Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Amalia Sofianidi | -- | 2671 | 2024-02-29 18:40:04 |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Sofianidi, A.; Karadimou, A.; Charpidou, A.; Syrigos, K.N. Lung Cancer Incidence and Socioeconomic Status. Encyclopedia. Available online: https://encyclopedia.pub/entry/55748 (accessed on 13 January 2026).
Sofianidi A, Karadimou A, Charpidou A, Syrigos KN. Lung Cancer Incidence and Socioeconomic Status. Encyclopedia. Available at: https://encyclopedia.pub/entry/55748. Accessed January 13, 2026.
Sofianidi, Amalia, Alexandra Karadimou, Andriani Charpidou, Konstantinos N. Syrigos. "Lung Cancer Incidence and Socioeconomic Status" Encyclopedia, https://encyclopedia.pub/entry/55748 (accessed January 13, 2026).
Sofianidi, A., Karadimou, A., Charpidou, A., & Syrigos, K.N. (2024, February 29). Lung Cancer Incidence and Socioeconomic Status. In Encyclopedia. https://encyclopedia.pub/entry/55748
Sofianidi, Amalia, et al. "Lung Cancer Incidence and Socioeconomic Status." Encyclopedia. Web. 29 February, 2024.
Copy Citation
Lung cancer treatment and patient care continue to advance, yet concerns persist about whether these improvements are equally accessible to all socioeconomic groups. Socioeconomic disparities exist in lung cancer incidence, screening, effective treatment, overall survival, and prognosis. One of the key contributing factors to low socioeconomic status that is amenable to change is low education. Lower educational attainment is oftentimes linked to various factors, including smoking habits, unhealthy lifestyle behaviors, lower paid and unhealthier occupations, exposure to environmental pollutants, and genetic-familial risks, all contributing to an elevated incidence of lung cancer.
lung cancer
socioeconomic status
education
incidence
prognosis
stigma
1. Introduction
Lung cancer ranks as the second-most frequently diagnosed malignancy in both men and women. However, it stands as the foremost cause of cancer-related mortality worldwide and surpassed the combined fatalities from breast, colorectal, and prostate cancers in 2020 [1]. This burden disproportionately affects people with lower socioeconomic status (SES). One of the first articles about the disparities regarding lung cancer was published in 1958, where it was stated that there was an increase in lung cancer mortality related to inequalities in different social classes and occupations [2]. Nowadays, it is possible that the existing inequalities are even greater as a result of the COVID-19 pandemic, which suddenly changed our routines, modified our health behaviors, and significantly disrupted the landscape of lung cancer care. More specifically, the stress associated with COVID-19 and the economic crisis was linked to an increase in smoking, predominantly in individuals from populations especially vulnerable to smoking. In addition, the pandemic lockdown resulted in delaying screening, diagnosis, and treatment of many cancers and in putting a halt to clinical trials [3].
According to the WHO, the social determinants of health are the nonmedical factors that influence health outcomes. These are the circumstances in which individuals come into existence, develop, work, reside, and age. They encompass the broader array of influences and systems that mold the conditions of everyday life. Studies indicate that social determinants play a more significant role in influencing health than healthcare alone. For instance, multiple research findings propose that social determinants of health contribute to approximately 30–55% of health outcomes. Social determinants of health play a crucial role in the existence of health inequities, which refer to unjust and preventable differences in health status observed within and among nations. Regardless of income level, a social gradient is evident in health outcomes, where individuals in lower social standing positions experience poorer health.
There are many factors that are related to low SES, such as education, income, environment, and occupation. All factors are connected to one another and influence each other. The key contributing and amenable-to-change factor is low education. Individuals with lower education levels often have limited access to resources such as information, healthcare services, and preventive measures, ignoring the importance of health literacy. Lower education levels may lead to employment in occupations with higher physical risks, exposure to environmental hazards, and lower job security, all of which can impact health negatively. Also, limited educational attainment is associated with low-pay occupations or unemployment that may contribute to chronic stress, which can adversely affect mental health and, in turn, impact physical health and exacerbate existing health conditions. Moreover, low income also relates to inhabiting environmentally degraded and unhealthy neighborhoods.
