research has shown that manipulating the entry site and psoas muscle traction direction may help reduce the risk of lumbar plexus injury [52].
3. The Oblique Lumbar Interbody Fusion (OLIF)
Oblique lumbar interbody fusion (OLIF), also known as the anterior to psoas (ATP) approach for interbody fusion, is a procedure which was first adopted by Meyer in 1997, and the term was officially coined by Silvestre et al. in 2012. Subsequently, Hynes further developed and popularized the technique [53]. This approach typically involves minimally invasive access into the disc space via the anatomical corridor between the psoas muscle and the great vessels (aorta and inferior vena cava) and is suitable for performing fusion of levels L2–L5 [54]. In addition, Hynes also developed the concept of OLIF L5–S1, which is essentially an anterior approach performed in a lateral decubitus position when the L5–S1 region needs to be accessed [53].
3.1. The Procedure of OLIF L2-L5 [53]
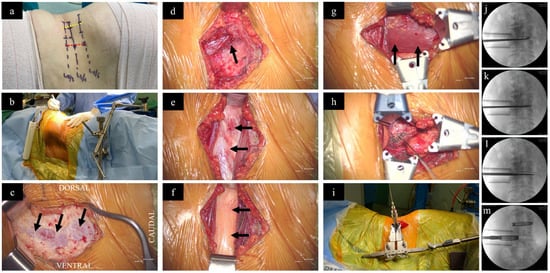
For OLIF L2–L5, similar to XLIF, the patient should be positioned in a right lateral decubitus position on a radiolucent table to expose the spine from the left side, as the working passage between the psoas muscle and the IVC is narrower on the right side [55][56]. Once positioned, the legs are slightly flexed. A line is drawn across the desired disc level from the anterior to the posterior. This determines the incision, which is typically made approximately 3–5 cm anterior to the midpoint of the line (Figure 1a). The fascia of the external oblique muscle is first encountered and incised using electrocautery, followed by gentle finger dissection of the external oblique, the internal oblique, and the transversalis muscles (Figure 1c–e). While working on the transversalis fascia, the finger’s force is directed obliquely and posteriorly to prevent entry into the peritoneal cavity. Once the retroperitoneal fat plane is reached, the space should be developed both cephalad and caudal to the desired disc level (Figure 1f), followed by anterior retraction of the peritoneal sac and posterior retraction of the anterior belly of psoas muscle to establish the working corridor (Figure 1g). After retraction of the psoas muscle, the disc space is visualized and a guide wire is inserted, followed by a series of dilations to create space pushing aside the surrounding tissues (Figure 1h,i). Subsequently, a retractor is positioned over the dilators and can be anchored to the vertebral body using a pin. The retractor blades are oriented such that it allows for an orthogonal maneuver (rotating the instruments in a manner that they are obliquely inserted but become direct lateral as they go deeper) during disc removal, sequential trialling, and final placement of the interbody cage. Annulotomy and discectomy is performed under X-ray guidance (Figure 1j,k). After completing the disc preparation, a contralateral annular release is performed using a blunt-tipped shaver or cobb elevator, as carried out during XLIF (Figure 1l). Sequential trials distract the disc space and allow indirect decompression. Finally, a wide-bodied interbody cage is placed within the disc space (Figure 2m). The procedure may be accompanied by lateral or posterior stabilization, contingent upon the indication, the requirement for direct decompression, and the surgeon’s preference [57][58].

Figure 1. Surgical steps of OLIF. (a) While the patient is in a right lateral decubitus position, the surgical disc levels are marked (lines drawn across each disc level from anterior to posterior) under X-ray guidance. The red dotted line (3–5 centimeters anterior to the mid-disc) represents the incision site for OLIF, as performed for this patient, in relation to the yellow dotted line connecting the mid points of the disc levels, which represent the incision site for XLIF. (b) Surgeon standing on the abdominal aspect. (c) After incision of skin and subcutaneous tissue, the external oblique fascia is first encountered (arrows). (d) Following dissection of the external oblique, the internal oblique muscle is carefully split (arrow). (e) The transversalis fascia beneath the internal oblique muscle is exposed (arrows) (f) Blunt dissection of the transversalis fascia reveals the retroperitoneal fat (arrows). (g) By finger dissection, a plane is developed pushing the retroperitoneal fat anteriorly to reach the psoas muscle (arrows). (h) Placement of a guide wire into the disc space. (i) Application of the dilators and specialized retractor assembly. Preparation of the disc space using (j) curette, (k) disc punch, and (l) contralateral annular release using Cobb. (m) Placement of cages filled with bone graft under X-ray guidance.
3.2. The Procedure of OLIF L5-S1 [53]
Surface marking is carried out with the help of X-ray guidance. A line is drawn across the L5-S1 disc level from the posterior to anterior and is extended onto the abdominal area. Subsequently, a second line is drawn from the center of the L5-S1 disc, projecting perpendicular to the floor onto the abdomen’s surface. Finally, approximately two finger-breadths anterior to the anterior superior iliac spine (ASIS), a third line is drawn connecting the first and second lines where the incision is made. The anatomical advantages of positioning the patient laterally enable abdominal contents to naturally fall away from the spine, resulting in a reduction in the need for peritoneal retraction. Dissection is performed as described for OLIF L2–L5. The common iliac artery pulse can be felt on the anterior border of the psoas, and the common iliac vein is medial to the artery. The adventitial layer containing the superior hypogastric plexus and sympathetic chain within is to be released by blunt dissection. After successfully releasing the adventitial layer, the left common iliac vein can be gently retracted laterally if needed.
