Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | RUXANDRA COSTEA | -- | 3128 | 2024-02-28 13:42:21 | | | |
| 2 | Rita Xu | Meta information modification | 3128 | 2024-02-29 03:39:50 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Costea, R.; Ene, I.; Pavel, R. Pig Sedation and Anesthesia. Encyclopedia. Available online: https://encyclopedia.pub/entry/55663 (accessed on 08 February 2026).
Costea R, Ene I, Pavel R. Pig Sedation and Anesthesia. Encyclopedia. Available at: https://encyclopedia.pub/entry/55663. Accessed February 08, 2026.
Costea, Ruxandra, Ioana Ene, Ruxandra Pavel. "Pig Sedation and Anesthesia" Encyclopedia, https://encyclopedia.pub/entry/55663 (accessed February 08, 2026).
Costea, R., Ene, I., & Pavel, R. (2024, February 28). Pig Sedation and Anesthesia. In Encyclopedia. https://encyclopedia.pub/entry/55663
Costea, Ruxandra, et al. "Pig Sedation and Anesthesia." Encyclopedia. Web. 28 February, 2024.
Copy Citation
Anesthesia plays a crucial role in ensuring the ethical treatment of research animals and obtaining reliable and accurate data. Pig anesthesia is a significant aspect of clinical veterinary practice, especially when performing surgical procedures, diagnostic imaging, various medical interventions, and scientific research procedures. Proper anesthesia protocols ensure that the animals are kept unconscious and do not experience pain or distress, which is not only ethically responsible but also needed by regulatory bodies and animal welfare standards.
sedation
anesthesia
pig
research models
1. Physical Examination
Anesthesia ensures the welfare of the animal, enables safe and effective procedures, and allows accurate data collection [1]. Pigs are commonly used in medical and scientific research as models for studying various aspects of human health, physiology, and disease due to their physiological and anatomical similarities to humans [2][3][4][5]. Pigs are known to be highly sensitive to stress; consequently, they should be conditioned at the research facility for approximately 7–14 days before anesthesia, in order to have time to adapt to the experimental environment, to avoid stress-induced respiratory disease or diarrhea [6][7].
Physical preanesthetic examination must be performed in a low-stress environment with a focus on evaluation of respiratory and cardiovascular system function. Age and maturity criteria should be considered when choosing a model. The majority of pigs utilized in research projects weigh 15 to 30 kg and are 8 to 12 weeks old [6]. The decision to withhold food and water preoperatively in pigs should involve consideration of the animals’ age, growth rate, breed, pregnancy status, clinical status, and the procedure to be performed. Food and water withdrawal regimens have a wide variation of 2–12 h, with particularly aggressive fasting regimes for gastrointestinal or abdominal surgery [8]. Although fasting may reduce the risk of regurgitation, fasting is recommended, as aspiration of regurgitated material can occur and may cause airway obstruction, irritation, and ultimately aspiration pneumonia. Aspiration of acidic stomach fluid may cause immediate reflexive airway closure and destruction of type II alveolar cells and pulmonary capillary lining cells. Consequently, pulmonary edema and hemorrhage may develop along with bronchospasm, dyspnea, hypoxemia, and cyanosis. Recovery from aspiration pneumonia, which may take a few days to develop, depends on the pH of the material aspirated. Swine tend to have very acidic stomach fluid with a pH as low as 1.5–2.5 [9][10]. Alfalfa and other types of hay can delay gastric emptying time, which means that vomiting and aspiration may still occur even after a 12-h fasting period. To avoid this, alfalfa or other forms of hay should be eliminated from the regular diet 2–3 days before general anesthesia [11]. Piglets, who are prone to hypoglycemia, should be denied suckling for only 1–2 h before anesthetic induction [9].
2. Recommendations for Injectable Administration
Injections should be performed slowly, if possible, to minimize pain associated with injection and tissue damage [12]. The dimensions of the needle must be selected with consideration of the size of the animal and the liquid consistency of the injectate (aqueous or oily). For subcutaneous (SC) or intramuscular injections (IM), an extension line can be used to connect the syringe and cannula to reduce the risk associated with any evasive movements of the pig [13]. As the skin of swine can only be tented to a minor degree, only small-volume SC injections can be delivered [14]. Two locations are suitable for SC injections and are recommended: the knee fold (body weight under 20 kg) or caudal to the ear base for larger pigs [13][15]. The muscles of the caudal thigh region, semimembranosus and semitendinosus, and the gluteal muscles of the cranial thigh are generally selected as suitable sites for large-volume intramuscular injections (IM), while for small volumes to be injected, it is preferred to access the dorsolateral neck region. The injection can be performed in a less stressful way for the pig if it is possible to feed it simultaneously [15][16]. Intravenous access (IV) can be challenging because pigs resist restraint and they have very few superficial veins accessible for IV injection or catheterization [9][16]. The auricular veins, jugular vein, and femoral vein are all commonly used for drawing blood or administering fluids in pigs. The auricular veins located on the lateral and medial dorsal ear margins offer the easiest access for intravenous injection [17][18]. Topical application of a eutectic mixture of lidocaine 2.5% and prilocaine 2.5% for anesthesia has been used for various procedures in human medicine and although studies in animals are limited, it appears to facilitate various procedures in veterinary medicine, including venipuncture [19]. Puncture of the ear vein requires physical restraint of the swine or heavy sedation. After occluding blood flow at the base of the ear, the vessels are easy to identify (Figure 1). Catheterization of the jugular or femoral veins can be challenging and should only be performed by experienced personnel [13][14].