2. Lung Cancer Incidence and SES
Several studies have shown an association between SES and incidence of lung cancer. Interestingly, this association is observed not only in underdeveloped countries but also in countries with excellent health systems. A registry-based study in Norway investigated the correlation between socioeconomic factors—utilizing income and education as indicators—and cancer incidence in Norway, a country recognized for its egalitarian principles, universal healthcare access, and high human development index. The most significant variations in incidence rate ratios (IRR) were observed in lung cancer. Individuals, both men and women, with a college or university education as their highest completed level showed a two- to threefold reduction in risk compared with those with primary school education (IRR for men: 0.40 (0.37–0.43), for women: 0.34 (0.31–0.37)) [4]. Low educational level is correlated with several factors, such as smoking habits, bad lifestyle behaviors, polluted neighborhoods, and genetic-familial risk, that lead to increased lung cancer incidence.
2.1. Smoking Habits
The link between active tobacco use and exposure to secondhand smoke in causing lung cancer is firmly established, with tobacco exposure contributing to approximately 80–95% of lung cancers [5]. Smoking continues to be the primary cause of avoidable illness and death on a worldwide scale [6], despite significant public health initiatives that have had a substantial impact on decreasing the prevalence of cigarette smoking. Nevertheless, smoking is not evenly spread throughout society. Instead, it is progressively clustering among individuals with the lowest education, income, and occupational status. As a result, it plays a pivotal role in contributing to health disparities, representing a significant portion of the variations in lung cancer incidence and mortality linked to SES [7][8].
Education stands out as one of the most robust sociodemographic indicators for predicting smoking prevalence and cessation. Lower education is associated with elevated smoking prevalence and reduced cessation rates [9]. An article published in JAMA in 1989 showed that, from 1974 to 1985, education took precedence over gender as the most influential sociodemographic factor associated with smoking [10]. The trend is the same 35 years later, with the prevalence of smoking being much higher among individuals without a high school diploma compared with those with a college degree [9]. In fact, marketing plays a big role in whether people try or use commercial tobacco products. The tobacco industry directs its marketing and advertising efforts towards communities with lower incomes and lower health literacy status. In the United States, it has been demonstrated that two major tobacco manufacturers specifically tailor their advertising to attract “working-class” youth. This further contributes to the initiation of smoking among young individuals from lower socioeconomic backgrounds at an earlier age [11] and leads to lower screening rates, increased diagnosis at advanced-stage disease, and lower rates of surgical intervention for early-stage disease observed in low SES communities [12].
In previous years, lower education was also correlated with lower rates of quitting smoking [13]. Minorities are less prone to receiving guidance to quit smoking, possessing awareness of accessible smoking cessation programs, engaging in smoking cessation initiatives, or utilizing pharmacotherapy to cease smoking [14]. However, recent data from the CDC state that people with lower incomes and less education try to quit using tobacco similarly to other sociodemographic groups. In 2015, an estimated 50% of adults who smoke cigarettes, and who also have not earned a high school diploma, tried to quit smoking, compared with 58% of those with some college education [15]. Notably, smokers in the lower socioeconomic class require additional support and assistance in quitting smoking, given that they are frequently more deeply addicted and more frequently exposed to nicotine [16].
The most recent globally supported data correlating low educational status with smoking and increased lung cancer incidence are from an analysis of data from 15 countries in the Lung Cancer Cohort Consortium. In both current and former smokers, a higher level of education demonstrated a remarkably consistent reduction in the risk of developing incident lung cancer across cohorts spanning four continents. This pattern persisted even after thorough adjustments for smoking were taken into account. When grouping by world region, the association between education and lung cancer incidence among currently/formerly smoking participants was similar for the US (hazard ratio (HR) = 0.88, 95% confidence interval (CI) 0.87–0.89), Europe (HR 0.89, 95% CI 0.88–0.91), and Asia (HR 0.91, 95% CI 0.86–0.96), but attenuated in the Australian cohort (HR 1.02, 95% CI 0.95–1.09 in all cohorts), except in the US Southern Community Cohort Study. This particular study, predominantly consisting of African Americans, exhibited a hazard ratio of 0.75 (95% CI 0.62–0.90) [17].