3.3. Advantages of OLIF over XLIF
There are several factors that make OLIF more convenient compared to XLIF. Firstly, the surgical oblique approach enables direct and extensive visualization of crucial structures, such as the ureters, major blood vessels, and most of the psoas muscle, while XLIF provides only limited visualization [59]. It also allows for the visualization of the anterior disc margin, facilitating easier estimation of cage location and, hence, better anterior placement of cages [60]. The biggest advantage OLIF has over XLIF is that no dissection of the psoas is involved [61][62][63]. This facilitates limited EMG neuromonitoring of the psoas during the procedure [59][64]. However, some patients will still experience hip flexion weakness due to prolonged psoas retraction. Some nerve branches supplying the psoas traverse the intervertebral disc obliquely prior to ramification within the muscle and are, therefore, vulnerable to injury when muscle fibers of the superficial layer of psoas are pulled away from vertebral bodies [65][66][67].
3.4. Surgical Outcomes following OLIF
OLIF has been shown to be able to achieve similar surgical outcomes as compared to XLIF by the principle of indirect decompression [68][69][70][71]. There was no significant difference between the fusion rates of OLIF and XLIF [72]. OLIF achieved a similar restoration of disc height as XLIF, which has been determined to be the most significant factor in lumbar lordosis recovery. Some papers even suggested that OLIF leads to a greater increase in posterior disc space as compared to XLIF, along with reduced cage shift rates [69][73]. OLIF has also been shown to be effective in achieving greater sagittal deformity correction and lower risk of motor deficits compared to XLIF [74]. On its own, OLIF was demonstrated to be effective in elderly patients above 65 years old, in terms of clinical outcomes and patient satisfaction rates [75].
3.5. Limitations of OLIF and Strategies to Overcome
Despite the use of wide interbody cages during OLIF and XLIF procedures, cage subsidence can still occur [76]. However, the incidence of subsidence is relatively lower than those observed after implanting smaller banana and bullet cages in other procedures, like PLIF and TLIF. Moreover, subsidence risk can be effectively reduced by taking into consideration several factors, such as a pre-existing bone health, conducting careful patient selection and evaluation of medical statuses, and practicing meticulous intraoperative techniques, such as avoiding aggressive endplate preparation [31][77]. The most frequently reported intraoperative complications are minor vascular injuries, mostly affecting segmental arteries, as well as endplate damage [78]. Other intraoperative complications, which occur in less than 1% of cases, include major vascular injury, vertebral body fracture, membrane laceration, and ureteral injury. The most common immediate postoperative complications are transient numbness or pain in the lower limbs, as well as temporary weakness and nerve deficits arising from sympathetic trunk injury [79][80].
4. Recent Advances
4.1. Single-Position Surgery (SPS)—LLIF with Posterior Stabilization (PS)
Despite the significant demonstrated benefits of XLIF and OLIF, one limitation that both share is the need to reposition the patient when additional posterior decompression and stabilization needs to be performed [81]. The first stage of the surgery requires the patient to be placed in the lateral decubitus position to access the intervertebral space, discectomy, and cage placement. This is followed by the second stage, which requires the patient to be placed in a prone position for posterior decompression and stabilization using implants [82]. While doing so, re-draping and repositioning the patient prolongs surgical duration and may not be suitable for patients with contraindications [83][84].
However, with the advent of single-position surgery (SPS), both XLIF and OLIF can be performed in a single position, predominantly the former, along with posterior stabilization (PS). This eliminates the need for patient repositioning, ultimately enhancing surgical efficiency and minimizing complications [82][85][86][87]. There are currently two main approaches to SPS: Lateral-SPS (L-SPS) and Prone–SPS (P-SPS), where the patient is placed either in the lateral decubitus or prone position, respectively, throughout the entire surgical duration. Both approaches are reported to have significant decreases in surgical times, with reductions from 60 min to up to 135 min, ultimately leading to a decrease in the duration of hospitalisation [83][87][88][89][90][91].
4.1.1. Lateral Single-Position Surgery (L-SPS)
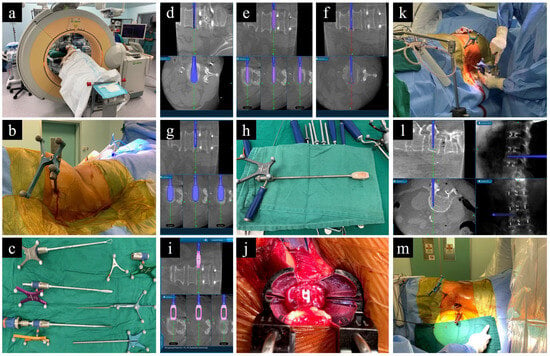
In the context of L-SPS, the patient is consistently placed in a lateral decubitus position during the XLIF or OLIF procedures, which includes the application of pedicle screws for posterior stabilization (Figure 2). However, while this approach eliminates the need for flipping the patient before addressing the posterior pedicle screws, a common drawback arises. Surgeons may lack familiarity with performing posterior stabilization in the lateral position. Basic tasks, such as laminectomy for posterior decompression and the insertion of pedicle screws, become challenging, ultimately limiting the size of the posterior construct. Furthermore, there is limited lordosis correction, compared to that which can be accomplished in a prone position. These drawbacks have resulted in greater incidences of facet joint violation and pedicle screw breach [92]. Addressing these challenges requires a different approach to patient positioning that mitigates the shortcomings of lateral positioning while retaining the benefits of single-position surgery.

