Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Bianca Diedericks | -- | 1560 | 2024-02-28 07:09:11 | | | |
| 2 | Lindsay Dong | Meta information modification | 1560 | 2024-02-29 08:55:51 | | | | |
| 3 | Lindsay Dong | Meta information modification | 1560 | 2024-03-01 09:21:19 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Diedericks, B.; Kok, A.; Mandiwana, V.; Lall, N. 7-Methyljuglone in Tuberculosis Treatment. Encyclopedia. Available online: https://encyclopedia.pub/entry/55595 (accessed on 08 February 2026).
Diedericks B, Kok A, Mandiwana V, Lall N. 7-Methyljuglone in Tuberculosis Treatment. Encyclopedia. Available at: https://encyclopedia.pub/entry/55595. Accessed February 08, 2026.
Diedericks, Bianca, Anna-Mari Kok, Vusani Mandiwana, Namrita Lall. "7-Methyljuglone in Tuberculosis Treatment" Encyclopedia, https://encyclopedia.pub/entry/55595 (accessed February 08, 2026).
Diedericks, B., Kok, A., Mandiwana, V., & Lall, N. (2024, February 28). 7-Methyljuglone in Tuberculosis Treatment. In Encyclopedia. https://encyclopedia.pub/entry/55595
Diedericks, Bianca, et al. "7-Methyljuglone in Tuberculosis Treatment." Encyclopedia. Web. 28 February, 2024.
Copy Citation
7-Methyljuglone (7-MJ) is a pure compound isolated from the roots of Euclea natalensis A. DC., a shrub indigenous to South Africa. It exhibits significant promise as a potential treatment for the highly communicable disease tuberculosis (TB), owing to its effective antimycobacterial activity against Mycobacterium tuberculosis.
tuberculosis (TB)
antimycobacterial
cytotoxicity
7-methyljuglone
1. Introduction
Tuberculosis (TB) is a highly infectious disease caused by the bacteria Mycobacterium tuberculosis. Tuberculosis is a complex and communicable disease that was the global primary source of death caused by a solitary infectious agent, which ranked above human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS) until 2019, when the coronavirus (COVID-19) pandemic broke out [1][2]. The COVID-19 pandemic has since caused a significant setback to the many years of headway in providing vital TB services and lowering the disease burden [2].
Using natural products, such as plants, as alternative therapies may assist in improving the therapeutic efficacy and, in some cases, can decrease some of the side effects experienced due to conventional drugs [3][4]. Medicinal plants are often promoted as natural and therefore harmless, which is true in most cases; however, they are not always free from adverse effects or toxicity [5]. Many very active medicinal plants and pure compounds isolated from medicinal plants have been found to possess a degree of toxicity [6].
2. 7-Methyljuglone
2.1. Introduction to Euclea natalensis A. DC.
Medicinal plants have been used for decades to treat numerous diseases. One such medicinal plant indigenous to South Africa, Euclea natalensis A. DC. (E. natalensis), has shown great cultural significance and traditional use as a potential therapeutic intervention in TB management [7]. Indigenous people throughout Southern Africa, particularly in the East and South Coast regions extending outward up to Mozambique, Swaziland, and Ethiopia, have been using this deciduous tree for the treatment of respiratory and dermatological ailments [7][8]. Euclea natalensis is traditionally employed as a medicine in 57% of the countries where it is indigenous [9]. In a study conducted by Lall et al. (2016), the ethanolic shoot extract of E. natalensis exhibited a minimum inhibitory concentration (MIC) value of 125 µg/mL, indicating moderate antimycobacterial ability [7]. This was further supported by in vivo studies where the mycobacterial load in infected mice decreased when treated with the ethanolic shoot extract [7]. Euclea natalensis belongs to the Ebenaceae family and is commonly known as Natal Ebony. This medicinally active plant is neither threatened nor endangered, and it is widely distributed. Therefore, the sustainability of the plant will not be immediately threatened by research initiatives conducted in a responsible manner [8].
