Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rafael González-Parra | -- | 3934 | 2024-02-27 19:46:09 | | | |
| 2 | Jessie Wu | -5 word(s) | 3929 | 2024-02-28 06:47:33 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
González-Parra, J.R.; Di Turo, F. Use of Plant Extracts as Sustainable Corrosion Inhibitors. Encyclopedia. Available online: https://encyclopedia.pub/entry/55573 (accessed on 07 February 2026).
González-Parra JR, Di Turo F. Use of Plant Extracts as Sustainable Corrosion Inhibitors. Encyclopedia. Available at: https://encyclopedia.pub/entry/55573. Accessed February 07, 2026.
González-Parra, Jesús Rafael, Francesca Di Turo. "Use of Plant Extracts as Sustainable Corrosion Inhibitors" Encyclopedia, https://encyclopedia.pub/entry/55573 (accessed February 07, 2026).
González-Parra, J.R., & Di Turo, F. (2024, February 27). Use of Plant Extracts as Sustainable Corrosion Inhibitors. In Encyclopedia. https://encyclopedia.pub/entry/55573
González-Parra, Jesús Rafael and Francesca Di Turo. "Use of Plant Extracts as Sustainable Corrosion Inhibitors." Encyclopedia. Web. 27 February, 2024.
Copy Citation
Corrosion inhibitors have traditionally been utilised to protect copper alloy sculptures from corrosion despite the recognised environmental and human health risks. Knowing the associated toxicity, ongoing extensive research seeks alternative substances for corrosion reduction, giving rise to the emergence of green inhibitors. In this pursuit, plant extract inhibitors have gained attention, particularly in the heritage field.
corrosion
cultural heritage
inhibitors
green chemistry
plant extracts
1. Introduction
Copper-based alloys play a crucial role in cultural heritage remains. Throughout history, copper-made artefacts have been continuously used and nowadays are used for the casting of artistic artworks. These types of objects hold immense cultural value and provide valuable technological insights into the cultures that develop them, enabling the identification of trade and technological routes. As a result, preserving these items for future generations is imperative. In contemporary times, there is a strong emphasis on environmental sustainability and health in various realms of research and technological applications. This sensitivity has a pervasive impact, affecting almost every field, including research and developments related to the preservation and restoration of cultural heritage (CH). Indeed, a multitude of substances and technologies find application in conservation science for diverse purposes, with coatings and inhibitors serving as just two examples of potentially harmful substances currently in use.
2. Plant Extracts
Tannic acid mixtures, often including orthophosphoric acid, are commonly applied as a final step in the conservation treatment process to coat archaeological iron. However, it is important to note that tannic acid has its limitations. In some cases, it can transform the surface colour from reddish to black, and its protective effect on iron has been found to be less effective [1][2]. Benzidia and collaborators conducted an experiment as detailed in their work titled “Investigation of Green Corrosion Inhibitor Based on Aloe vera (L.) Burm. F. for the Protection of Bronze B66 in 3% NaCl” [3]. They explored the application of tannins extracted from Aloe vera to inhibit the corrosion of bronze B66 in a chlorine-rich medium, simulating a marine environment.
Tannic acid, a polyphenol tannin, exists in the form of an amorphous powder that is highly soluble in water [4]. Its chemical formula (C76H52O46) indicates that it consists of a mixture of polygallolyl glucoses or polygallolyl quinic acid esters ranging from 2 to 12 in number [5]. Specifically, tannic acid is a type of gallotannin containing glucose esters of phenolcarboxylic acids, and is widely used in various fields, ranging from medicine to dyeing [6][7].
In the field of metal conservation, tannic acid has shown inhibitory properties, initially on iron and subsequently on copper. Given the favourable outcomes observed with iron, researchers began investigating its application in other metals, including copper and its alloys. Leveraging the chemical characteristics of tannic acid, Benzidia et al. successfully applied tannic acid to bronze B66, which closely resembles the composition of archaeological bronze [3]. It is essential to note that during these experimental phases, it was not feasible to apply innovative products directly to original materials without first ensuring the product’s safety and effectiveness on this type of metal. The results demonstrated that the Aloe vera extract effectively inhibits the corrosion of bronzes immersed in a 3% NaCl solution, with the level of protection correlating with the concentration of the plant extract. SEM-EDS analysis was instrumental in illustrating the formation of corrosion products after 24 h of immersion without protection and, conversely, the active inhibition provided by the Aloe vera extract when applied to bronze B66.
In their work [8], Kusmierek and E. Chrzescijanska evaluated the effectiveness of tannic acid, which is found in various plants, as a corrosion inhibitor for use in preserving Polish cultural heritage. In the procedure, as shown in Figure 1, the plants collected were dried at room temperature for 13 days and then crushed into a fine powder. The powdered pods were then placed in 250 mL of methanol for 5 days, and the extraction (25 × 30 mL) was repeated until the plant materials were exhausted. The extract obtained was concentrated using a rotary evaporator below 55 °C. The extract was evaporated, resulting in a solid form for the preparation of the stock inhibitor concentration. Their research confirmed the inhibitory properties of such compounds, particularly on copper and brass. The inhibitors were then added to water solutions at concentrations ranging from 0.25 to 25 mg/dm3. The corrosion behaviour was assessed using linear polarization resistance and weight loss methods. The results clearly demonstrated that tannic acid was an effective protective agent for Cu surfaces, with the highest inhibition efficiency observed in solutions with a concentration of 10 mg/dm3. Furthermore, the immersion method was tested, and the highest inhibition performance was achieved after 5 immersions in a solution containing 100 mg/dm3. The corrosion rate was strongly related to the inhibitor concentration. Surprisingly, the protection of brass was found to be more effective than that observed for other alloys such as Inconel and steel with a concentration above 20 mg/dm3.