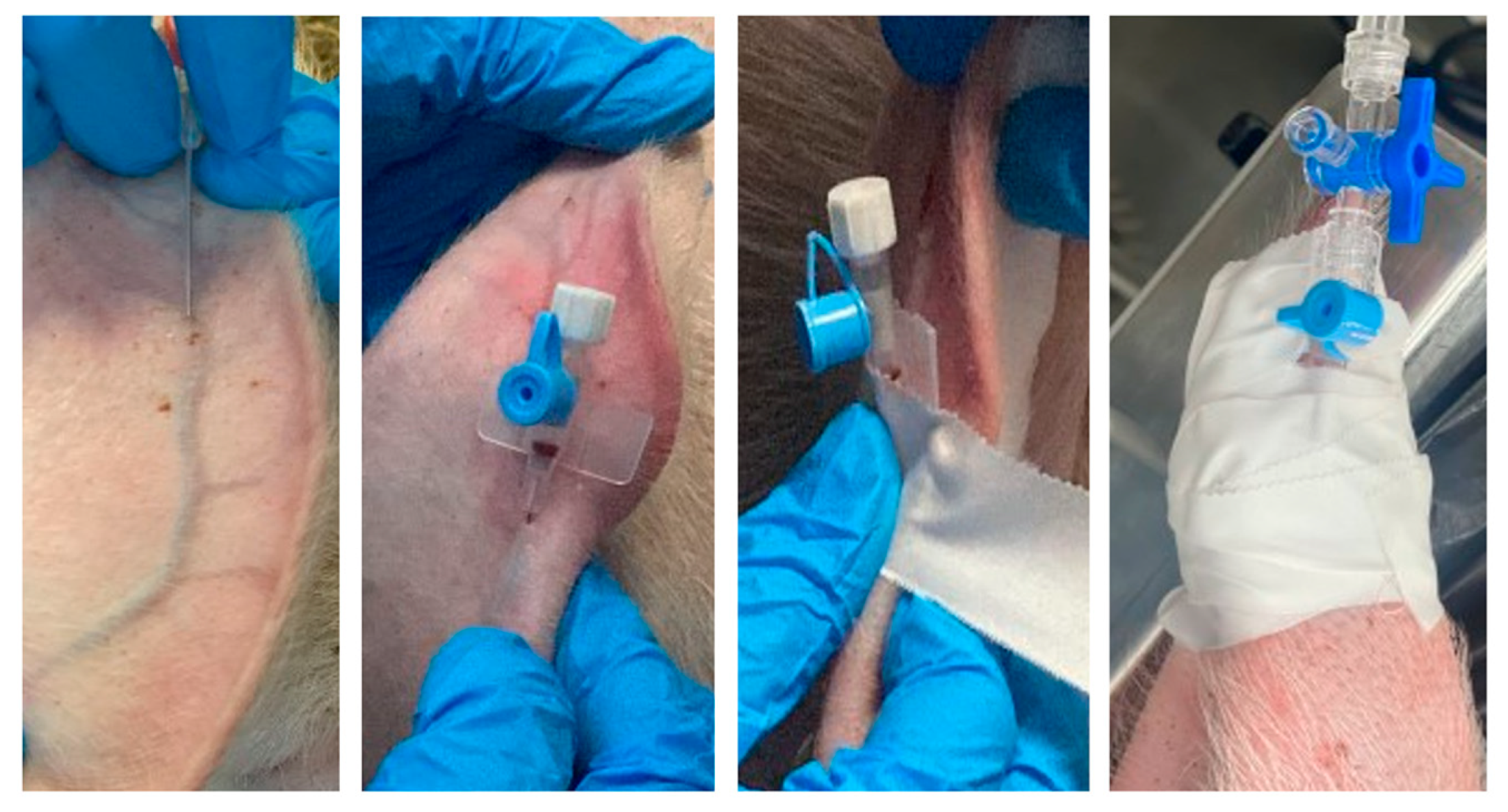
Figure 1. Mounting and fixing a peripheral catheter in the auricular vein.
3. Sedation and Premedication
Sedation is often suitable for minor procedures, such as physical examination and diagnostic imaging. or it represents premedication for anesthesia. The choice of an appropriate sedative protocol should be based on the procedure’s type, animal health status, age, and size. Other factors, such as the desired level of sedation and the duration of the procedure, influence the selection of medication. Sedatives should be administered at the lowest effective dose to minimize the risk of adverse effects and calculated based on the pig’s weight. After sedation, pigs may still need some level of physical restraint to ensure the safety of both the animal and the people involved in the procedure [14][20].