2.2. Environmental and Occupational Exposure
According to a study by the World Health Organization (WHO), maintaining healthy working and living environments has the potential to prevent at least 1.7 million cancer deaths each year [18]. While smoking is the main cause of lung cancer, there are other environmental risk factors that contribute to the pathogenesis of lung cancer [19]. These factors contribute to the pathogenesis of lung cancer in nonsmokers or intensify the deleterious effects of smoke on the lung [20]. The International Agency for Research on Cancer (IARC) has categorized air pollution as a Group 1 carcinogen, meaning it is confirmed to be carcinogenic to humans. This classification, based on IARC’s 2013 findings, is associated with over 220,000 annual global deaths from lung cancer and is linked to reduced survival rates post-diagnosis [21][22]. At the ESMO Congress in 2022, it was reported that very small pollutant particles in the air may trigger lung cancer in people who have never smoked. These particles, which are typically found in vehicle exhaust and smoke from fossil fuels, are associated with non-small cell lung cancer (NSCLC) risk and drive mutations in EGFR and KRAS genes [23].
Education and the environment are strongly connected to one another. It is well established that populations with lower SES are more likely to be exposed to higher levels of air pollution than those with higher SES [24][25][26]. Individuals with lower incomes may find themselves residing in more affordable regions where land values have decreased, such as areas near major roadways or industrial zones. This can result in heightened exposure levels and, consequently, an increased risk of disease. The issue of indoor air pollution stemming from combustion byproducts is especially worrisome in developing nations and rural areas, where wood and charcoal are frequently utilized for cooking and heating. Research indicates that implementing proper ventilation in these cooking spaces can lead to a reduction in lung cancer risk of up to 50% [27].
Education can influence individual behaviors and attitudes towards the environment. Environmental education refers to the adoption of educational methods aimed at fostering citizens’ understanding of their ethical connection to the environment, enhancing their awareness of environmental protection, skills, attitudes, and values, and guiding them to prioritize environmental concerns and undertake actions conducive to fostering a process of civic education conducive to sustainable development. Individuals with lower levels of education may have limited awareness and understanding of environmental issues, including climate change, pollution, and resource depletion. This lack of awareness can contribute to unsustainable behaviors and practices that harm the environment. On the same basis, individuals with low education levels may lack the necessary skills to work in environmentally friendly industries or to adopt sustainable practices in their daily lives, such as energy efficiency or waste reduction [28].
Asbestos is also carcinogenic and can cause lung cancer and mesothelioma [29]. People from lower SES are more likely to work in mines, being exposed to asbestos every day. Numerous epidemiological studies have been conducted on workers exposed to asbestos [30], but there is a limited number of studies focusing on the health effects of asbestos exposure in household and residential settings. Primary concerns regarding household exposure involve the immediate family members of asbestos workers, stemming from dust brought home on clothing. Household sources of asbestos exposure include the degradation, installation, removal, and repair of products containing asbestos. Radon exposure is also associated with an increased risk of lung cancer. The duration spent in subterranean environments, such as basements or mines, particularly in regions with elevated uranium concentrations, is linked to radon exposure. Individuals working underground in metal or uranium mining are recognized to face a significantly heightened risk of squamous cell carcinoma in the lungs and other organs [31][32][33].