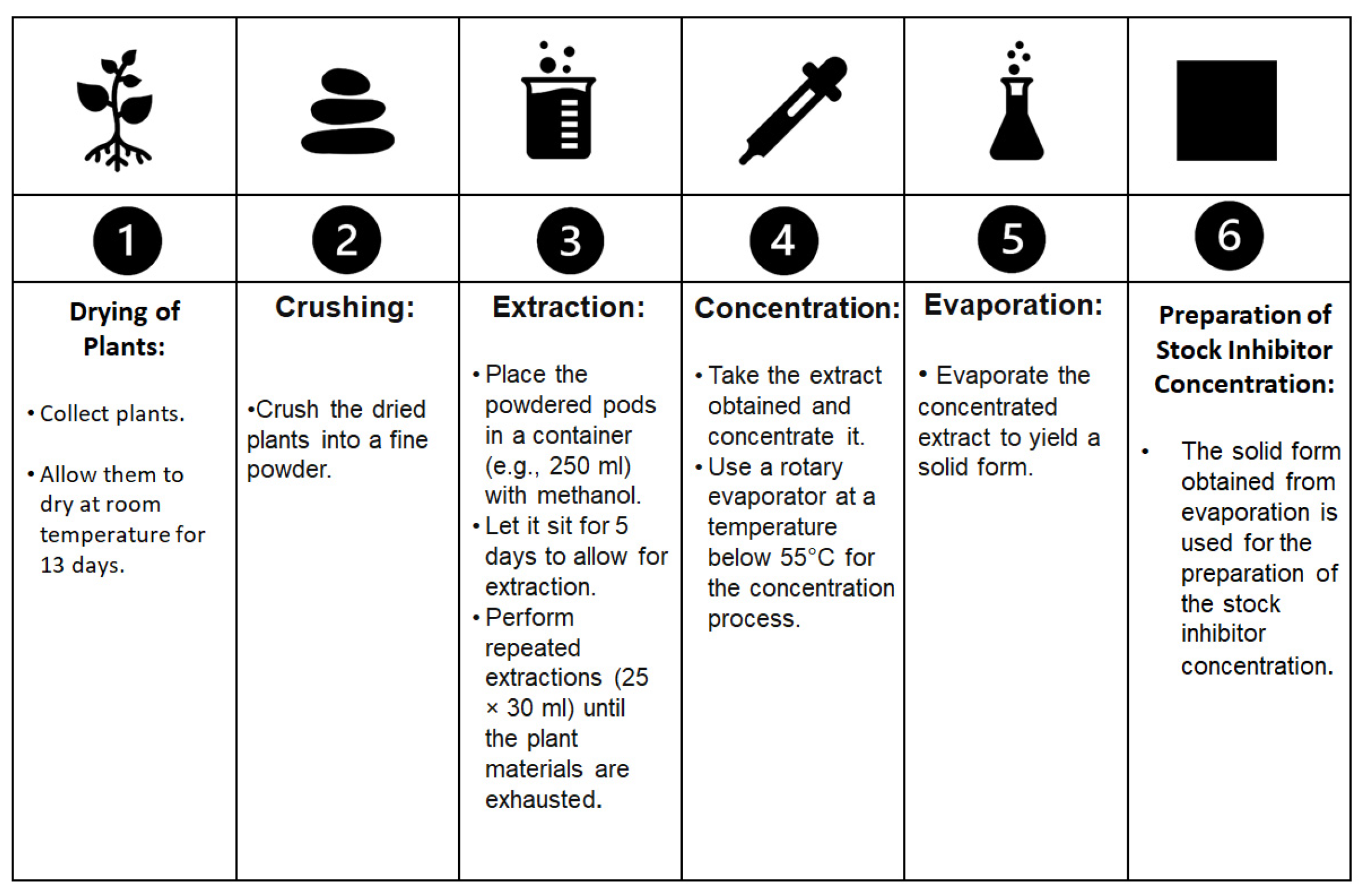
Figure 1. The extraction procedure described by E. Kusmierek and E. Chrzescijanska in their work [8] that was used to obtain an inhibitor for brass, starting from Aloe vera.
Fouda and collaborators [9] conducted experiments using Ceratonia siliqua as a corrosion inhibitor for copper and α-brass in 1 M HNO3 solutions. To prepare the inhibitor, they collected fresh plants, dried them at room temperature for 13 days, and subsequently crushed them to obtain a fine powder. The powdered pods were then immersed in 250 mL of methanol for 5 days, followed by repeated extractions (25 cycles of 30 mL each) until all the plant material was exhausted. The resulting extract was concentrated using a rotary evaporator at a temperature below 55 °C, eventually yielding a solid form suitable for preparing the stock inhibitor concentration. The procedure is schematised in Figure 2. Also, the extracted inhibitor was added to a 70% HNO3 solution in concentrations of 50 to 300 ppm. The analytical procedure they used resembled previous work involving gravimetric and electrochemical analyses to assess the level of protection. The results obtained using a combination of weight loss analysis, electrochemical impedance spectroscopy (EIS), and polarization curves indicated that Ceratonia siliqua exhibited effective corrosion protection for both copper and brass, acting by increasing the energy barrier for the corrosion of copper and brass dissolution. Similar to tannic acid, the level of protection provided by C. siliqua increased with concentration but decreased with temperature because of the corrosion inhibition performed through the physical adsorption mechanism. and desorption was more prone to occur as the temperature increased. Of note, the metals adsorbed the inhibitor in accordance with the Langmuir adsorption isotherm, involving a spontaneous and exothermic reaction, as evidenced by the negative values of free energy. C. siliqua primarily acted as a cathodic inhibitor in their research. These findings suggest that further investigations into the application of C. siliqua on archaeological metals would be worthwhile.