Stress during handling and restraint can lead to increased vocalizations, making the process of injection of sedative drugs challenging. Small pigs (<10 kg) may be more easily restrained compared to larger ones and less prone to stress-related vocalizations [1].
Multiple classes of agents may be considered for sedation in pigs. Short, minimally invasive procedures may require lighter sedation with a focus on anxiolysis, achieved through benzodiazepines and alpha-2 adrenoreceptor agonists. Some examples of sedation protocols include azaperone, acepromazine, diazepam, midazolam, xylazine, and medetomidine, used alone or in combination. For more invasive surgeries, a combination of sedatives, analgesics, and anesthetics may be employed to ensure deep sedation, pain control, and a stable anesthetic plane. When deeper sedation is necessary, ketamine can be added to the combinations. The combination of tiletamine and zolazepam produces heavy sedation and immobilization with a relatively small volume of injection, making it particularly suitable for larger animals [1]. If pain is present or anticipated for the procedure, the protocols may also include opioids such as buprenorphine, morphine, or methadone.
Premedication refers to the administration of medications prior to the induction of anesthesia, minimizing stress and anxiety, providing pre-emptive analgesia, and optimizing the induction and maintenance of anesthesia. The ideal premedication agent must be effective with predictable results and fast onset, easy to administer, reversible, and offer analgesia and muscle relaxation with minimum cardiovascular and respiratory depression. Medication and protocols will be decided based on the preanesthetic evaluation (ASA status, temperament, procedure, level of pain expected), anesthetist’s level of experience, and equipment available [12].
Protocols for premedication usually include multiple agents, to achieve the maximum effect with minimum secondary effects. The use of the anticholinergics glycopyrrolate and atropine has the potential to reduce salivation and bronchial secretions, but should be performed with caution considering their cardiovascular effects [14].
In the authors’ practice, the most common combination for sedative drugs used for pigs includes IM administration of ketamine (10–20 mg/kg), xylazine (1–2 mg/kg), and midazolam (0.1–0.2 mg/kg), with alternative combinations that include medetomidine or dexmedetomidine [20]. The lower doses are usually used for sedation and the higher are intended for anesthetic premedication.
3.1. Butyrophenones
Azaperone is a neuroleptic sedative medication that belongs to the class of butyrophenone derivatives. It is widely used in pigs to provide sedation, reduce anxiety, calm animals, and combat aggression and stress in pigs [21][22]. Azaperone works at central adrenergic dopamine D2 receptors located in the reticular activating system, leading to its sedative and anti-anxiety effects [23]. Vasodilation, hypotension, and hypothermia may occur following the administration of azaperone so it should not be used in debilitated, hypovolemic, or hypotensive pigs. It can also be used for maiden sows after their first litter to reduce the rejection of piglets [24]. Azaperone given alone by the intramuscular route has a rapid onset of action (5–20 min) with a duration of action of 2–6 h (maximal effects within 30 min), while intravenous injection often results in excitation [9]. Oral or intranasal administration of azaperone at a dose of 4 mg/kg induces sedation in piglets that is clinically comparable to an intramuscular administration of 2 mg/kg [25][26]. Deeper sedation with fewer adverse effects can be achieved by combining azaperone with ketamine and butorphanol [24][27] or azaperone with ketamine and an alpha-2 adrenoreceptor agonist [28][29]. Susceptible Pietrain pigs were protected against halothane-induced malignant hyperthermia with azaperone at doses of 0.5–2 mg/kg IM [11][30].
3.2. Phenothiazines
Acepromazine (0.11–1.1 mg/kg IM, IV, SC) is commonly used alone for tranquilization [31]. This drug decreases spontaneous motor activity and may cause hypotension and hypothermia [9]. The recommended dose of 0.1–0.4 mg/kg IV or IM may be used in combination with other drugs to improve the quality of premedication [29]. The combination of acepromazine with ketamine or tiletamine/zolazepam produces reliable sedation and muscle relaxation [22]. Acepromazine 1.1–1.65 mg/kg IM has been reported to reduce the incidence of malignant hyperthermia related to anesthesia [30][32].
3.3. Benzodiazepines
Benzodiazepines are a class of sedative and anxiolytic drugs that are commonly used in both human and veterinary medicine. They work by enhancing the effects of a neurotransmitter called gamma-aminobutyric acid (GABA), which leads to sedative, anxiolytic (anti-anxiety), muscle relaxant, and anticonvulsant effects [33]. Midazolam, when compared with diazepam, is water-soluble, is absorbed rapidly, has a higher affinity for receptors, stronger potency, and quicker onset with a shorter duration of effect [9]. Diazepam and midazolam can be used in combination with ketamine, alpha-2 adrenoreceptor agonists, and opioids. When used in combination with ketamine, muscle relaxation will be improved during anesthesia [22], and when used in combination with alfaxalone (5 mg/kg IM), muscle relaxation and sedation levels increase [9]. Intranasal administration of midazolam (0.2 mg/kg) provides reliable sedation (effect in 3–4 min) [34]. Less commonly used benzodiazepines include flurazepam 2 mg/kg IV [33] and lorazepam 0.1 mg/kg [14]. Flumazenil 0.02–0.08 mg/kg is a selective benzodiazepine antagonist reversal agent that can be used to counteract the effects of benzodiazepines in cases of overdose or adverse reactions, or to facilitate recovery from sedation or anesthesia [35].