Figure 2. O-arm-based navigation-assisted L-SPS. (a) Patient positioning. (b) Reference frame on PSIS. (c) Navigated instruments for disc preparation. (d–f) Preparation of disc using cobb, shaver, and curette. (g) Trailing. (h,i) Navigated cage. (j) Intraoperative visualization of cage placement. (k–m) Application of pedicle screws under navigation guidance while patient is in lateral position.
4.1.2. Prone Single-Position Surgery (P-SPS)
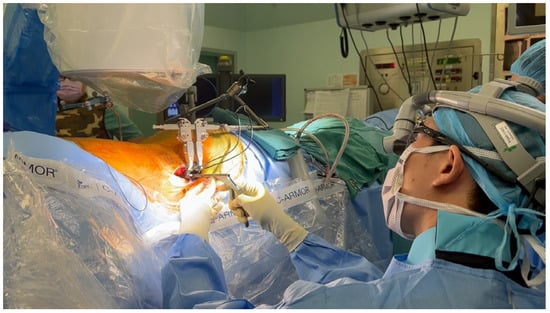
P-SPS overcomes the aforementioned downsides of L-SPS, being procedurally similar to L-SPS, except that the patient is placed in a prone position instead of laterally (Figure 3). This positioning offers a more familiar and spacious area for the surgeon to operate, facilitating easier pedicle screw placement and posterior decompression, as deemed necessary [93][94][95]. Furthermore, studies have demonstrated that adopting a prone position enables enhanced correction of sagittal plane imbalance attributed to an augmented lumbar lordosis [96][97], resulting in better segmental lordosis correction when compared to L-SPS [98].

Figure 3. Prone single-position surgery (P-SPS), with the surgeon working on the lateral approach while the patient is in a prone position, facilitating the possibility of simultaneous posterior pedicle screw fixation.
4.2. Robot-Assisted L-SPS and P-SPS
The use of robots in spine surgery is gaining popularity, evolving from the era of computer-assisted navigation. With the adoption of a preoperative planning software and robotic guidance for pedicle screw placement, there is an enhanced ability to adhere to and execute the surgical plan with the utmost accuracy [99]. This improves the likelihood of success and reduces the potential for significant complications. Studies have also emphasized the potential for decreased blood loss and shorter perioperative hospital stays achievable through the use of robots [100]. Scholars use the Mazor X Stealth Edition Robot (Medtronic). In short, following preoperative planning, the procedure begins with establishing a stable bed and securing the patient for robotic precision throughout the surgery. Subsequently, the robotic arm performs a 3D mapping of the operative field, and the patient is registered with the O-arm or fluoroscopy, independently registering each vertebra and correlating them with the previously obtained CT scan. The reference frame is secured in place, and snapshot tracker registration is performed. For screw application, the robotic arm moves along the preplanned trajectory with precision, enabling the use of instrumentation through the arm. Navigated instruments are then introduced through the robotic arm into the pedicle, preparing it for screw application, with the robotic arm maintaining a fixed trajectory [101].
References
- Howorth, M.B. Evolution of Spinal Fusion. Ann. Surg. 1943, 117, 278–289.
- Albee, F. The fundamental principles involved in the use of the bone graft in surgery. Am. J. Med. Sci. 1915, 149, 313–325.
- Polikeit, A.; Ferguson, S.J.; Nolte, L.P.; Orr, T.E. The importance of the endplate for interbody cages in the lumbar spine. Eur. Spine J. 2003, 12, 556–561.
- Patel, D.V.; Yoo, J.S.; Karmarkar, S.S.; Lamoutte, E.H.; Singh, K. Interbody options in lumbar fusion. J. Spine Surg. 2019, 5 (Suppl. S1), S19–S24.
- Cloward, R.B. Posterior lumbar interbody fusion updated. Clin. Orthop. Relat. Res. 1985, 193, 16–19.
- Lane, J.D., Jr.; Moore, E.S., Jr. Transperitoneal Approach to the Intervertebral Disc in the Lumbar Area. Ann. Surg. 1948, 127, 537–551.
- Harms, J.; Rolinger, H. A one-stager procedure in operative treatment of spondylolistheses: Dorsal traction-reposition and anterior fusion (author’s transl). Z. Orthop. Ihre Grenzgeb. 1982, 120, 343–347.
- Cole, C.D.; McCall, T.D.; Schmidt, M.H.; Dailey, A.T. Comparison of low back fusion techniques: Transforaminal lumbar interbody fusion (TLIF) or posterior lumbar interbody fusion (PLIF) approaches. Curr. Rev. Musculoskelet. Med. 2009, 2, 118–126.
- Audat, Z.; Moutasem, O.; Yousef, K.; Mohammad, B. Comparison of clinical and radiological results of posterolateral fusion, posterior lumbar interbody fusion and transforaminal lumbar interbody fusion techniques in the treatment of degenerative lumbar spine. Singap. Med. J. 2012, 53, 183–187.
- De Kunder, S.L.; van Kuijk, S.M.J.; Rijkers, K.; Caelers, I.; van Hemert, W.L.W.; de Bie, R.A.; van Santbrink, H. Transforaminal lumbar interbody fusion (TLIF) versus posterior lumbar interbody fusion (PLIF) in lumbar spondylolisthesis: A systematic review and meta-analysis. Spine J. 2017, 17, 1712–1721.