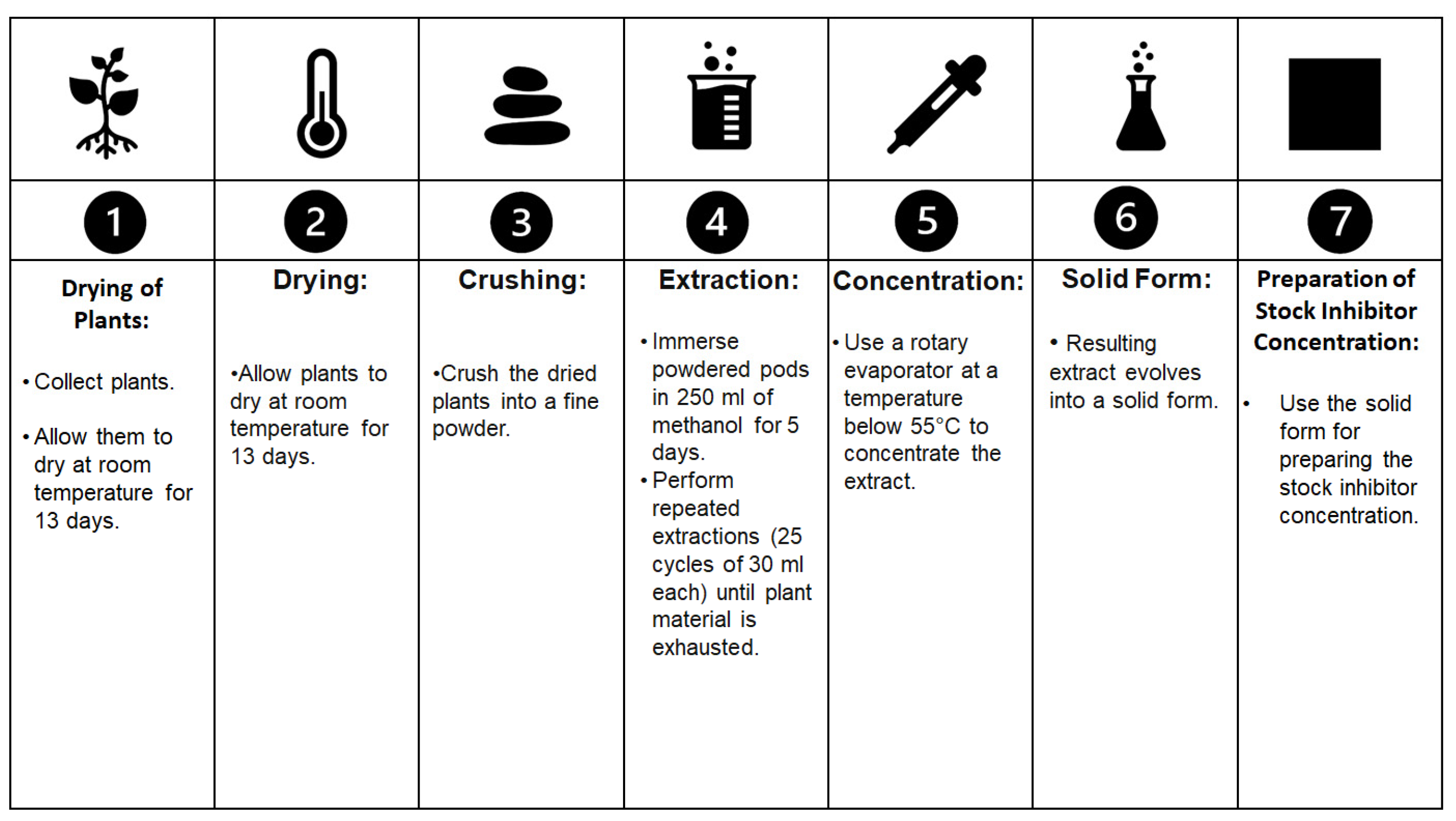
Figure 2. The extraction procedure described by Fouda and collaborators [9] that was used to extract inhibitors from Ceratonia siliqua.
Fascinating investigations involving novel plant extracts have emerged from experiments with acacia fruit extracts (Robinia pseudoacacia L.) [10]. This research aimed to assess the viability of Robinia pseudoacacia L. extracts as Cu-10Sn corrosion inhibitors in saline media. To extract the inhibitors, fruit samples were collected, dried, and ground to obtain 30 g of powder. Later, the collected powder was soaked in 100 mL of double-distilled water and constantly shaken for 24 h at room temperature. The liquid was mechanically filtered to separate extracts and powder. Also, the extract was centrifuged at 2500 rpm and 4 °C and dewatered using a vacuum rotary device. The powder was diluted ranging from 200 to 1800 ppm. This dissolution was applied to as-cast Cu-10Sn bronze alloy with a composition similar to historical ones. These bronze samples underwent corrosion in a 0.5 M sodium chloride solution for four consecutive weeks. The researchers discovered that the green inhibitor derived from the acacia fruit aqueous extract could effectively inhibit bronze corrosion, increasing its inhibitory properties with concentration. Also, the highest corrosion protection was obtained after two weeks of constant immersion. For higher immersion times, the inhibitory efficiency decreased. However, at higher concentrations, it exhibited fungus growth, which could diminish the effectiveness of the acacia fruit aqueous extract. Based on the cathodic and anodic slopes, the authors indicated that the inhibitor corresponds to a mixed-type corrosion inhibitor. While this research provides preliminary results, it identifies Robinia pseudoacacia L. as a promising candidate warranting further study.