3.4. Alpha-2 Adrenoreceptor Agonists
Alpha-2 adrenoreceptor agonists are a class of medications that activate specific receptors in the body. These medications have various effects, including sedation, analgesia, muscle relaxation, and vasoconstriction. Alpha-2 adrenoreceptor agonists are often used for sedation, preanesthetic medication, and pain management in pigs, alone or as part of a balanced anesthesia protocol in combination with other medication, such as anesthetics and analgesics [36]. Pigs are more resistant to alpha-2 adrenoceptor agonists than ruminants and other domestic animals and require a higher dosage for mild to moderate sedation [36][37]. While alpha-2 agonists have beneficial effects, they can also cause side effects such as bradycardia, decreased respiratory rate, hypotension, decreased gastrointestinal motility, and hypothermia. Reversal agents (e.g., atipamezole, yohimbine, tolazoline, vatinoxan) are available to antagonize the effects of the alpha-2 adrenoceptor agonists [22].
Intramuscular administration of medetomidine at doses ranging from 0.04 to 0.08 mg/kg induced sedation and muscle relaxation, with an increasing effect observed at higher doses [38]. However, increasing the dose above 0.1 mg/kg did not further intensify sedation or muscle relaxation, but instead prolonged the duration of these effects. Medetomidine (0.04 mg/kg IV or 0.08 mg/kg IM) in combination with ketamine has been utilized in pigs for short-term anesthesia [38]. Medetomidine, when combined with butorphanol (0.2 mg/kg IM) and ketamine (10 mg/kg IM), produced prolonged anesthesia in pigs compared to a combination of xylazine (2 mg/kg IM), butorphanol (0.2 mg/kg IM), and ketamine (10 mg/kg IM). The achieved muscle relaxation was adequate for tracheal intubation, but moderate cardiovascular depression was observed after using the combination of medetomidine, butorphanol, and ketamine for anesthesia [39]. In a specific study involving young pigs, the administration of a combination of 0.08 mg/kg medetomidine and 0.2 mg/kg butorphanol did not provide adequate sedation to facilitate blood sampling in all animals [40].
3.5. Dissociative Anesthetics
Ketamine is an NMDA (N-methyl D aspartate) receptor antagonist drug that can be used for sedation in pigs. It works by antagonizing the effects of the neurotransmitter glutamate, resulting in sedation, analgesia, and dissociation from the environment. Ketamine is often used in combination with other medications to achieve the desired level of sedation or anesthesia. Ketamine can cause side effects such as increased muscle tone, muscle fasciculations, poor muscle relaxation, and analgesia when used alone [22]. Occasionally, pigs may experience a period of disorientation and ataxia during recovery from ketamine sedation and might need a comfortable environment to prevent injury during this phase. These effects can be managed and minimized through appropriate dosing and the use of ketamine combined with other medications [41]. In healthy animals, ketamine has a good analgesic effect and only slightly modifies heart rate. When ketamine is administered alone, the ability of the swallowing reflex is unaffected, but excitation and excessive salivation can develop during anesthesia and recovery [24]. Tiletamine is a dissociative anesthetic used in veterinary medicine in combination with zolazepam (Telazol® tiletamine/zolazepam) to induce sedation or anesthesia in pigs. Tiletamine is approximately twice as potent as ketamine and has a longer duration of action [42]. Telazol® (tiletamine/zolazepam, 4.4 mg/kg) and xylazine (2.2 mg/kg) IM provide rapid sedation and can be used for sedation and induction [37]. Pigs often experience prolonged and rough recovery characterized by swimming motions, with repeated attempts to right themselves when recovering from Telazol anesthesia, similar to that observed when ketamine is used alone [30][43]. Studies have shown that tiletamine and zolazepam are both eliminated more slowly in pigs than in other species and that tiletamine has a longer effect than zolazepam in pigs [43]. Flumazenil can be used to antagonize zolazepam, but care should be granted to avoid residual effects of tiletamine leading to excitation, muscular tone, and fasciculations [35][44].