- Allain, J.; Dufour, T. Anterior lumbar fusion techniques: ALIF, OLIF, DLIF, LLIF, IXLIF. Orthop. Traumatol. Surg. Res. 2020, 106, S149–S157.
- Malham, G.M.; Parker, R.M.; Ellis, N.J.; Blecher, C.M.; Chow, F.Y.; Claydon, M.H. Anterior lumbar interbody fusion using recombinant human bone morphogenetic protein-2: A prospective study of complications. J. Neurosurg. Spine 2014, 21, 851–860.
- Lindley, E.M.; McBeth, Z.L.; Henry, S.E.; Cooley, R.; Burger, E.L.; Cain, C.M.; Patel, V.V. Retrograde ejaculation after anterior lumbar spine surgery. Spine (Phila Pa 1976) 2012, 37, 1785–1789.
- Sasso, R.C.; Kenneth Burkus, J.; LeHuec, J.C. Retrograde ejaculation after anterior lumbar interbody fusion: Transperitoneal versus retroperitoneal exposure. Spine (Phila Pa 1976) 2003, 28, 1023–1026.
- Inamasu, J.; Guiot, B.H. Vascular injury and complication in neurosurgical spine surgery. Acta Neurochir. 2006, 148, 375–387.
- Mobbs, R.J.; Phan, K.; Malham, G.; Seex, K.; Rao, P.J. Lumbar interbody fusion: Techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J. Spine Surg. 2015, 1, 2–18.
- Okuda, S.; Miyauchi, A.; Oda, T.; Haku, T.; Yamamoto, T.; Iwasaki, M. Surgical complications of posterior lumbar interbody fusion with total facetectomy in 251 patients. J. Neurosurg. Spine 2006, 4, 304–309.
- Hosono, N.; Namekata, M.; Makino, T.; Miwa, T.; Kaito, T.; Kaneko, N.; Fuji, T. Perioperative complications of primary posterior lumbar interbody fusion for nonisthmic spondylolisthesis: Analysis of risk factors. J. Neurosurg. Spine 2008, 9, 403–407.
- Wangaryattawanich, P.; Kale, H.A.; Kanter, A.S.; Agarwal, V. Lateral Lumbar Interbody Fusion: Review of Surgical Technique and Postoperative Multimodality Imaging Findings. AJR Am. J. Roentgenol. 2021, 217, 480–494.
- Abdoli, S.; Sui, J.; Ziegler, K.; Katz, S.; Burnham, W.; Ochoa, C. The periumbilical incision for anterior lumbar interbody fusions. J. Vasc. Surg. Cases Innov. Tech. 2020, 6, 384–387.
- Reisener, M.J.; Pumberger, M.; Shue, J.; Girardi, F.P.; Hughes, A.P. Trends in lumbar spinal fusion-a literature review. J. Spine Surg. 2020, 6, 752–761.
- Pimenta, L. Less-invasive lateral lumbar interbody fusion (XLIF) surgical technique: Video lecture. Eur. Spine J. 2015, 24 (Suppl. S3), 441–442.
- Pimenta, L. Lateral endoscopic transpsoas retroperitoneal approach for lumbar spine surgery. In Proceedings of the VIII Brazilian Spine Society Meeting, Belo Horizonte, Brazil, 4 May 2001.
- Ozgur, B.M.; Aryan, H.E.; Pimenta, L.; Taylor, W.R. Extreme Lateral Interbody Fusion (XLIF): A novel surgical technique for anterior lumbar interbody fusion. Spine J. 2006, 6, 435–443.
- Rodgers, W.B.; Gerber, E.J.; Patterson, J. Intraoperative and early postoperative complications in extreme lateral interbody fusion: An analysis of 600 cases. Spine (Phila Pa 1976) 2011, 36, 26–32.
- Ozgur, B.M.; Agarwal, V.; Nail, E.; Pimenta, L. Two-year clinical and radiographic success of minimally invasive lateral transpsoas approach for the treatment of degenerative lumbar conditions. SAS J. 2010, 4, 41–46.
- Caputo, A.M.; Michael, K.W.; Chapman, T.M., Jr.; Massey, G.M.; Howes, C.R.; Isaacs, R.E.; Brown, C.R. Clinical outcomes of extreme lateral interbody fusion in the treatment of adult degenerative scoliosis. Sci. World J. 2012, 2012, 680643.
- Patel, V.C.; Park, D.K.; Herkowitz, H.N. Lateral transpsoas fusion: Indications and outcomes. Sci. World J. 2012, 2012, 893608.
- Youssef, J.A.; McAfee, P.C.; Patty, C.A.; Raley, E.; DeBauche, S.; Shucosky, E.; Chotikul, L. Minimally invasive surgery: Lateral approach interbody fusion: Results and review. Spine (Phila Pa 1976) 2010, 35 (Suppl. S26), S302–S311.
- Formica, M.; Berjano, P.; Cavagnaro, L.; Zanirato, A.; Piazzolla, A.; Formica, C. Extreme lateral approach to the spine in degenerative and post traumatic lumbar diseases: Selection process, results and complications. Eur. Spine J. 2014, 23 (Suppl. S6), 684–692.
- Kotheeranurak, V.; Jitpakdee, K.; Lin, G.X.; Mahatthanatrakul, A.; Singhatanadgige, W.; Limthongkul, W.; Yingsakmongkol, W.; Kim, J.S. Subsidence of Interbody Cage Following Oblique Lateral Interbody Fusion: An Analysis and Potential Risk Factors. Glob. Spine J. 2021, 13, 1981–1991.