2.2. Pure Compounds Isolated from Euclea natalensis A. DC.
Throughout the Ebenaceae family, in species such as Euclea natalensis A. DC., Diospyros mespiliformis Hochst., Diospyros ferrea (Willd.) Bak., and Diospyros tricolor (Schumach. and Thonn.) Hiern, the presence of naphthoquinones is widespread, and these compounds possess significant antitubercular activity, among other properties. Five of the most biologically active naphthoquinones isolated from the root of E. natalensis include diospyrin, neodiospyrin, isodiospyrin, shinanolone, and 7-methyljuglone (7-MJ) [7]. Among all the pure compounds isolated from E. natalensis, 7-MJ exhibited the most promising antimycobacterial activity [7].
2.3. 7-Methyljuglone as a Potential Antimycobacterial Therapeutic Agent
The 7-MJ isolated from the root chloroform extracts of E. natalensis exhibited MIC values of 0.50 µg/mL against M. tuberculosis and 1.57 µg/mL against M. smegmatis [10][11]. The crude E. natalensis chloroform root extracts had MIC values of 8 µg/mL and 7.33 µg/mL on M. tuberculosis and M. smegmatis, respectively [10]. Intracellularly, 7-MJ showed an EC90 (90% maximal effective concentration) of 0.57 µg/mL for the growth inhibition of M. tuberculosis in mouse macrophage (J774A.1) cells [12]. The inhibitory effect of 7-MJ on M. tuberculosis intracellularly (EC90) in J774A.1 cells and extracellularly (MIC) is comparable to that of streptomycin (EC90 = 1.11 µg/mL and MIC = 0.625 µg/mL) and ethambutol (EC90 = 1.62 µg/mL and MIC = 1.25 µg/mL). In a synergistic study, it was shown that 7-MJ also has the ability to improve the activity of isoniazid and rifampicin, as they showed fractional inhibitory concentrations of 0.25 and 0.5, respectively, with 7-MJ [12][13]. 7-Methyljuglone (Figure 1A) is a monomer of diospyrin (Figure 1B), wherein diospyrin showed a MIC value of 100 µg/mL against M. tuberculosis, which is 200-fold higher when compared to 7-MJ [14].
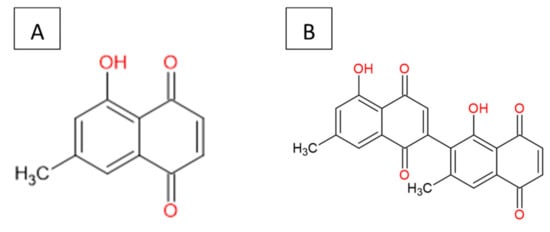
Figure 1. Chemical structure: (A) 7-methyljuglone and (B) diospyrin.
2.4. Mechanism of Action of 7-Methyljuglone
7-Methyljuglone has a chemical structure very similar to that of menaquinone. Menaquinone is a natural redox cycler found in the Mycobacterium family. It is responsible within the respiratory chain for mediating electron transfer between different membrane-bound enzymes [15][16]. Mammals and most bacteria make use of ubiquinone to fulfill the function of electron transport. Mycobacterium tuberculosis, however, lacks ubiquinone and only has the ability to utilize menaquinone in its electron transport chain. This makes it an appealing drug target, seeing as it lacks a human homologue [17]. In a study carried out by Van der Kooy et al. (2006), it was postulated that the mechanism of action of 7-MJ is that of an inhibitory interaction with the enzymes found within the mycobacterial electron transport chain. Due to the structural similarities found between 7-MJ and menaquinone (Figure 2), the electron flow can then potentially be reduced or halted due to the imbalance in the redox potential through the incorporation of 7-MJ [11].
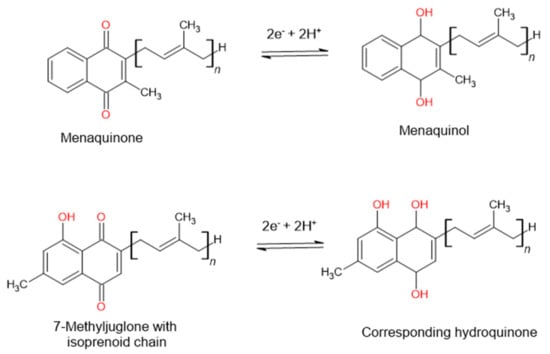
Figure 2. The postulated mechanism of action of 7-methyljuglone will disrupt the electron transport chain and therefore decrease or stop the electron flow in the bacterium, as suggested by Van der Kooy et al. (2006).