3.6. Opioids
Opioids are a class of medication commonly used for pain management and sedation in pigs, acting by binding to specific receptors in the nervous system (opioid receptors), which results in pain relief, sedation, and other effects [20][23]. Opioids can be used for sedation in pigs, particularly for pain management and calming effects. Opioids can be used in combination with other sedatives, anesthetics, or analgesics to achieve the desired level of sedation and pain control; pure µ agonists result in a strong analgesic effect, and partial μ agonists can be used in protocols for moderate pain along with μ- antagonists/K-agonists. Opioids can cause side effects such as vocalization, excitations, respiratory depression, decreased heart rate, and constipation. Butorphanol, administered at 0.2 mg/kg intramuscularly, resulted in important behavioral changes in piglets, resembling panic attacks, which have not been described in this species before [45]. The administration of buprenorphine did not decrease piglet vocalizations during the castration procedure but proved to be highly successful in mitigating pain behaviors [46]. In the post-surgery recovery, buprenorphine alleviated pain related to different surgical procedures, but had reduced effectiveness in addressing pain symptoms associated with inflammation, organ failure, or systemic disease when compared to pain associated with surgical incisions, orthopedic, dental, or ophthalmic procedures [47]. Buprenorphine has a relatively long duration of effect and low rate of side effects, but doses higher than 0.01 mg/kg must be used bearing in mind a possible respiratory depression [13]. Fentanyl, a short-acting opioid, can be used in pigs as a constant intravenous infusion at rates varying from 10 to 100 µg/kg/h without major side effects [36]. Boluses of morphine and fentanyl infusions will decrease the minimum alveolar concentration (MAC) levels of isoflurane [30]. Fentanyl and buprenorphine can also be used as transdermal patches, providing long-term analgesia, with a reduced incidence of side effects [48]. An example of ensuring preoperative and postoperative analgesia is represented by the protocol consisting of epidural morphine (0.1 mg/kg) prior to abdominal surgery, and a transdermal fentanyl patch (50 mg/h) postoperatively, which contributes to almost immediate restoration of normal activity levels and weight gain after recovery from general anesthesia [49]. Reversal agents available (e.g., antagonist naloxone 0.5–2 mg/kg IV [31]) can counteract negative side effects of opioids and can be used in unexpected reactions or overdose. In these cases, analgesic effects will also be reversed.
3.7. Alfaxalone
Alfaxalone is a neurosteroid anesthetic agent used for sedation, induction, and maintenance of anesthesia, with a rapid onset and relatively short duration of action. Alfaxalone can be administered both IV and IM in pigs [50][51]. Alfaxalone can cause side effects such as respiratory depression, decreased heart rate, and a decrease in blood pressure. Alfaxalone has been used in pigs to induce and maintain anesthesia with minimal cardiovascular effects [45][52]. A combination of alfaxalone and dexmedetomidine can be used to maintain long-duration total intravenous anesthesia in pigs [53][54].
3.8. Local Anesthetics
Lidocaine and bupivacaine are local anesthetic medications commonly used for various purposes in pigs, including local anesthesia for surgical procedures, postoperative pain management, and nerve blocks [55]. While local anesthetics are generally well-tolerated, some pigs may experience hypersensitivity or allergic reactions to the medications [23]. Careful observation of adverse reactions is important [56]. Lidocaine is widely used intravenously in different species to provide analgesia and as an adjunct to general anesthesia. In one experimental model of lung transplantation, intravenous lidocaine was associated with an attenuation of the histological markers of lung damage in the early stages of reperfusion [57]. Administration of lidocaine may help to prevent lung injury during surgery with one lung ventilation, reducing the expression of proinflammatory cytokines and lung apoptosis [58].
3.9. Neurokinin-1 (NK-1) Receptor Antagonists—Maropitant
Maropitant is a potent, selective neurokinin (NK-1) receptor antagonist primarily administered before anesthetic premedication (1 mg/kg q 24 h, IM) as an antiemetic medication [59]. The MAC of sevoflurane is decreased by maropitant, indicating a potential role as an adjunct visceral analgesic, as demonstrated in other animals [60]. Thus, there is a potential for future applications for swine.
3.10. Non-Depolarizing Neuromuscular Blocking Agents (NMBs)
In biomedical research, the use of non-depolarizing neuromuscular blocking agents (NMBs) involves profound muscle relaxation and prevents accidental awareness in conditions of inadequate anesthesia or analgesia. NMBs are widely recommended for tracheal intubation, which is relatively difficult in swine. Studies are quite controversial regarding the achievement of these objectives [61]. When using NMBs, pigs must be unconscious and controlled ventilation must be used. NMBs are not recommended for routine use or without advanced monitoring, which includes measuring arterial blood pressure and neuromuscular blockade assessment with a peripheral nerve stimulator. The NMBs can be administrated as boluses or continuous-rate infusions. Reversal of the neuromuscular blockade involves administration of an acetylcholinesterase inhibitor (neostigmine, edrophonium), which can also generate side effects such as bradycardia and gastrointestinal stimulation. To reduce parasympathetic stimulation, it is recommended to administer an anticholinergic (atropine, glycopyrrolate) before the antagonization of the NMBs. The most common NMBs used are pancuronium, vecuronium, atracurium, and rocuronium. Although monitoring of neuromuscular blockade is possible in pigs, neuromuscular blockade is rarely objectively monitored and is often administered based on clinical signs such as the return of spontaneous ventilation [62].