- Tempel, Z.J.; McDowell, M.M.; Panczykowski, D.M.; Gandhoke, G.S.; Hamilton, D.K.; Okonkwo, D.O.; Kanter, A.S. Graft subsidence as a predictor of revision surgery following stand-alone lateral lumbar interbody fusion. J. Neurosurg. Spine 2018, 28, 50–56.
- Zhao, L.; Xie, T.; Wang, X.; Yang, Z.; Pu, X.; Lu, Y.; Zeng, J. Clinical and radiological evaluation of cage subsidence following oblique lumbar interbody fusion combined with anterolateral fixation. BMC Musculoskelet. Disord. 2022, 23, 214.
- Amorim-Barbosa, T.; Pereira, C.; Catelas, D.; Rodrigues, C.; Costa, P.; Rodrigues-Pinto, R.; Neves, P. Risk factors for cage subsidence and clinical outcomes after transforaminal and posterior lumbar interbody fusion. Eur. J. Orthop. Surg. Traumatol. 2022, 32, 1291–1299.
- Marchi, L.; Abdala, N.; Oliveira, L.; Amaral, R.; Coutinho, E.; Pimenta, L. Radiographic and clinical evaluation of cage subsidence after stand-alone lateral interbody fusion. J. Neurosurg. Spine 2013, 19, 110–118.
- Lang, G.; Navarro-Ramirez, R.; Gandevia, L.; Hussain, I.; Nakhla, J.; Zubkov, M.; Hartl, R. Elimination of Subsidence with 26-mm-Wide Cages in Extreme Lateral Interbody Fusion. World Neurosurg. 2017, 104, 644–652.
- Alimi, M.; Lang, G.; Navarro-Ramirez, R.; Perrech, M.; Berlin, C.; Hofstetter, C.P.; Moriguchi, Y.; Elowitz, E.; Hartl, R. The Impact of Cage Dimensions, Positioning, and Side of Approach in Extreme Lateral Interbody Fusion. Clin. Spine Surg. 2018, 31, E42–E49.
- Fernandes, R.J.R.; Gee, A.; Kanawati, A.J.; Siddiqi, F.; Rasoulinejad, P.; Zdero, R.; Bailey, C.S. Evaluation of the contact surface between vertebral endplate and 3D printed patient-specific cage vs commercial cage. Sci. Rep. 2022, 12, 12505.
- Le, T.V.; Baaj, A.A.; Dakwar, E.; Burkett, C.J.; Murray, G.; Smith, D.A.; Uribe, J.S. Subsidence of polyetheretherketone intervertebral cages in minimally invasive lateral retroperitoneal transpsoas lumbar interbody fusion. Spine (Phila Pa 1976) 2012, 37, 1268–1273.
- Sharma, A.K.; Kepler, C.K.; Girardi, F.P.; Cammisa, F.P.; Huang, R.C.; Sama, A.A. Lateral lumbar interbody fusion: Clinical and radiographic outcomes at 1 year: A preliminary report. J. Spinal Disord. Tech. 2011, 24, 242–250.
- Hijji, F.Y.; Narain, A.S.; Bohl, D.D.; Ahn, J.; Long, W.W.; DiBattista, J.V.; Kudaravalli, K.T.; Singh, K. Lateral lumbar interbody fusion: A systematic review of complication rates. Spine J. 2017, 17, 1412–1419.
- Epstein, N.E. Review of Risks and Complications of Extreme Lateral Interbody Fusion (XLIF). Surg. Neurol. Int. 2019, 10, 237.
- Khajavi, K.; Shen, A.; Lagina, M.; Hutchison, A. Comparison of clinical outcomes following minimally invasive lateral interbody fusion stratified by preoperative diagnosis. Eur. Spine J. 2015, 24 (Suppl. S3), 322–330.
- Uribe, J.S.; Isaacs, R.E.; Youssef, J.A.; Khajavi, K.; Balzer, J.R.; Kanter, A.S.; Kuelling, F.A.; Peterson, M.D.; Group, S.D.S. Can triggered electromyography monitoring throughout retraction predict postoperative symptomatic neuropraxia after XLIF? Results from a prospective multicenter trial. Eur. Spine J. 2015, 24 (Suppl. S3), 378–385.
- O’Brien, J.R. Nerve Injury in Lateral Lumbar Interbody Fusion. Spine (Phila Pa 1976) 2017, 42 (Suppl. S7), S24.
- Abel, N.A.; Januszewski, J.; Vivas, A.C.; Uribe, J.S. Femoral nerve and lumbar plexus injury after minimally invasive lateral retroperitoneal transpsoas approach: Electrodiagnostic prognostic indicators and a roadmap to recovery. Neurosurg. Rev. 2018, 41, 457–464.
- Cummock, M.D.; Vanni, S.; Levi, A.D.; Yu, Y.; Wang, M.Y. An analysis of postoperative thigh symptoms after minimally invasive transpsoas lumbar interbody fusion. J. Neurosurg. Spine 2011, 15, 11–18.
- Tohmeh, A.G.; Rodgers, W.B.; Peterson, M.D. Dynamically evoked, discrete-threshold electromyography in the extreme lateral interbody fusion approach. J. Neurosurg. Spine 2011, 14, 31–37.
- Lee, Y.P.; Regev, G.J.; Chan, J.; Zhang, B.; Taylor, W.; Kim, C.W.; Garfin, S.R. Evaluation of hip flexion strength following lateral lumbar interbody fusion. Spine J. 2013, 13, 1259–1262.