Another possibility is that 7-MJ can bind to the Men enzymes (MenA [1,4-Dihydroxy-2-naphthoate isoprenyltransferase], MenB [1,4-Dihydroxy-2-naphthoyl-CoA synthase], MenC [O-Succinylbenzoate synthase], MenD [2-Succinyl-5-enolpyruvyl-6-hydroxy-3-cyclohexadiene-1-carboxylate synthase], MenE [O-Succinylbenzoate synthase], MenF [Isochorismate synthase], MenG [Demethylmenaquinone methyltransferase], MenH [Demethylmenaquinone methyltransferase], and MenI [1,4-Dihydroxy-2-naphthoyl-CoA hydrolase]), which are responsible for the synthesis of menaquinone and therefore inhibit the addition of the hydrophobic sidechain [11][18]. This inhibition will influence the production of adenosine triphosphate (ATP) and lead to a detrimental effect on the bacterium [11].
2.5. The Sustainability of 7-Methyljuglone
There is currently concern regarding the sustainable availability of 7-MJ, as a study performed by Lall et al. (2005) reported a very low yield of 0.03% isolated from the root of E. natalensis [12]. In literature, leaves are generally removed from the aerial parts since the leaves are not used by the indigenous communities for the treatment of TB. There is currently no literature that suggests that other plant parts of E. natalensis have any significant antimycobacterial activity [9][19]. This can potentially place pressure on the current Euclea populations due to the roots being the most bioactive plant part identified and the plant part where 7-MJ is predominantly found. This has since led to the artificial synthesis of 7-MJ and its derivatives, of which the activity on M. tuberculosis was compared to that of the parent compound in a structure-activity bioassay. However, out of the 19 derivatives tested, 7-MJ was still the most active and selective antitubercular agent [16]. This indicates that 7-MJ can be considered as a potential antimycobacterial drug. However, in a study carried out by Kishore et al. (2014), 7-MJ has been shown to be cytotoxic to two human cell lines, namely, peripheral blood mononuclear cells (PBMCs) and human macrophages (U937) [20].
2.6. The Cytotoxic Effects of 7-Methyljuglone
7-Methyljuglone has been shown to have very promising antimycobacterial properties; however, 7-MJ has also been shown to be cytotoxic to various cancer and non-cancer cell lines, as summarized in Table 1. A compound that exhibits a half-maximal inhibitory concentration (IC50) of less than 10 μM is reasoned to have in vitro cytotoxic activity against cancer cells [21].
Table 1. The cytotoxic effect of 7-methyljuglone on cancerous and non-cancerous cell lines [12][20][22].
| Cell Lines | IC50 (µM) |
|---|---|
| Cancerous Cell Lines | |
| Human breast cancer (MCF-7) | 27.2 |
| Immortal human (HeLa) | 66.6 |
| Spindle-shaped N-cadherin +CD45− osteoblastic (SNO) | 81.4 |
| Human prostate cancer (DU145) | 11.9 |
| Human oral epidermoid carcinoma (KB) | 4.1 |
| Human lung cancer (Lu1) | 13.2 |
| Hormone-dependent human prostate cancer (LNCaP) | 3.7 |
| Leukemia (HL60) | 8.8 |
| Non-cancerous Cell Lines | |
| Peripheral blood mononuclear cells (PBMC) | 18.4 |
| Human histiocytic lymphoma (U937) | Between 5.31 and 26.6 |
| Umbilical vein endothelial cells (HUVEC) | 5.7 |
| Green monkey kidney (Vero) | 80.4 |
| Mouse macrophage (J774A.1) | 20.8 |
In a study conducted by Kishore et al. (2014), the exact cytotoxic IC50 value of 7-MJ on U937 cells was not reported; however, it was reported that the IC50 lies between 1 and 5 µg/mL (5.31 and 26.6 µM) [20]. 7-Methyljuglone can be regarded as a promising cytotoxic compound against respective cancerous cell lines such as DU145, KB, Lu1, LNCaP, and HL60 [20][22]. It, however, does not seem to have selectivity toward cancerous cell lines in comparison with non-cancerous cell lines, as it showed similar in vitro cytotoxicity against most of the non-cancerous cell lines previously tested. The lack of selectivity exhibited by 7-MJ implies that this drug will not be effective in targeting cancer cells specifically and is therefore not a good contender for cancer treatment. Achieving selectivity for cancer cells is a key goal in the drug development of anti-cancer drugs: to maximize efficacy while minimizing toxicity and adverse effects on normal tissues [23]. For the use of 7-MJ as a potential anti-TB drug, the selectivity of cancerous versus non-cancerous cells does not influence its efficacy but does raise drug safety concerns [24]. Its cytotoxicity toward the HUVEC cell line also suggested that pregnant women would need to practice caution when taking 7-MJ [22].