In France in 2015, lung cancer stood out as the predominant form of cancer linked to occupational exposures. Among men, there were 5621 cases (constituting 89% of all work-related cases), while among women, there were 294 cases (making up 80%) [34]. Many epidemiological studies conducted in various countries have identified a notably elevated mortality rate for lung cancer among construction workers [35][36][37][38]. Construction workers face an increased risk of developing lung cancer with prolonged exposure, a risk not evident in supervisors, engineers, and higher-ranking officials in construction. This distinction is attributed to the lower exposure levels of the latter group compared with those working directly on construction sites. An analysis of data from a multicenter case-control study of lung cancer conducted in six Central and Eastern European countries showed that exposures in the workplace, such as to diesel exhaust and welding fumes, and to a lesser extent, crystalline silica, contribute to the risk associated with educational levels in the development of lung cancer. Between 13.4% and 14.8% of the association between education and lung cancer is mediated by occupational exposure [39]. Another French analysis showed that considering socio-occupational groups, the aggregate attributable fraction for three occupational carcinogens (asbestos, silica, diesel motor exhaust) reached 26.7% (95% CI 22.5; 30.8) for blue-collar workers, whereas it was minimal, at 0.2% (95% CI −1.35; 1.64), for managers [34]. In other words, it was proven that a portion of the increased likelihood of lung cancer among individuals with lower educational levels is attributed to their occupational exposure to carcinogens.
2.3. Genetic Predisposition
Genome-wide association studies (GWAS) have identified variants in multiple chromosomal regions linked to an elevated hereditary risk of lung cancer. These encompass the 5p15 locus, housing the gene for telomerase reverse transcriptase (TERT) [40], the 6p21 locus, responsible for regulating G-protein signaling [41], and the 15q25–26 loci, demonstrated to enhance nicotine dependence and susceptibility to lung cancer [42]. A study conducted in China explored the potential causal association between an increased number of years in education and a reduced risk of lung cancer, utilizing a two-sample Mendelian randomization (MR) study. It was demonstrated that genetically predicted higher educational attainment was related to significantly lower odds of lung cancer. Using conventional MR analysis, one standard deviation (SD) of longer education, specifically 3.6 years of additional education (due to genetic predisposition across 73 single nucleotide polymorphisms), was associated with a 52% lower risk of lung cancer [odds ratio (OR) 0.48, 95% CI 0.34, 0.66, p = 1.02 × 10−5]. A genetic inclination towards extended education was also linked to reduced smoking, a lower body mass index, and a favorable blood lipid profile [43].
2.4. Familial Socioeconomic Position
The socioeconomic status of the family during childhood plays a crucial role in predicting certain chronic diseases. Researchers in Denmark tried to investigate whether familial factors shared among siblings account for the correlation between education and the risk of lung cancer. Using the valid information of millions of siblings born in Denmark between 1950 and 1979, it was shown that family factors shared by siblings, such as exposure to secondhand smoke from parents, confounded some of the association between education and lung cancer incidence [44]. Wang et al. performed a meta-analysis of 13 studies in order to investigate whether childhood SES, defined as the education level or socioeconomic position of parents, and/or childhood housing conditions, influenced lung cancer mortality. Poorer childhood SES was associated with increased lung cancer risk (HR 1.25, 95% CI 1.10, 1.43) [45]. However, this meta-analysis was still in the preprint stage and had not been externally reviewed.
2.5. Infections
The process of lung carcinogenesis has been associated with inflammation and cellular damage occurring during respiratory infections. Tuberculosis (TB) increases the risk of lung cancer onset and progression according to a meta-analysis, expressing an OR of lung cancer development of 1.76 [46]. On the other hand, HIV also increases the risk of lung cancer by two to four times, regardless of smoking status [47]. In fact, in the US, lung cancer has emerged as the primary cause of mortality among individuals with HIV, especially after the introduction of antiretroviral therapies that limited the incidence of AIDS-defining infection [47][48][49]. Patients infected with TB and HIV are more likely to be minorities, of low SES, and smokers.
Figure 1 provides a schematic presentation of the factors that contribute to lung cancer health disparities.