- Epstein, N.E. High neurological complication rates for extreme lateral lumbar interbody fusion and related techniques: A review of safety concerns. Surg. Neurol. Int. 2016, 7 (Suppl. S25), S652–S655.
- Ahmadian, A.; Deukmedjian, A.R.; Abel, N.; Dakwar, E.; Uribe, J.S. Analysis of lumbar plexopathies and nerve injury after lateral retroperitoneal transpsoas approach: Diagnostic standardization. J. Neurosurg. Spine 2013, 18, 289–297.
- Nojiri, H.; Okuda, T.; Takano, H.; Gomi, M.; Takahashi, R.; Shimura, A.; Tamagawa, S.; Hara, T.; Ohara, Y.; Ishijima, M. Elimination of Lumbar Plexus Injury by Changing the Entry Point and Traction Direction of the Psoas Major Muscle in Transpsoas Lateral Lumbar Spine Surgery. Medicina 2023, 59, 730.
- Woods, K.R.; Billys, J.B.; Hynes, R.A. Technical description of oblique lateral interbody fusion at L1-L5 (OLIF25) and at L5-S1 (OLIF51) and evaluation of complication and fusion rates. Spine J. 2017, 17, 545–553.
- Li, R.; Li, X.; Zhou, H.; Jiang, W. Development and Application of Oblique Lumbar Interbody Fusion. Orthop. Surg. 2020, 12, 355–365.
- Wang, K.; Zhang, C.; Wu, H.; Chen, Z.; Chou, D.; Jian, F. The Anatomic Characteristics of the Retroperitoneal Oblique Corridor to the L1-S1 Intervertebral Disc Spaces. Spine (Phila Pa 1976) 2019, 44, E697–E706.
- Razzouk, J.; Ramos, O.; Mehta, S.; Harianja, G.; Wycliffe, N.; Danisa, O.; Cheng, W. Anterior-To-Psoas Approach Measurements, Feasibility, Non-Neurological Structures at Risk and Influencing Factors: A Bilateral Analysis From L1-L5 Using Computed Tomography Imaging. Oper. Neurosurg. 2023, 25, 52–58.
- Li, H.D.; Zhong, L.; Min, J.K.; Fang, X.Q.; Jiang, L.S. Oblique lateral interbody fusion combined with lateral plate fixation for the treatment of degenerative diseases of the lumbar spine: A retrospective study. Medicine 2022, 101, e28784.
- Wang, W.; Xiao, B.; Wang, H.; Qi, J.; Gu, X.; Yu, J.; Ye, X.; Xu, G.; Xi, Y. Oblique lateral interbody fusion stand-alone vs. combined with percutaneous pedicle screw fixation in the treatment of discogenic low back pain. Front. Surg. 2022, 9, 1013431.
- Phan, K.; Mobbs, R.J. Oblique Lumbar Interbody Fusion for Revision of Non-union Following Prior Posterior Surgery: A Case Report. Orthop. Surg. 2015, 7, 364–367.
- Chung, H.W.; Lee, H.D.; Jeon, C.H.; Chung, N.S. Comparison of surgical outcomes between oblique lateral interbody fusion (OLIF) and anterior lumbar interbody fusion (ALIF). Clin. Neurol. Neurosurg. 2021, 209, 106901.
- Ohtori, S.; Orita, S.; Yamauchi, K.; Eguchi, Y.; Ochiai, N.; Kishida, S.; Kuniyoshi, K.; Aoki, Y.; Nakamura, J.; Ishikawa, T.; et al. Mini-Open Anterior Retroperitoneal Lumbar Interbody Fusion: Oblique Lateral Interbody Fusion for Lumbar Spinal Degeneration Disease. Yonsei Med. J. 2015, 56, 1051–1059.
- Sato, J.; Ohtori, S.; Orita, S.; Yamauchi, K.; Eguchi, Y.; Ochiai, N.; Kuniyoshi, K.; Aoki, Y.; Nakamura, J.; Miyagi, M.; et al. Radiographic evaluation of indirect decompression of mini-open anterior retroperitoneal lumbar interbody fusion: Oblique lateral interbody fusion for degenerated lumbar spondylolisthesis. Eur. Spine J. 2017, 26, 671–678.
- Silvestre, C.; Mac-Thiong, J.M.; Hilmi, R.; Roussouly, P. Complications and Morbidities of Mini-open Anterior Retroperitoneal Lumbar Interbody Fusion: Oblique Lumbar Interbody Fusion in 179 Patients. Asian Spine J. 2012, 6, 89–97.
- Gragnaniello, C.; Seex, K. Anterior to psoas (ATP) fusion of the lumbar spine: Evolution of a technique facilitated by changes in equipment. J. Spine Surg. 2016, 2, 256–265.
- Abe, K.; Orita, S.; Mannoji, C.; Motegi, H.; Aramomi, M.; Ishikawa, T.; Kotani, T.; Akazawa, T.; Morinaga, T.; Fujiyoshi, T.; et al. Perioperative Complications in 155 Patients Who Underwent Oblique Lateral Interbody Fusion Surgery: Perspectives and Indications From a Retrospective, Multicenter Survey. Spine (Phila Pa 1976) 2017, 42, 55–62.
- Zeng, Z.Y.; Xu, Z.W.; He, D.W.; Zhao, X.; Ma, W.H.; Ni, W.F.; Song, Y.X.; Zhang, J.Q.; Yu, W.; Fang, X.Q.; et al. Complications and Prevention Strategies of Oblique Lateral Interbody Fusion Technique. Orthop. Surg. 2018, 10, 98–106.