Another noteworthy study, reported by Rahal et al. [11], presented the results of using an olive leaf extract as a corrosion inhibitor for pure copper in 0.5 M NaCl solution. To extract the inhibitor compound, olive leaves were air-dried at room temperature for one month in the absence of light. The leaves were ground, and the dissolution of 100 g of olive leaf powder per litre of distilled water was prepared. The primary compound identified was oleuropein, and three solutions with varying concentrations of this compound (2.42 mmol L−1, 1.21 mmol L−1, 0.48 mmol L−1) were tested. The samples were immersed for 24 h in a completely static 0.5 M NaCl solution at room conditions. The authors reported that the corrosion rate of the samples decreased as the inhibitor concentration increased. This olive leaf extract strongly modified the oxygen cathodic reduction reaction, indicating a cathodic inhibitor type. Furthermore, the inhibition efficiency exceeded 86% for the highest solution concentration and decreased with temperature in a similar manner to that previously indicated for Ceratonia siliqua.
Another eco-friendly alternative for copper protection was described by Gao et al., as detailed in their work [12]. In their study, a Saccharum officiniarum leaf extract was tested to identify its corrosion inhibition properties for copper in acidic media. The inhibitor extract was obtained by crushing dried leaves to obtain a fine powder. An alcoholic solution of leaf extract was obtained after 48 h of static immersion and subsequently dried in a rotary evaporating flask. Various amounts of the extract (ranging from 100 mg/L to 500 mg/L) were dissolved in a 0.5 M H2SO4 solution. The results indicated that the leaf extract formed a protective film on the copper surface, increasing the charge transfer resistance as the concentration increased. Also, Saccharum officiniarum acted as a mixed corrosion inhibitor. The inhibition efficiency reached 93% for the highest inhibitor concentration.
Elshahawi and collaborators [13] reported the efficiency of a Jatropha extract as a green corrosion inhibitor for bronze archaeological artefacts in a 3.5% NaCl solution. The Jatropha extract was obtained using cold pressing and was added by brushing the surface at concentrations of 10 ppm, 20 ppm, 30 ppm, 40 ppm, and 50 ppm. The authors conducted a series of electrochemical tests, correlating the results with the weight loss method and a salt spray exposure chamber. The electrochemical results indicated a mixed-type corrosion behaviour. All the results indicated that the addition of 30 ppm of the extract demonstrated the highest inhibition efficiency, reaching 86%. Also, as temperature increased the inhibitory efficiency decreased due to the desorption process that led to the detachment of the inhibitor molecules on the metallic surface, as evidenced by the studies [3][9]. It is relevant to note that this inhibitor was applied by brushing on an Egyptian archaeological bronze mirror with patina layers.
An interesting study reported by Tan et al. [14] aimed to identify the corrosion inhibitor properties of papaya leaf extract for copper in sulfuric acid media. The corrosion inhibitor was obtained by drying fresh papaya leaves in an oven for 24 h. Later, the leaves were powdered and added to 1 L of ultrapure water that was heated and evaporated to 200 mL and filtered with fine gauze. Again, the dissolution was heated to evaporate until 50 mL. The plant extract corrosion inhibitor was placed in a refrigerator for 10 h to completely freeze. The inhibitor was placed in a freeze-drying device for 24 h to finally obtain 5.23 g of dry powder. The plant extract was diluted in 0.5 mol/L of sulfuric acid with concentrations of 10, 20, 100, and 150 mg/L. The results showed that the addition of the plant extract corrosion inhibitor modified the kinetics of copper dissolution due to the adsorbed inhibitor layer. However, the icorr values of the cathodic and anodic branches of potentiodynamic polarization curves were reduced, indicating a mixed-type corrosion inhibitor behaviour. Also, a higher inhibition efficiency (93%) was obtained at 25 °C with 150 mg/L. The adsorption isotherm analysis indicated that this papaya-based inhibitor attached to the surface through a physico-chemical adsorption mechanism. These results indicated that the papaya leaf extracts could attach to the copper surface strongly, providing a dense barrier film inhibiting corrosion. More studies on this matter will be valuable. Another work by the same author [15] studied the corrosion inhibition properties of Pasiflora edulia Sims leaf extracts in protecting copper in a H2SO4 solution. To obtain the corrosion inhibitor, fresh Pasiflora edulia Sims leaves were dried in an oven at 343 K for 24 h and then ground into a fine powder. Later, 100 g of powder was added to the dissolution of 500 mL of ultrapure water. Also, the dissolution was heated and evaporated until it reached 200 mL and was then filtered. The dissolution was frozen for 24 h and then placed in a freeze-drying oven for 48 h. The Pasiflora edulia Sims leaf extract was dissolved in 0.5 M H2SO4 with concentrations of 100, 200, 400, 600, and 800 mg/L. The results showed that the inhibitor attached to the copper surface and increased the charge transfer resistance. Also, the corrosion inhibitory properties were strongly dependent on the inhibitor concentration. With a concentration of 600 mg/L, the diffusive processes became weaker, indicating the formation of a closed protective film on the Cu surface. The maximum inhibitory efficiency reached was 96.5% for a concentration of 800 mg/L. This type of inhibitor acted by modifying the cathodic behaviour and showed a physico-chemical adsorption model.