References
- Flecknell, P. Laboratory Animal Anaesthesia; Academic Press: Cambridge, MA, USA, 2015; pp. 238–239.
- Sullivan, T.P.; Eaglstein, W.H.; Davis, S.C.; Mertz, P. The Pig as a Model for Human Wound Healing. Wound Repair Regen. 2001, 9, 66–76.
- Kuzmuk, K.N.; Schook, L.B. Pigs as a Model for Biomedical Sciences. In The Genetics of the Pig; CABI: Wallingford, UK, 2011; pp. 426–444.
- Lunney, J.K.; Van Goor, A.; Walker, K.E.; Hailstock, T.; Franklin, J.; Dai, C. Importance of the Pig as a Human Biomedical Model. Sci. Transl. Med. 2021, 13, eabd5758.
- Clark, S.C.; Sudarshan, C.D.; Khanna, R.; Roughan, J.V.; Flecknell, P.A.; Dark, J.H. A New Porcine Model of Reperfusion Injury after Lung Transplantation. Lab. Anim. 1999, 33, 135–142.
- Smith, A.C.; Swindle, M.M. Preparation of swine for the laboratory. ILAR J. 2006, 47, 358–363.
- Grandin, T. Minimizing Stress in Pig Handling in the Research Lab. Lab Anim. 1986, 15, 15–20.
- Bradbury, A.G.; Clutton, R.E. Review of practices reported for preoperative food and water restriction of laboratory pigs (Sus scrofa). J. Am. Assoc. Lab. Anim. Sci. 2016, 55, 35–40.
- Anderson, D.E.; Mulon, P.Y. Anesthesia and Surgical Procedures in Swine. In Diseases of Swine; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019; pp. 171–196.
- DeRouchey, J.; Goodband, B.; Tokach, M.; Dritz, S.; Nelssen, J. Digestive System of the Pig: Anatomy and Function. N. Am. Vet. Commun. Conf. 2009, 23, 375–376.
- Lin, H. Perioperative Monitoring and Management of Complications. In Farm Animal Anesthesia: Cattle, Small Ruminants, Camelids, and Pigs; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2022; pp. 135–158.
- Portier, K.; Ida, K.K. The ASA Physical Status Classification: What Is the Evidence for Recommending Its Use in Veterinary Anesthesia?—A Systematic Review. Front. Vet. Sci. 2018, 5, 204.
- Kaiser, G.M.; Heuer, M.M.; Frühauf, N.R.; Kühne, C.A.; Broelsch, C.E. General Handling and Anesthesia for Experimental Surgery in Pigs. J. Surg. Res. 2006, 130, 73–79.
- Smith, A.C.; Ehler, W.J.; Swindle, M.M. Anesthesia and Analgesia in Swine. In Anesthesia and Analgesia in Laboratory Animals; Elsevier: Amsterdam, The Netherlands, 1997; pp. 313–336.
- Hedenqvist, P. Laboratory animal analgesia, anesthesia, and euthanasia. In Handbook of Laboratory Animal Science; CRC Press: Boca Raton, FL, USA, 2021; pp. 343–378.
- Xanthos, T.; Bassiakou, E.; Koudouna, E.; Tsirikos-Karapanos, N.; Lelovas, P.; Papadimitriou, D.; Dontas, I.; Papadimitriou, L. Baseline Hemodynamics in Anesthetized Landrace–Large White Swine: Reference Values for Research in Cardiac Arrest and Cardiopulmonary Resuscitation Models. J. Am. Assoc. Lab. Anim. Sci. 2007, 46, 21–25.
- Dyce, K.M.; Sack, W.O.; Wensing, C.J.G. Textbook of Veterinary Anatomy; Saunders Company: Philadelphia, PA, USA, 2002; pp. 400–401.
- Singh, B. Dyce, Sack, and Wensing’s Textbook of Veterinary Anatomy; Saunders: St. Louis, MI, USA, 2018.
- Erkert, R.S.; MacAllister, C.G. Use of a eutectic mixture of lidocaine 2.5% and prilocaine 2.5% as a local anesthetic in animals. J. Am. Vet. Med. Assoc. 2005, 226, 1990–1992.
- Costea, R.; Tudor, R.; Degan, A.; Girdan, G. Anesthesia Complications Related to Swine Experimental Invasive Surgical Procedures. Sci. Works. Ser. C Vet. Med. 2019, 65, 2065-1295.
- Gruen, M.E.; Sherman, B.L.; Papich, M.G. Drugs Affecting Animal Behavior; John Willey & Sons: Hoboken, NJ, USA, 2018.
- Lin, H. Injectable Anesthetics and Field Anesthesia. In Farm Animal Anesthesia; John Wiley & Sons: Hoboken, NJ, USA, 2022; pp. 60–100. ISBN 978-1-119-67266-1.
- Golan, D.E.; Tashjian, A.H.; Armstrong, E.J. Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011.
- Hodgkinson, O. Practical Sedation and Anaesthesia in Pigs. Practice 2007, 29, 34–39.