- Mahan, M.A.; Sanders, L.E.; Guan, J.; Dailey, A.T.; Taylor, W.; Morton, D.A. Anatomy of psoas muscle innervation: Cadaveric study. Clin. Anat. 2017, 30, 479–486.
- Yingsakmongkol, W.; Jitpakdee, K.; Varakornpipat, P.; Choentrakool, C.; Tanasansomboon, T.; Limthongkul, W.; Singhatanadgige, W.; Kotheeranurak, V. Clinical and Radiographic Comparisons among Minimally Invasive Lumbar Interbody Fusion: A Comparison with Three-Way Matching. Asian Spine J. 2022, 16, 712–722.
- Li, H.M.; Zhang, R.J.; Shen, C.L. Differences in radiographic and clinical outcomes of oblique lateral interbody fusion and lateral lumbar interbody fusion for degenerative lumbar disease: A meta-analysis. BMC Musculoskelet. Disord. 2019, 20, 582.
- Rabau, O.; Navarro-Ramirez, R.; Aziz, M.; Teles, A.; Mengxiao Ge, S.; Quillo-Olvera, J.; Ouellet, J. Lateral Lumbar Interbody Fusion (LLIF): An Update. Glob. Spine J. 2020, 10 (Suppl. S2), 17S–21S.
- Liu, Y.; Park, C.W.; Sharma, S.; Kotheeranurak, V.; Kim, J.S. Endoscopic anterior to psoas lumbar interbody fusion: Indications, techniques, and clinical outcomes. Eur. Spine J. 2023, 32, 2776–2795.
- Xu, D.S.; Walker, C.T.; Godzik, J.; Turner, J.D.; Smith, W.; Uribe, J.S. Minimally invasive anterior, lateral, and oblique lumbar interbody fusion: A literature review. Ann. Transl. Med. 2018, 6, 104.
- He, W.; He, D.; Tian, W. Evaluation of lumbar fusion using the anterior to psoas approach for the treatment of L5/S1 spondylolisthesis. Medicine 2020, 99, e20014.
- Verma, R.; Virk, S.; Qureshi, S. Interbody Fusions in the Lumbar Spine: A Review. HSS J. 2020, 16, 162–167.
- Jin, C.; Jaiswal, M.S.; Jeun, S.S.; Ryu, K.S.; Hur, J.W.; Kim, J.S. Outcomes of oblique lateral interbody fusion for degenerative lumbar disease in patients under or over 65 years of age. J. Orthop. Surg. Res. 2018, 13, 38.
- Bhatti, A.U.R.; Cesare, J.; Wahood, W.; Alvi, M.A.; Onyedimma, C.E.; Ghaith, A.K.; Akinnusotu, O.; El Sammak, S.; Freedman, B.A.; Sebastian, A.S.; et al. Assessing the differences in operative and patient-reported outcomes between lateral approaches for lumbar fusion: A systematic review and indirect meta-analysis. J. Neurosurg. Spine 2022, 37, 498–514.
- Uribe, J.S. Neural anatomy, neuromonitoring and related complications in extreme lateral interbody fusion: Video lecture. Eur. Spine J. 2015, 24 (Suppl. S3), 445–446.
- Pham, M.H.; Hassan, O.; Diaz-Aguilar, L.D.; Lehman, R.A. Complications Associated With Oblique Lumbar Interbody Fusion at L5-S1: A Systematic Review of the Literature. Neurosurg. Pract. 2021, 2, okab018.
- Chang, S.Y.; Lee, W.S.; Mok, S.; Park, S.C.; Kim, H.; Chang, B.S. Anterior Thigh Pain Following Minimally Invasive Oblique Lateral Interbody Fusion: Multivariate Analysis from a Prospective Case Series. Clin. Orthop. Surg. 2022, 14, 401–409.
- Rutter, G.; Phan, K.; Smith, A.; Stewart, F.; Seex, K.; Gragnaniello, C. Morphometric anatomy of the lumbar sympathetic trunk with respect to the anterolateral approach to lumbar interbody fusion: A cadaver study. J. Spine Surg. 2017, 3, 419–425.
- Thomas, J.A.; Menezes, C.; Buckland, A.J.; Khajavi, K.; Ashayeri, K.; Braly, B.A.; Kwon, B.; Cheng, I.; Berjano, P. Single-position circumferential lumbar spinal fusion: An overview of terminology, concepts, rationale and the current evidence base. Eur. Spine J. 2022, 31, 2167–2174.
- Ziino, C.; Konopka, J.A.; Ajiboye, R.M.; Ledesma, J.B.; Koltsov, J.C.B.; Cheng, I. Single position versus lateral-then-prone positioning for lateral interbody fusion and pedicle screw fixation. J. Spine Surg. 2018, 4, 717–724.
- Ziino, C.; Arzeno, A.; Cheng, I. Analysis of single-position for revision surgery using lateral interbody fusion and pedicle screw fixation: Feasibility and perioperative results. J. Spine Surg. 2019, 5, 201–206.
- Blizzard, D.J.; Thomas, J.A. MIS Single-position Lateral and Oblique Lateral Lumbar Interbody Fusion and Bilateral Pedicle Screw Fixation: Feasibility and Perioperative Results. Spine (Phila Pa 1976) 2018, 43, 440–446.