With the focus on reducing the cytotoxic effect of 7-MJ, nanotechnology may be a promising alternative to consider. As a system to deliver therapeutic agents, nanotechnology can provide advantages such as drastically reducing the size of the drug taken up, which will result in a higher surface-to-volume ratio, protecting the drug moiety within the nanoparticle against degradation, and reducing the toxic effects of the therapeutic agent [25].
References
- Patel, S.; Sodha, M.N. Tuberculosis: Current scenario & challenges in India. World J. Pharm. Res. 2022, 11, 359–365.
- WHO Global Tuberculosis Report 2021. World Health Organization. 2021. Available online: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2021 (accessed on 1 March 2022).
- Khameneh, B.; Fazly Bazzaz, B.S.; Amani, A.; Rostami, J.; Vahdati-Mashhadian, N. Combination of anti-tuberculosis drugs with vitamin C or NAC against different Staphylococcus aureus and Mycobacterium tuberculosis strains. Microb. Pathog. 2016, 93, 83–87.
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The traditional medicine and modern medicine from natural products. Molecules 2016, 21, 559.
- Bnouham, M.; Zahra Merhfour, F.; Elachoui, M.; Legssyer, A.; Mekhfi, H.; Lamnaouer, D.; Ziyyat, A. Toxic effects of some medicinal plants used in Moroccan traditional medicine. Moroc. J. Biol. 2006, 2, 21–30.
- Fennell, C.W.; Lindsey, K.L.; McGaw, L.J.; Sparg, S.G.; Stafford, G.I.; Elgorashi, E.E.; Grace, O.M.; van Staden, J. Assessing African medicinal plants for efficacy and safety: Pharmacological screening and toxicology. J. Ethnopharmacol. 2004, 94, 205–217.
- Lall, N.; Kumar, V.; Meyer, D.; Gasa, N.; Hamilton, C.; Matsabisa, M.; Oosthuizen, C. In vitro and In vivo antimycobacterial, hepatoprotective and immunomodulatory activity of Euclea natalensis and its mode of action. J. Ethnopharmacol. 2016, 194, 740–748.
- Oosthuizen, C.B.; Lall, N. Chapter 16—Euclea natalensis. In Underexplored Medicinal Plants from Sub-Saharan Africa: Plants with Therapeutic Potential for Human Health; Academic Press: Cambridge, MA, USA, 2020; pp. 111–116.
- Maroyi, A. Review of ethnomedicinal uses, phytochemistry and pharmacological properties of Euclea natalensis A. DC. Molecules 2017, 22, 2128.
- McGaw, L.J.; Lall, N.; Hlokwe, T.M.; Michel, A.L.; Meyer, J.J.M.; Eloff, J.N. Purified compounds and extracts from Euclea species with antimycobacterial activity against Mycobacterium bovis and fast-growing mycobacteria. Biol. Pharm. Bull. 2008, 31, 1429–1433.
- Van der Kooy, F.; Meyer, J.J.M.; Lall, N. Antimycobacterial activity and possible mode of action of newly isolated neodiospyrin and other naphthoquinones from Euclea natalensis. S. Afr. J. Bot. 2006, 72, 349–352.
- Lall, N.; Meyer, J.J.M.; Wang, Y.; Bapela, N.B.; Van Rensburg, C.E.J.; Fourie, B.; Franzblau, S.G. Characterization of intracellular activity of antitubercular constituents from the roots of Euclea natalensis. Pharm. Biol. 2005, 43, 353–357.