Myrthus communis was studied by Dahmani et al. [16] to inhibit the corrosion of copper in 0.5 M sulfuric acid. The plant extraction inhibitor was obtained by collecting fresh leaves and drying them in the shade at room conditions for 3 weeks, followed by the obtention of the essential oil using hydrodistillation for 2 h. The resulting volatile extract was dried and stored at 4 °C. The plant extract corrosion inhibitor was added in concentrations of 0.5 to 2 g/L. The inhibitor addition diminished the anodic and cathodic corrosion currents. However, this inhibitor relied on the mixed-type corrosion inhibitor and attached to the surface through a physisorption mechanism. Again, the inhibition properties depended on the inhibitor concentration, reaching a maximum efficiency of 93.2% for the concentration of 2 g/L. An interesting point in their study was that the inhibition properties remained almost constant after 16 h of constant immersion with the maximum concentration.
Cao et al. [17] studied the properties of Dimocarpus longan Lour leaf plant extract in inhibiting the corrosion of Cu in 0.5 M sulfuric acid media. The route of obtention of the corrosion inhibitor consisted of collecting the fresh leaves, which were dried and pulverised. The powder was placed into a baker containing the sulfuric acid 0.5 M solution for 36 h. The supernatant was decanted into a baker, freeze-dried, and kept in a dissector until it was used. The concentrations tested were 50 mg/L to 400 mg/L, which were added to a corrosive 0.5 M sulfuric acid solution. The addition of the plant extract corrosion inhibitor led to a decrease in the cathodic corrosion current, preferentially inhibiting the precipitating of oxygen at the cathodic active sites at the copper surface. The inhibition efficiency of this plant extract corrosion inhibitor was 98% for a concentration of 400 mg/L. In this case, a chemisorption mechanism was indicated.
In general, the route of obtention of the plant extract corrosion inhibitors is similar for almost all the studies reviewed. The route consists of a set of progressive stages, which are represented in Figure 3. As a first step, the plant is collected in fresh conditions, dried, and powdered and then added to a solvent (distilled water, methanol, and sulfuric acid). Later, the dissolution is concentrated using evaporation, centrifugation, or freeze-drying. Finally, the powder that can be added to the corrosive test solution is obtained. In special cases, Dahmani et al. [16] obtained a plant extract corrosion inhibitor using essential oils that were dried and stored in a powder form, which was added to the corrosive solution. Elshahawi et al. [13] drew upon the cold pressing method and applied an inhibitor directly by brushing the metallic surface.
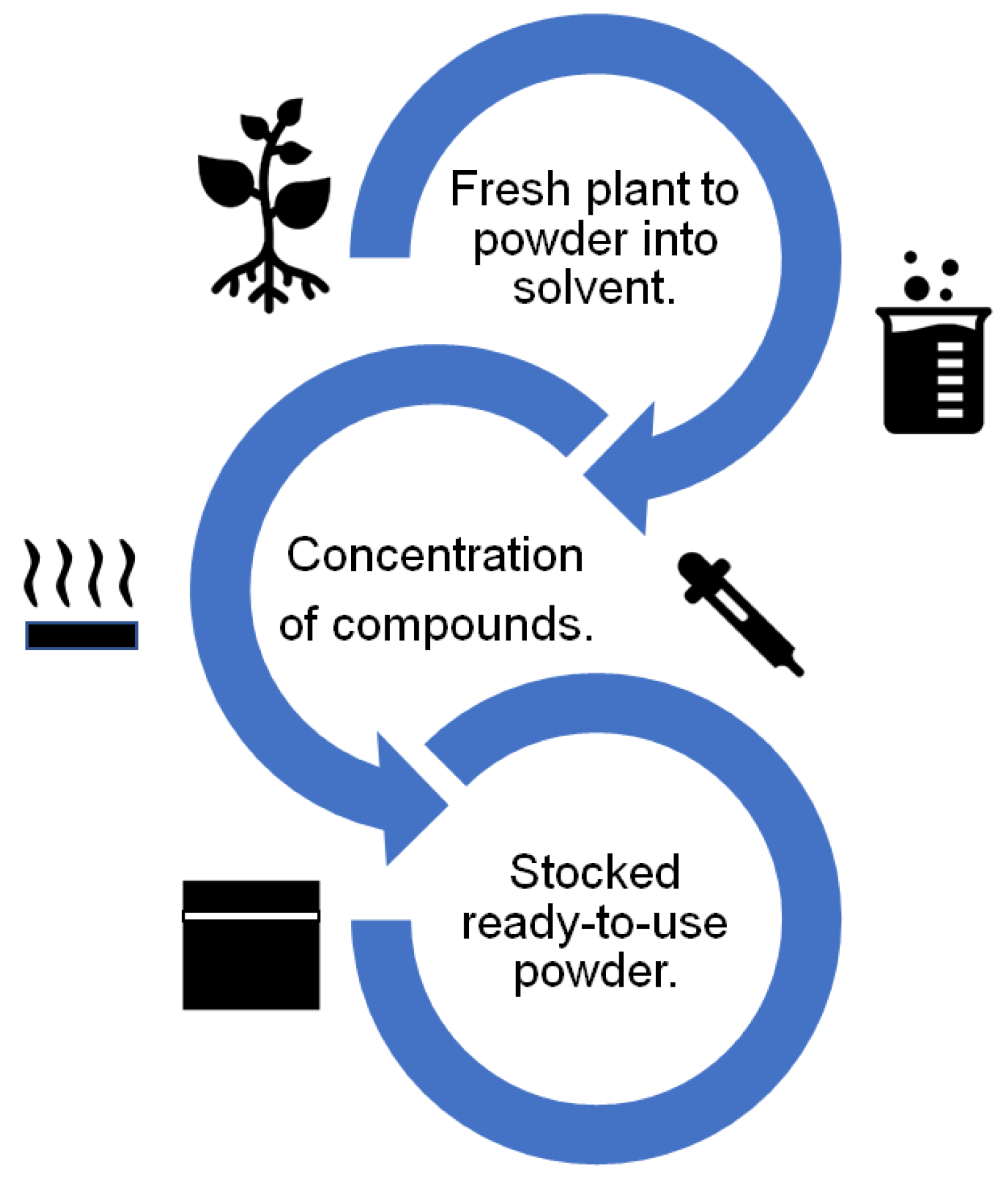
Figure 3. Schematic representation of the route of obtention of plant extract corrosion inhibitors.