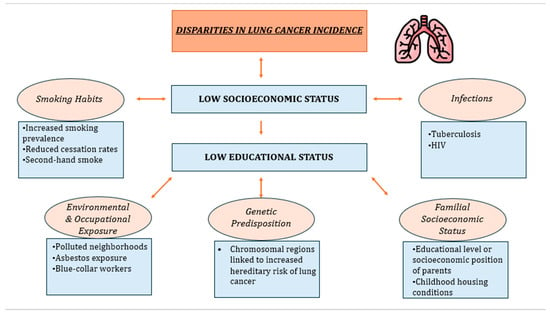
Figure 1. The key contributing factor to low SES is low education. Low educational level is associated with several factors, such as smoking habits, bad lifestyle behaviors, polluted neighborhoods, and genetic-familial risk, that lead to increased lung cancer incidence.
3. Conclusions
It is nowadays well established that there are socioeconomic inequalities regarding lung cancer incidence, screening, effective treatment, overall survival, and prognosis, which are primarily attributed to low education. Low educational level is correlated with several factors, such as smoking habits, bad lifestyle behaviors, polluted neighborhoods, and genetic-familial risk, that lead to increased lung cancer incidence. The battle against lung cancer is relentless. It is time that pivotal actions towards narrowing the disparities in patient care are initiated in an effort to ensure that prioritizing the defeat of cancer is not only a priority for Europe but for the entire world.
References
- International Agency for Research on Cancer. GLOBOCAN Lung Cancer Facts Sheet 2020; IARC: Lyon, France, 2021.
- Herdan, G. The Increase in the Mortality Due to Cancer of the Lung in the Light of the Distribution of the Disease among the Different Social Classes and Occupations. Br. J. Cancer 1958, 12, 492–506.
- Wiley, R.C.; Oliver, A.C.; Snow, M.B.; Bunn, J.Y.; Barrows, A.J.; Tidey, J.W.; Lee, D.C.; Sigmon, S.C.; Gaalema, D.E.; Heil, S.H.; et al. The Impact of the Covid-19 Pandemic on Smoking among Vulnerable Populations. Nicotine Tob. Res. 2023, 25, 282–290.
- Larsen, I.K.; Myklebust, T.Å.; Babigumira, R.; Vinberg, E.; Møller, B.; Ursin, G. Education, Income and Risk of Cancer: Results from a Norwegian Registry-Based Study. Acta Oncol. 2020, 59, 1300–1307.
- Warren, G.W.; Cummings, K.M. Tobacco and Lung Cancer: Risks, Trends, and Outcomes in Patients with Cancer. Am. Soc. Clin. Oncol. Educ. Book 2013, 33, 359–364.
- Samet, J.M. Tobacco Smoking: The Leading Cause of Preventable Disease Worldwide. Thorac. Surg. Clin. 2013, 23, 103–112.
- Wardle, J.; Steptoe, A. Socioeconomic Differences in Attitudes and Beliefs about Healthy Lifestyles. J. Epidemiol. Community Health 2003, 57, 440–443.
- Shankar, A.; Yau, C.; Wallbridge, I.G.; Saini, D.; Prasad, C.P.; Singh, P.; Kaur, J.; Roy, S.; Sinha, P. The Intersection of Tobacco Use, Health Disparities, and Inequalities in Lung Cancer Treatment and Survival. Indian J. Med. Paediatr. Oncol. 2022, 43, 289–293.
- Cao, P.; Jeon, J.; Tam, J.; Fleischer, N.L.; Levy, D.T.; Holford, T.R.; Meza, R. Smoking Disparities by Level of Educational Attainment and Birth Cohort in the US. Am. J. Prev. Med. 2023, 64, S22–S31.
- Fiore, M.C.; Novotny, T.E.; Pierce, J.P.; Hatziandreu, E.J.; Patel, K.M.; Davis, R.M. Trends in Cigarette Smoking in the United States: The Changing Influence of Gender and Race. JAMA 1989, 261, 49–55.