- Svoboda, M.; Fajt, Z.; Mruvčinská, M.; Vašek, J.; Blahová, J. The Effects of Buccal Administration of Azaperone on the Sedation Level and Biochemical Variables of Weaned Piglets. Acta Vet. Brno 2021, 90, 47–56.
- Svoboda, M.; Blahova, J.; Jarkovsky, J.; Zacharda, A.; Hajkova, S.; Vanhara, J.; Vasek, J. Efficacy of the Intranasal Application of Azaperone for Sedation in Weaned Piglets. Vet. Med. 2023, 68, 145–151.
- Nussbaumer, I.; Indermühle, N.; Zimmermann, W.; Leist, Y. Piglet Castration by Injection Anaesthesia: Experience with the Azaperone, Butorphanol and Ketamine Combination. SAT Schweiz. Arch. Für Tierheilkd. 2011, 153, 33–35.
- Short, C.E. Preanesthetic medications in ruminants and swine. Vet. Clin. N. Am. Food Anim. Pract. 1986, 2, 553–566.
- Swine, L.M. Veterinary Anesthesia and Analgesia: The Fifth Edition of Lumb and Jones; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 928–940.
- Moon, P.F.; Smith, L.J. General Anesthetic Techniques in Swine. Vet. Clin. N. Am. Food Anim. Pract. 1996, 12, 663–691.
- Swindle, M.M. Swine in the Laboratory: Surgery, Anesthesia, Imaging, and Experimental Techniques; CRC Press: Boca Raton, FL, USA, 2007.
- McGrath, C.J.; Rempel, W.E.; Addis, P.B.; Crimi, A.J. Acepromazine and Droperidol Inhibition of Halothane-Induced Malignant Hyperthermia (Porcine Stress Syndrome) in Swine. Am. J. Vet. Res. 1981, 42, 195–198.
- Lacoste, L.; Bouquet, S.; Ingrand, P.; Caritez, J.C.; Carretier, M.; Debaene, B. Intranasal Midazolam in Piglets: Pharmacodynamics (0.2 vs. 0.4 mg/kg) and Pharmacokinetics (0.4 mg/kg) with Bioavailability Determination. Lab. Anim. 2000, 34, 29–35.
- de Souza Dantas, L.M.; Crowell-Davis, S.L. Benzodiazepines. In Veterinary Psychopharmacology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019; pp. 67–102.
- Lee, J.Y.; Kim, M.C. Anesthesia of Growing Pigs with Tiletamine-Zolazepam and Reversal with Flumazenil. J. Vet. Med. Sci. 2012, 74, 335–339.
- Thurmon, J.C.; Smith, G.W. Swine. In Lumb and Jones’ Veterinary Anesthesia and Analgesia, 4th ed.; Tranquili, W.J., Thurmon, J.C., Grimm, K.A., Eds.; Blackwell Publishing: Ames, IA, USA, 2007; pp. 747–764.
- Riebold, T.; Geiser, D.; Goble, D.O. Anesthetic agents and ancillary drugs. In Large Animal Anesthesia; Iowa State University Press: Ames, IA, USA, 1995; pp. 11–64.
- Nishimura, R.; Kim, H.; Matsunaga, S.; Sakaguchi, M.; Sasaki, N.; Tamura, H.; Takeuchi, A. Antagonism of Medetomidine Sedation by Atipamezole in Pigs. J. Vet. Med. Sci. 1992, 54, 1237–1240.
- Sakaguchi, M.; Nishimura, R.; Sasaki, N.; Ishiguro, T.; Tamura, H.; Takeuchi, A. Anesthesia Induced in Pigs by Use of a Combination of Medetomidine, Butorphanol, and Ketamine and Its Reversal by Administration of Atipamezole. Am. J. Vet. Res. 1996, 57, 529–534.
- Ugarte, C.E.; O’Flaherty, K. The use of a medetomidine, butorphanol and atropine combination to enable blood sampling in young pigs. N. Z. Vet. J. 2005, 53, 249–252.
- Bettschart-Wolfensberger, R.; Stauffer, S.; Hässig, M.; Flaherty, D.; Ringer, S.K. Racemic Ketamine in Comparison to S-Ketamine in Combination with Azaperone and Butorphanol for Castration of Pigs. Schweiz. Arch. Tierheilkd. 2013, 155, 669–675.
- Lester, P.A.; Moore, R.M.; Shuster, K.A.; Myers, D.D. Anesthesia and Analgesia. In The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents; Elsevier: Amsterdam, The Netherlands, 2012; pp. 33–56.
- Kumar, A.; Mann, H.J.; Remmel, R.P. Pharmacokinetics of Tiletamine and Zolazepam (Telazol®) in Anesthetized Pigs. J. Vet. Pharmacol. Ther. 2006, 29, 587–589.
- Swindle, M.M.; Sistino, J.J.; Perioperative Care. Swine in the Laboratory: Surgery, Anesthesia, Imaging, and Experimental Techniques; CRC Press: Boca Raton, FL, USA, 2015; p. 39.