Once a corrosion inhibitor has been obtained, it can be applied to a metallic sample by directly immersing the object in a solution containing the plant extract corrosion inhibitor or by brushing the inhibitor solution onto the surface of the sample, as shown in Figure 4. The corrosion system is then sealed with a protective coating, as shown in Figure 3. It is worth noting that only a few works deal with the direct application of the inhibitor on metallic samples [8][13]; in the majority of the studies reviewed, the inhibitor was added to the corrosive test solution.
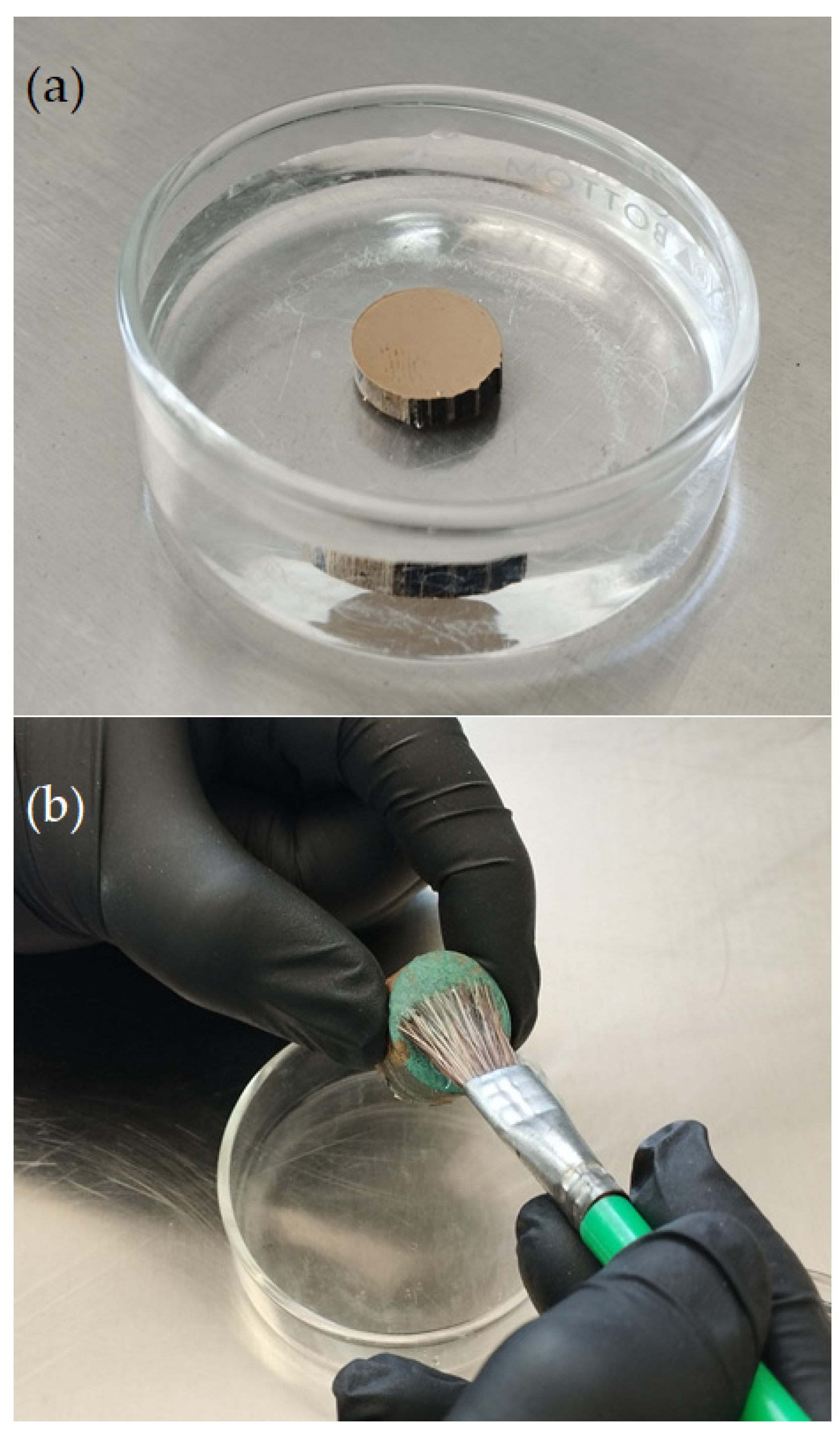
Figure 4. Examples of the application of plant extract corrosion inhibitors to cultural heritage copper-based alloys: (a) direct immersion and (b) brush application of the inhibitor-containing solution.
As expected by the diverse origin of plant extract corrosion inhibitors, different adsorbed molecules provide corrosion protection to the substrate. It is observed that different compounds can provide corrosion protection; however, the following premise is maintained: corrosion inhibitor molecules contain N, S, and O groups that can provide bonding sites for attaching the inhibitor layers to the metallic surface and exert corrosion protection through the barrier effect of the molecule into the metallic surface. To properly understand the protective effect of the active compounds, quantum chemical analysis tools such as DFT can shed light on the electronic behaviour, preferential attachment sites, and molecules responsible for corrosion inhibition. The work of Gao et al. [12] is a clear example of this powerful analysis tool since molecular simulation results allowed the authors to determine that N and O were preferential sites to form coordination bonds to the copper surface. Also, based on the molecular dipole, the authors determined that HMA and DHC molecules have higher corrosion performance. Similarly, Tan et al. [15] determined that the most favourable molecules for corrosion inhibition were PTH and DMT.