- Barbeau, E.M.; Leavy-Sperounis, A.; Balbach, E.D. Smoking, Social Class, and Gender: What Can Public Health Learn from the Tobacco Industry about Disparities in Smoking? Tob. Control 2004, 13, 115–120.
- Brown-Johnson, C.G.; England, L.J.; Glantz, S.A.; Ling, P.M. Tobacco Industry Marketing to Low Socioeconomic Status Women in the USA. Tob. Control 2014, 23, e139–e146.
- Kotz, D.; West, R. Explaining the Social Gradient in Smoking Cessation: It’s Not in the Trying, but in the Succeeding. Tob. Control 2009, 18, 43–46.
- Babb, S.; Malarcher, A.; Schauer, G.; Asman, K.; Jamal, A. Quitting Smoking among Adults—United States, 2000–2015. MMWR Morb. Mortal. Wkly. Rep. 2017, 65, 1457–1464.
- Lebrun-Harris, L.A.; Fiore, M.C.; Tomoyasu, N.; Ngo-Metzger, Q. Cigarette Smoking, Desire to Quit, and Tobacco-Related Counseling among Patients at Adult Health Centers. Am. J. Public Health 2015, 105, 180–188.
- Siahpush, M.; McNeill, A.; Borland, R.; Fong, G.T. Socioeconomic Variations in Nicotine Dependence, Self-Efficacy, and Intention to Quit across Four Countries: Findings from the International Tobacco Control (ITC) Four Country Survey. Tob. Control 2006, 15 (Suppl. S3), iii71–iii75.
- Onwuka, J.U.; Zahed, H.; Feng, X.; Alcala, K.; Johansson, M.; Robbins, H.A.; Consortium, L.C.C. Abstract 1950: Socioeconomic Status and Lung Cancer Incidence: An Analysis of Data from 15 Countries in the Lung Cancer Cohort Consortium. Cancer Res. 2023, 83, 1950.
- WHO. An Estimated 12.6 Million Deaths Each Year ARE Attributable to Unhealthy Environments. 2019. Available online: https://www.who.int/news-room/detail/15-03-2016-an-estimated-12-6-million-deaths-each-year-are-attributable-to-unhealthy-environments (accessed on 20 November 2023).
- International Agency for Research on Cancer. Health Impacts of Chemicals. World Health Organization. 2016. Available online: https://www.who.int/publications/i/item/WHO-FWC-PHE-EPE-16.01-eng (accessed on 20 November 2023).
- Corrales, L.; Rosell, R.; Cardona, A.F.; Martín, C.; Zatarain-Barrón, Z.L.; Arrieta, O. Lung Cancer in Never Smokers: The Role of Different Risk Factors Other than Tobacco Smoking. Crit. Rev. Oncol. Hematol. 2020, 148, 102895.
- Loomis, D.; Grosse, Y.; Lauby-Secretan, B.; Ghissassi, F.E.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Baan, R.; Mattock, H.; Straif, K. The Carcinogenicity of Outdoor Air Pollution. Lancet Oncol. 2013, 14, 1262–1263.
- Lelieveld, J.; Evans, J.S.; Fnais, M.; Giannadaki, D.; Pozzer, A. The Contribution of Outdoor Air Pollution Sources to Premature Mortality on a Global Scale. Nature 2015, 525, 367–371.
- Swanton, C.; Hill, W.; Lim, E.; Lee, C.; Weeden, C.E.; Augustine, M.; Chen, K.; Kuan, F.-C.; Marongiu, F.; Rodrigues, F.; et al. LBA1 Mechanism of Action and an Actionable Inflammatory Axis for Air Pollution Induced Non-Small Cell Lung Cancer: Towards Molecular Cancer Prevention. Ann. Oncol. 2022, 33, S1413.
- Hajat, A.; MacLehose, R.F.; Rosofsky, A.; Walker, K.D.; Clougherty, J.E. Confounding by Socioeconomic Status in Epidemiological Studies of Air Pollution and Health: Challenges and Opportunities. Environ. Health Perspect. 2021, 129, 65001.