- Bapela, N.B.; Lall, N.; Fourie, P.B.; Franzblau, S.G.; Van Rensburg, C.E.J. Activity of 7-methyljuglone in combination with antituberculous drugs against Mycobacterium tuberculosis. Phytomedicine 2006, 13, 630–635.
- Lall, N.; Das Sarma, M.; Hazra, B.; Meyer, J.J.M. Antimycobacterial activity of diospyrin derivatives and a structural analogue of diospyrin against Mycobacterium tuberculosis in vitro. J. Antimicrob. Chemother. 2003, 51, 435–438.
- Garbe, T.R. Co-induction of methyltransferase Rv0560c by naphthoquinones and fibric acids suggests attenuation of isoprenoid quinone action in Mycobacterium tuberculosis. Can. J. Microbiol. 2004, 50, 771–778.
- Mahapatra, A.; Mativandlela, S.P.N.; Binneman, B.; Fourie, P.B.; Hamilton, C.J.; Meyer, J.J.M.; van der Kooy, F.; Houghton, P.; Lall, N. Activity of 7-methyljuglone derivatives against Mycobacterium tuberculosis and as subversive substrates for mycothiol disulfide reductase. Bioorganic Med. Chem. 2007, 15, 7638–7646.
- Truglio, J.J.; Theis, K.; Feng, Y.; Gajda, R.; Machutta, C.; Tonge, P.J.; Kisker, C. Crystal Structure of Mycobacterium tuberculosis MenB, a Key Enzyme in Vitamin K2 Biosynthesis. J. Biol. Chem. 2003, 278, 42352–42360.
- Zhang, Z.; Liu, L.; Liu, C.; Sun, Y.; Zhang, D. New aspects of microbial vitamin K2 production by expanding the product spectrum. Microb. Cell Fact. 2021, 20, 84.
- Bapela, J.M.; Kuete, V.; Du Toit, E.; Meyer, M.J.J.; Lall, N. Fertilization-induced changes in growth parameters and antimycobacterial activity of Euclea natalensis (Ebenaceae). S. Afr. J. Bot. 2008, 74, 244–250.
- Kishore, N.; Binneman, B.; Mahapatra, A.; Van De Venter, M.; Du Plessis-Stoman, D.; Boukes, G.; Houghton, P.; Meyer, J.J.M.; Lall, N. Cytotoxicity of synthesized 1,4-naphthoquinone analogues on selected human cancer cell lines. Bioorganic Med. Chem. 2014, 22, 5013–5019.
- Kuete, V.; Ngameni, B.; Wiench, B.; Krusche, B.; Horwedel, C.; Ngadjui, B.T.; Efferth, T. Cytotoxicity and mode of action of four naturally occuring flavonoids from the genus dorstenia: Gancaonin Q, 4-hydroxylonchocarpin, 6-prenylapigenin, and 6,8-diprenyleriodictyol. Planta Med. 2011, 77, 1984–1989.
- Mbaveng, A.T.; Kuete, V. Review of the chemistry and pharmacology of 7-Methyljugulone. Afr. Health Sci. 2014, 14, 201–205.
- Ioele, G.; Chieffallo, M.; Occhiuzzi, M.A.; De Luca, M.; Garofalo, A.; Ragno, G.; Grande, F. Anticancer Drugs: Recent Strategies to Improve Stability Profile, Pharmacokinetic and Pharmacodynamic Properties. Molecules 2022, 27, 5436.
- Jacobs, R.E.A.; Gu, P.; Chachoua, A. Reactivation of pulmonary tuberculosis during cancer treatment. Int. J. Mycobacteriol. 2015, 4, 337–340.
- Nabi, B.; Rehman, S.; Aggarwal, S.; Baboota, S.; Ali, J. Nano-based anti-tubercular drug delivery: An emerging paradigm for improved therapeutic intervention. Drug Deliv. Transl. Res. 2020, 10, 1111–1121.
More
Information
Subjects:
Infectious Diseases; Plant Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
508
Revisions:
3 times
(View History)
Update Date:
01 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