- Pavlovsky, V.H.; Corona, D.; Hug, P.J.; Kümmerlen, D.; Graage, R.; Bettschart-Wolfensberger, R. Butorphanol induces anxiety-like behaviour and distress in piglets. Schweiz. Arch. Für Tierheilkd. 2021, 163, 485–491.
- Viscardi, A.V.; Turner, P.V. Efficacy of buprenorphine for management of surgical castration pain in piglets. BMC Vet. Res. 2018, 14, 318.
- Rodriguez, N.A.; Cooper, D.M.; Risdahl, J.M. Antinociceptive activity of and clinical experience with buprenorphine in swine. J. Am. Assoc. Lab. Anim. Sci. 2001, 40, 17–20.
- Lujan, S.O.; Habre, W.; Daali, Y.; Pan, Z.; Kronen, P.W. Plasma concentrations of transdermal fentanyl and buprenorphine in pigs (Sus scrofa domesticus). Vet. Anaesth. Analg. 2017, 44, 665–675.
- Malavasi, L.M.; Nyman, G.; Augustsson, H.; Jacobson, M.; Jensen-Waern, M. Effects of epidural morphine and transdermal fentanyl analgesia on physiology and behaviour after abdominal surgery in pigs. Lab. Anim. 2006, 40, 16–27.
- Keates, H. Induction of Anaesthesia in Pigs Using a New Alphaxalone Formulation. Vet. Rec. 2003, 153, 627–628.
- Santos, M.; de Lis, B.T.B.; Tendillo, F.J. Effects of Intramuscular Dexmedetomidine in Combination with Ketamine or Alfaxalone in Swine. Vet. Anaesth. Analg. 2016, 43, 81–85.
- Bigby, S.E.; Carter, J.E.; Bauquier, S.; Beths, T. The Use of Alfaxalone for Premedication, Induction and Maintenance of Anaesthesia in Pigs: A Pilot Study. Vet. Anaesth. Analg. 2017, 44, 905–909.
- Kat, I.; Ahern, B.J.; Dhanani, J.; Whitten, G.; Cowling, N.; Goodwin, W. Long Duration Anaesthesia in Pigs with an Infusion of Alfaxalone and Dexmedetomidine. Vet. Med. Sci. 2022, 8, 2418–2421.
- Lervik, A.; Toverud, S.F.; Krontveit, R.; Haga, H.A. A Comparison of Respiratory Function in Pigs Anaesthetised by Propofol or Alfaxalone in Combination with Dexmedetomidine and Ketamine. Acta Vet. Scand. 2020, 62, 14.
- Clarke, K.W.; Trim, C.M. Veterinary Anaesthesia E-Book; Elsevier Health Sciences: Oxford, UK, 2013.
- Satas, S.; Johannessen, S.I.; Hoem, N.-O.; Haaland, K.; Sorensen, D.R.; Thoresen, M. Lidocaine Pharmacokinetics and Toxicity in Newborn Pigs. Anesth. Analg. 1997, 85, 306.
- Romera, A.; Cebollero, M.; Romero-Gómez, B.; Carricondo, F.; Zapatero, S.; García-Aldao, U.; Martín-Albo, L.; Ortega, J.; Vara, E.; Garutti, I.; et al. Effect of intravenous lidocaine on inflammatory and apoptotic response of ischemia-reperfusion injury in pigs undergoing lung resection surgery. BioMed Res. Int. 2021, 2021, 6630232.
- Garutti, I.; Rancan, L.; Simón, C.; Cusati, G.; Sanchez-Pedrosa, G.; Moraga, F.; Olmedilla, L.; Lopez-Gil, M.T.; Vara, E. Intravenous lidocaine decreases tumor necrosis factor alpha expression both locally and systemically in pigs undergoing lung resection surgery. Anesth. Analg. 2014, 119, 815–828.
- Smith, J.S.; Gebert, J.E.; Ebner, L.S.; Bennett, K.O.; Collins, R.J.; Hampton, C.E.; Kleine, S.A.; Mulon, P.-Y.; Smith, C.K.; Seddighi, R. Pharmacokinetics of Intramuscular Maropitant in Pigs (Sus scrofa domesticus). J. Vet. Pharmacol. Ther. 2023, 46, 158–164.
- Hay Kraus, B.L. Spotlight on the perioperative use of maropitant citrate. Vet. Med. Res. Rep. 2017, 8, 41–51.
- Bradbury, A.G.; Clutton, R.E. Are neuromuscular blocking agents being misused in laboratory pigs? Br. J. Anaesth. 2016, 116, 476–485.
- Pedersen, K.; Kruhøffer, L.L.; Lykkesfeldt, J.; Kousholt, B.S. Comparison of the neuromuscular effects of two infusion rates of rocuronium in anesthetized pigs. Acta Vet. Scand. 2022, 64, 38.
More
Information
Subjects:
Veterinary Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Revisions:
2 times
(View History)
Update Date:
29 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