- Miao, Q.; Chen, D.; Buzzelli, M.; Aronson, K.J. Environmental Equity Research: Review with Focus on Outdoor Air Pollution Research Methods and Analytic Tools. Arch. Environ. Occup. Health 2015, 70, 47–55.
- Hajat, A.; Hsia, C.; O’Neill, M.S. Socioeconomic Disparities and Air Pollution Exposure: A Global Review. Curr. Environ. Health Rep. 2015, 2, 440–450.
- Alberg, A.J.; Brock, M.V.; Ford, J.G.; Samet, J.M.; Spivack, S.D. Epidemiology of Lung Cancer: Diagnosis and Management of Lung Cancer, 3rd Ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2013, 143, e1S–e29S.
- Fang, W.-T.; Hassan, A.; LePage, B.A. Introduction to Environmental Education. In The Living Environmental Education; Springer: Berlin/Heidelberg, Germany, 2023; ISBN 978-981-19-4233-4.
- Uguen, M.; Dewitte, J.-D.; Marcorelles, P.; Loddé, B.; Pougnet, R.; Saliou, P.; De Braekeleer, M.; Uguen, A. Asbestos-Related Lung Cancers: A Retrospective Clinical and Pathological Study. Mol. Clin. Oncol. 2017, 7, 135–139.
- Vicari, K.; Ribeiro, I.M.; Aguiar, B.F.; Brey, C.; Boller, S.; Miranda, F.M.D. Occupational Characterization of Workers Exposed to Asbestos: An Integrative Review. Rev. Bras. Med. Trab. 2022, 20, 650–658.
- Lubin, J.H.; Boice, J.D.; Edling, C.; Hornung, R.W.; Howe, G.R.; Kunz, E.; Kusiak, R.A.; Morrison, H.I.; Radford, E.P.; Samet, J.M. Lung Cancer in Radon-Exposed Miners and Estimation of Risk from Indoor Exposure. J. Natl. Cancer Inst. 1995, 87, 817–827.
- Schubauer-Berigan, M.K.; Daniels, R.D.; Pinkerton, L.E. Radon Exposure and Mortality among White and American Indian Uranium Miners: An Update of the Colorado Plateau Cohort. Am. J. Epidemiol. 2009, 169, 718–730.
- Chaitanya Thandra, K.; Barsouk, A.; Saginala, K.; Sukumar Aluru, J.; Barsouk, A. Epidemiology of Lung Cancer. Współczesna Onkol. 2021, 25, 45–52.
- Counil, E.; Roblin, A.; Ismail, W.; Paris, C.; Luce, D. O-80 Towards Occupational Health Equity Metrics Estimating the Burden of Lung Cancer Attributed to Three Occupational Carcinogens by Socio-Economic Position. Occup. Environ. Med. 2023, 80, A36–A37.
- Dement, J.M.; Ringen, K.; Welch, L.S.; Bingham, E.; Quinn, P. Mortality of Older Construction and Craft Workers Employed at Department of Energy (DOE) Nuclear Sites. Am. J. Ind. Med. 2009, 52, 671–682.
- Stocks, S.J.; McNamee, R.; Carder, M.; Agius, R.M. The Incidence of Medically Reported Work-Related Ill Health in the UK Construction Industry. Occup. Environ. Med. 2010, 67, 574–576.
- Thuret, A.; Geoffroy-Perez, B.; Luce, D.; Goldberg, M.; Imbernon, E. A 26-Year Cohort Mortality Study of French Construction Workers Aged 20 to 64 Years. J. Occup. Environ. Med. 2007, 49, 546–556.
- Veglia, F.; Vineis, P.; Overvad, K.; Boeing, H.; Bergmann, M.; Trichopoulou, A.; Trichopoulos, D.; Palli, D.; Krogh, V.; Tumino, R.; et al. Occupational Exposures, Environmental Tobacco Smoke, and Lung Cancer. Epidemiol. Camb. Mass 2007, 18, 769–775.