Despite the powerful results provided by this computational molecular analysis, this analysis technique is not widespread in the plant extract inhibitor community, making the proper identification of the active compound a hard task and leading to the consideration that corrosion protection results from the synergic effect of several molecules.
It is interesting to note that most of these studies assess the inhibitory efficiency of plant extract corrosion inhibitors applied on clean and polished metallic surfaces. However, these conditions do not accurately represent the typical conditions of copper-based cultural heritage, which naturally develops a corrosion product layer grown over years of exposition to a corrosive environment or may be intentionally formed by the artist as part of the concept of their artwork. Only in the case of the Jatropha extract [13] was a patinated surface used, but the authors did not provide the electrochemical characterisation of these archaeological samples. The inhibitory efficiency of the Jatropha extract applied on a patinated bronze may differ from that obtained for a polished bronze surface where the molecule can properly attach to the active sites. Ben Chanouf et al. [18] indicated that the corrosion product layer modifies the corrosion inhibitor behaviour. The corrosion-inhibitory properties of the reviewed plant extracts depend strongly on their concentration. A higher concentration of corrosion inhibitor molecules promotes the formation of a dense layer that effectively protects the metallic surface, but the patina layer restricts the metallic surface exposed surface area. Thus, because corrosion inhibitors are greatly applied over patinated surfaces, further research should be conducted to identify the interaction of plant extract corrosion inhibitors on surfaces covered with stable corrosion product layers.
Since corrosion is an electrochemical process, it is necessary to mainly characterise the corrosion inhibitory response using electrochemical techniques, and the works reviewed mainly establish their conclusion based on electrochemical impedance spectroscopy and potentiodynamic polarisation curves. It is necessary to consider that the electrochemical response of a sample depends on the concentration of the electrolyte, pH, temperature, composition of the sample, and immersion time. Then, to properly compare the inhibitory efficiency of these studies, similar conditions must be applied. The exposure time of the works considered varies during a lapse time of 30 min to 24 h. However, for the main corpus of the papers reviewed, the electrochemical characterisation was performed after 30–60 min of constant immersion to reach the OCP and maintain the OCP potential during EIS tests. Only the works of Rahal [11] and Pourzaghan [10] evaluate the inhibition response of the plant-extracted corrosion inhibitors after 24 h and 672 h of constant immersion. The concentration of the electrolyte was kept from 0.5 to 1 M for H2SO4 and 0.5 M NaCl to 3.5 wt%. of NaCl. The testing temperature was at room conditions; however, the influence of temperature was studied by heating the inhibitor container from 15 to 80 °C. As shown, the use of plant extract corrosion inhibitors allows for obtaining high inhibition efficiency values, indicating that the contained molecules can successfully protect the metallic substrates. In addition, these inhibition values were obtained at higher test concentrations. It is also noted that the extraction method is relatively safe for the operators because of the use of water and methanol as extraction solvents. This is an advantageous feature that makes the process sustainable, with the exception of the works [12][17], where acidic solutions were used. Despite the different parameters used, it can be said that the highest corrosion inhibitory efficiency for copper in H2SO4 media was obtained for Pasiflora edulia Sims leaf extracts (96.5%) [15], and for chloride media, the Jatropha extract showed the highest inhibition efficiency (86%) [13]. As stated above, it is necessary to consider that the inhibitory properties can vary because there are no reports about the performance of these plant extract corrosion inhibitors on patinated surfaces.
The above suggests that plant extract corrosion inhibitors are suitable for protecting copper and copper-based alloys from corrosion in outdoor exposure conditions such as acid rain or chloride media. This is due to the presence of atoms (N, S, O) in the extracted molecules that promote attachment to the metallic surface, as well as high dipolar moments and unsaturated functional groups [15]. However, researchers consider that, at this point, it is difficult to relate inhibition efficiency to a single compound because the extraction methods are not oriented towards purifying and concentrating a specific molecule and the inhibitory response is associated with a synergistic response of the molecules extracted. Some works such as those reported by [11][14][15] indicate which molecule was mainly identified; however, the lack of information in the other studies reviewed prevents a complete understanding of the relationship between the inhibitor compound and its inhibitory efficiency. On the other hand, Kokalji et al. [19] proposed a valuable method for identifying the inhibition properties of corrosion inhibitor molecules. They introduced the term “inhibition power” due to the lack of linearity in the classical inhibition efficiency and the negative correlation among the classical molecular electronic parameters. The future application of this methodology will be useful in this field because can allow researchers to elucidate new sets of plant extract corrosion inhibitors.
The study of green corrosion inhibitors derived from plant extracts represents a promising frontier in the field of materials conservation, particularly for metals like bronze and copper. Researchers have explored a variety of plant-based inhibitors, such as tannic acid, Aloe vera, Ceratonia siliqua, acacia fruit, olive leaf extract, Saccharum officiniarum, Jatropha extract, papaya leaves, Pasiflora edulia Sims, and Dimocarpus longan by assessing their effectiveness in inhibiting corrosion under different conditions. These studies have provided valuable insights into the potential of eco-friendly inhibitors to protect cultural heritage materials. While challenges such as concentration optimisation and temperature sensitivity persist, the continued research and development of green corrosion inhibitors offer a sustainable and environmentally friendly approach to preserving our cultural heritage for generations to come.