- Kim, B.D.; Hsu, W.K.; De Oliveira, G.S., Jr.; Saha, S.; Kim, J.Y. Operative duration as an independent risk factor for postoperative complications in single-level lumbar fusion: An analysis of 4588 surgical cases. Spine (Phila Pa 1976) 2014, 39, 510–520.
- Cheng, P.; Zhang, X.B.; Zhao, Q.M.; Zhang, H.H. Efficacy of Single-Position Oblique Lateral Interbody Fusion Combined with Percutaneous Pedicle Screw Fixation in Treating Degenerative Lumbar Spondylolisthesis: A Cohort Study. Front. Neurol. 2022, 13, 856022.
- Guiroy, A.; Carazzo, C.; Camino-Willhuber, G.; Gagliardi, M.; Fernandes-Joaquim, A.; Cabrera, J.P.; Menezes, C.; Asghar, J. Single-Position Surgery versus Lateral-Then-Prone-Position Circumferential Lumbar Interbody Fusion: A Systematic Literature Review. World Neurosurg. 2021, 151, e379–e386.
- Godzik, J.; Ohiorhenuan, I.E.; Xu, D.S.; de Andrada Pereira, B.; Walker, C.T.; Whiting, A.C.; Turner, J.D.; Uribe, J.S. Single-position prone lateral approach: Cadaveric feasibility study and early clinical experience. Neurosurg. Focus. 2020, 49, E15.
- Drazin, D.; Kim, T.T.; Johnson, J.P. Simultaneous Lateral Interbody Fusion and Posterior Percutaneous Instrumentation: Early Experience and Technical Considerations. Biomed. Res. Int. 2015, 2015, 458284.
- Keorochana, G.; Muljadi, J.A.; Kongtharvonskul, J. Perioperative and Radiographic Outcomes Between Single-Position Surgery (Lateral Decubitus) and Dual-Position Surgery for Lateral Lumbar Interbody Fusion and Percutaneous Pedicle Screw Fixation: Meta-Analysis. World Neurosurg. 2022, 165, e282–e291.
- Ouchida, J.; Kanemura, T.; Satake, K.; Nakashima, H.; Ishikawa, Y.; Imagama, S. Simultaneous single-position lateral interbody fusion and percutaneous pedicle screw fixation using O-arm-based navigation reduces the occupancy time of the operating room. Eur. Spine J. 2020, 29, 1277–1286.
- Hiyama, A.; Katoh, H.; Sakai, D.; Tanaka, M.; Sato, M.; Watanabe, M. Facet joint violation after single-position versus dual-position lateral interbody fusion and percutaneous pedicle screw fixation: A comparison of two techniques. J. Clin. Neurosci. 2020, 78, 47–52.
- Lopez, G.; Sayari, A.J.; Phillips, F. Single-Position Anterior Column Lateral Lumbar Interbody Fusion. Int. J. Spine Surg. 2022, 16, S17–S25.
- Pimenta, L.; Pokorny, G.; Amaral, R.; Ditty, B.; Batista, M.; Moriguchi, R.; Filho, F.M.; Taylor, W.R. Single-Position Prone Transpsoas Lateral Interbody Fusion Including L4L5: Early Postoperative Outcomes. World Neurosurg. 2021, 149, e664–e668.
- Smith, T.G.; Joseph, S.A., Jr.; Ditty, B.; Amaral, R.; Tohmeh, A.; Taylor, W.R.; Pimenta, L. Initial multi-centre clinical experience with prone transpsoas lateral interbody fusion: Feasibility, perioperative outcomes, and lessons learned. N. Am. Spine Soc. J. 2021, 6, 100056.
- Barkay, G.; Wellington, I.; Mallozzi, S.; Singh, H.; Moss, I.L. The Prone Lateral Approach for Lumbar Fusion-A Review of the Literature and Case Series. Medicina 2023, 59, 251.
- Martirosyan, N.L.; Uribe, J.S.; Randolph, B.M.; Buchanan, R.I. Prone Lateral Lumbar Interbody Fusion: Case Report and Technical Note. World Neurosurg. 2020, 144, 170–177.
- Amaral, R.; Moriguchi, R.; Pokorny, G.; Arnoni, D.; Barreira, I.; Marcelino, F.; Pokorny, J.; Pimenta, L. Comparison of segmental lordosis gain of prone transpsoas (PTP) vs. lateral lumbar interbody fusion. Arch. Orthop. Trauma. Surg. 2023, 143, 5485–5490.
- Lieberman, I.H.; Kisinde, S.; Hesselbacher, S. Robotic-Assisted Pedicle Screw Placement During Spine Surgery. JBJS Essent. Surg. Tech. 2020, 10, e0020.
- Asada, T.; Simon, C.Z.; Lu, A.Z.; Adida, S.; Dupont, M.; Parel, P.M.; Zhang, J.; Bhargava, S.; Morse, K.W.; Dowdell, J.E.; et al. Robot-Navigated Pedicle Screw Insertion Can Reduce Intraoperative Blood Loss and Length of Hospital Stay: Analysis of 1633 Patients Utilizing Propensity Score Matching. Spine J. 2023, 24, 118–124.
- Sinkov, V.; Lockey, S.D.; Cunningham, B.W. Single Position Lateral Lumbar Interbody Fusion With Posterior Instrumentation Utilizing Computer Navigation and Robotic Assistance: Retrospective case review and surgical technique considerations. Glob. Spine J. 2022, 12, 75S–81S.
More
Information
Subjects:
Orthopedics
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
489
Revisions:
3 times
(View History)
Update Date:
05 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