- Collatuzzo, G.; Teglia, F.; Boffetta, P. Role of Occupation in Shaping Cancer Disparities. Cancers 2022, 14, 4259.
- Landi, M.T.; Chatterjee, N.; Yu, K.; Goldin, L.R.; Goldstein, A.M.; Rotunno, M.; Mirabello, L.; Jacobs, K.; Wheeler, W.; Yeager, M.; et al. A Genome-Wide Association Study of Lung Cancer Identifies a Region of Chromosome 5p15 Associated with Risk for Adenocarcinoma. Am. J. Hum. Genet. 2009, 85, 679–691.
- Yokota, J.; Shiraishi, K.; Kohno, T. Genetic Basis for Susceptibility to Lung Cancer: Recent Progress and Future Directions. Adv. Cancer Res. 2010, 109, 51–72.
- Thorgeirsson, T.E.; Geller, F.; Sulem, P.; Rafnar, T.; Wiste, A.; Magnusson, K.P.; Manolescu, A.; Thorleifsson, G.; Stefansson, H.; Ingason, A.; et al. A Variant Associated with Nicotine Dependence, Lung Cancer and Peripheral Arterial Disease. Nature 2008, 452, 638–642.
- Zhou, H.; Zhang, Y.; Liu, J.; Yang, Y.; Fang, W.; Hong, S.; Chen, G.; Zhao, S.; Zhang, Z.; Shen, J.; et al. Education and Lung Cancer: A Mendelian Randomization Study. Int. J. Epidemiol. 2019, 48, 743–750.
- Søndergaard, G.; Mortensen, L.H.; Andersen, A.-M.N.; Andersen, P.K.; Dalton, S.O.; Osler, M. Social Inequality in Breast, Lung and Colorectal Cancers: A Sibling Approach. BMJ Open 2013, 3, e002114.
- Wang, R.; Li, C.; Huo, Z.; Ge, F.; Zhong, R.; Jiang, Y.; Wen, Y.; Su, Z.; Liang, H.; Cheng, B.; et al. Family Socioeconomic Position and Lung Cancer Risk: A Meta-Analysis and a Mendelian Randomization Study. 2020. Available online: https://www.researchsquare.com/article/rs-89906/v1 (accessed on 20 November 2023).
- Brenner, D.R.; McLaughlin, J.R.; Hung, R.J. Previous Lung Diseases and Lung Cancer Risk: A Systematic Review and Meta-Analysis. PLoS ONE 2011, 6, e17479.
- Sigel, K.; Wisnivesky, J.; Gordon, K.; Dubrow, R.; Justice, A.; Brown, S.T.; Goulet, J.; Butt, A.A.; Crystal, S.; Rimland, D.; et al. HIV as an Independent Risk Factor for Incident Lung Cancer. AIDS 2012, 26, 1017–1025.
- D’Jaen, G.A.; Pantanowitz, L.; Bower, M.; Buskin, S.; Neil, N.; Greco, E.M.; Cooley, T.P.; Henry, D.; Stem, J.; Dezube, B.J.; et al. Human Immunodeficiency Virus-Associated Primary Lung Cancer in the Era of Highly Active Antiretroviral Therapy: A Multi-Institutional Collaboration. Clin. Lung Cancer 2010, 11, 396–404.
- Silverberg, M.J.; Lau, B.; Achenbach, C.J.; Jing, Y.; Althoff, K.N.; D’Souza, G.; Engels, E.A.; Hessol, N.A.; Brooks, J.T.; Burchell, A.N.; et al. Cumulative Incidence of Cancer among Persons with HIV in North America: A Cohort Study. Ann. Intern. Med. 2015, 163, 507–518.
More
Information
Subjects:
Health Policy & Services
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
887
Revision:
1 time
(View History)
Update Date:
29 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