References
- Verma, C.; Ebenso, E.E.; Bahadur, I.; Quraishi, M.A. An Overview on Plant Extracts as Environmental Sustainable and Green Corrosion Inhibitors for Metals and Alloys in Aggressive Corrosive Media. J. Mol. Liq. 2018, 266, 577–590.
- Argyropoulos, V.; Rameau, J.J.; Dalard, F.; Degrigny, C. Testing Hostacor It as a Corrosion Inhibitor for Iron in Polyethylene Glycol Solutions. Stud. Conserv. 1999, 44, 49–57.
- Benzidia, B.; Hammouch, H.; Dermaj, A.; Benassaoui, H.; Abbout, S.; Hajjaji, N. Investigation of Green Corrosion Inhibitor Based on Aloe vera (L.) Burm. F. for the Protection of Bronze B66 in 3% NaCl. Anal. Bioanal. Electrochem. 2019, 11, 165–177.
- Li, M.; Jia, X.; Yang, J.; Deng, J.; Zhao, G. Effect of Tannic Acid on Properties of Soybean (Glycine Max) Seed Ferritin: A Model for Interaction between Naturally-Occurring Components in Foodstuffs. Food Chem. 2012, 133, 410–415.
- Gülçin, I.; Huyut, Z.; Elmastaş, M.; Aboul-Enein, H.Y. Radical Scavenging and Antioxidant Activity of Tannic Acid. Arab. J. Chem. 2010, 3, 43–53.
- Rahman, Z.; Zidan, A.S.; Khan, S.R.; Reddy, I.K.; Khan, M.A. Cholorpheniramine Tannate Complexes: Physicochemical, Chemometric, and Taste Masking Evaluation. Int. J. Pharm. 2012, 436, 582–592.
- Vankar, P.S.; Shanker, R.; Verma, A. Enzymatic Natural Dyeing of Cotton and Silk Fabrics without Metal Mordants. J. Clean. Prod. 2007, 15, 1441–1450.
- Kusmierek, E.; Chrzescijanska, E. Tannic Acid as Corrosion Inhibitor for Metals and Alloys. Mater. Corros. 2015, 66, 169–174.
- Fouda, A.S.; Shalabi, K.; Idress, A.A. Ceratonia Siliqua Extract as a Green Corrosion Inhibitor for Copper and Brass in Nitric Acid Solutions. Green Chem. Lett. Rev. 2015, 8, 17–29.
- Pourzarghan, V.; Fazeli, B. The Use of Robinia Pseudoacacia L Fruit Extract as a Natural Corrosion Inhibitor in the Protection of Copper-Based Objects. Herit. Sci. 2021, 9, 75.
- Rahal, C.; Masmoudi, M.; Abdelhedi, R.; Sabot, R.; Jeannin, M.; Bouaziz, M.; Refait, P. Olive Leaf Extract as Natural Corrosion Inhibitor for Pure Copper in 0.5 M NaCl Solution: A Study by Voltammetry around OCP. J. Electroanal. Chem. 2016, 769, 53–61.
- Gao, Z.; Sun, P.; Du, L.; Zhang, X.; Bai, J.; Xing, H.; Yan, Y. Saccharum Officinarum Leaf Extract as Corrosion Inhibitor of Copper Corrosion in Sulphuric Acid Solution: Experiments and Theoretical Calculations. Int. J. Electrochem. Sci. 2021, 16, 211126.
- Elshahawi, A.; Rifai, M.; Hamid, Z.A. Corrosion Inhibition of Bronze Alloy by Jatropha Extract in Neutral Media for Application on Archaeological Bronze Artifacts. Egypt. J. Chem. 2022, 65, 869–878.
- Tan, B.; Xiang, B.; Zhang, S.; Qiang, Y.; Xu, L.; Chen, S.; He, J. Papaya leaves extracts as a novel eco-fiendly corrosion inhibitor for Cu in H2SO4 medium. J. Colloid Interface Sci. 2021, 582, 918–931.
- Tan, B.; Lan, W.; Zhang, S.; Deng, H.; Qiang, Y.; Fu, A.; Ran, Y.; Xiong, J.; Marzouki, R.; Li, W. Passiflora edulia Sims leaves Extract as renewable and degradable inhibitor for copper in sulfuric acid solution. Colloids Surf. A Physicochem. Eng. Asp. 2022, 645, 128892.
- Dahmani, K.; Galai, M.; Ouakki, M.; Elgendy, A.; Ez-Zrioulli, R.; Lachhab, R.; Briche, S.; Cherkaoui, M. Corrosion inhibition of copper in sulfuric acid via environmentally friendly inhibitor (Myrtus Communis): Combining experimental and theoretical methods. J. Mol. Liq. 2022, 347, 117982.
- Cao, L. Dimocarpus longan Lour leaf extract as green corrosion inhibitor for copper in sulfuric acid solution. Int. J. Electrochem. Sci. 2022, 17, 2.
- Ben Channouf, R.; Souissi, N.; Bellakhal, N. Juniperus communis Extract Effect on Bronze Corrosion in Natural 0.5M Chloride Medium. J. Mater. Sci. Eng. 2015, 3, 21–29.
- Kokalj, A.; Lozinsek, M.; Kapun, B.; Taheri, P.; Neupane, S.; Losada-Pérez, P.; Xie, C.; Milosev, I. Simplistic correlations between molecular electronic properties and inhibition efficiencies: Do they really exist? Corros. Sci. 2021, 179, 108856.
More
Information
Subjects:
Chemistry, Applied
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
2 times
(View History)
Update Date:
28 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




