
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hong Geng | -- | 2567 | 2024-02-27 16:50:53 | | | |
| 2 | Wendy Huang | Meta information modification | 2567 | 2024-02-29 08:51:18 | | |
Video Upload Options
Salt Lakes, having a salt concentration higher than that of seawater and hosting unique extremophiles, are predominantly located in drought-prone zones worldwide, accumulating diverse salts and continuously emitting salt dust or aerosols. Salt Lake aerosols are produced through various processes, including lake-water spray, evaporation-induced salt crystallization, wind-driven dust emissions, microbial activities, chemical reactions, and anthropogenic influences. The primary mechanism involves the breaking of wind-driven waves at the lake surface. As the wind blows across the water, it generates waves. When these waves reach a critical size, they break and release tiny droplets into the air, which become airborne particles and form aerosols.
1. Introduction
2. Chemical Composition of Salt Lake Aerosols
| Site | Sampling Period | Size Fraction | Na+ | NH4+ | K+ | Mg2+ | Ca2+ | Cl− | NO3− | SO42− | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Urmia Lake | Jan–Sep (2013) | TSP-(PM10) (Mean value) |
1.99 | 0.87 | 0.47 | 0.17 | 2.09 | 1.88 | 2.81 | 4.20 | [28] |
| Nov (2007), (Sample A) | TSP | 0.13 | 0.13 | 0.04 | 0.02 | 0.13 | 0.11 | 0.13 | 0.39 | ||
| Lake Eyre | Sample (E) | TSP | 0.14 | 0.20 | 0.04 | 0.02 | 0.23 | 0.08 | 0.17 | 0.58 | [29] |
| Qinghai Lake | June–Sep (2010) | PM2.5 | 0.13 | - | 0.12 | 0.06 | 0.23 | 0.07 | 0.38 | 4.45 | [33] |
| TSP | 0.48 | - | 0.13 | 0.26 | 0.72 | 0.39 | 1.3 | 5.04 |
3. Health Effects of Salt Lake Aerosols
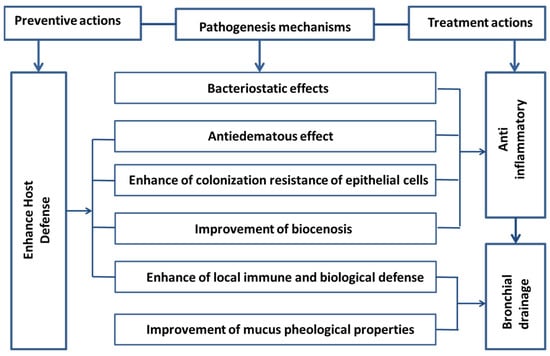
References
- Wang, Z.; Luo, P.; Zha, X.; Xu, C.; Kang, S.; Zhou, M.; Nover, D.; Wang, Y. Overview assessment of risk evaluation and treatment technologies for heavy metal pollution of water and Soil. J. Clean. Prod. 2022, 379, 134043.
- Grythe, H.; Ström, J.; Krejci, R.; Quinn, P.; Stohl, A. A review of sea-spray aerosol source functions using a large global set of sea salt aerosol concentration measurements. Atmos. Chem. Phys. 2014, 14, 1277–1297.
- Wang, X.; Hua, T.; Zhang, C.; Lang, L.; Wang, H. Aeolian salts in Gobi deserts of the western region of Inner Mongolia: Gone with the dust aerosols. Atmos. Res. 2012, 118, 1–9.
- Chen, J.; Li, C.; Ristovski, Z.; Milic, A.; Gu, Y.; Islam, M.S.; Wang, S.; Hao, J.; Zhang, H.; He, C.; et al. A review of biomass burning: Emissions and impacts on air quality, health and climate in China. Sci. Total Environ. 2017, 579, 1000–1034.
- Wang, X.; Hua, T.; Zhang, C.; Qian, G.; Luo, W. Salts in the clay playas of China’s arid regions: Gone with the wind. Environ. Earth Sci. 2012, 68, 623–631.
- Gill, T.E. Eolian sediments generated by anthropogenic disturbance of playas: Human impacts on the geomorphic system and geomorphic impacts on the human system. Geomorphology 1996, 17, 207–228.
- Zhang, X.; Zhuang, G.; Yuan, H.; Rahn, K.A.; Wang, Z.; An, Z. Aerosol particles from dried salt-lakes and saline soils carried on dust storms over Beijing. Terr. Atmos. Ocean. Sci. 2009, 20, 619–628.
- Abuduwaili, J.; Gabchenko, M.V.; Junrong, X. Eolian transport of salts—A case study in the area of Lake Ebinur (Xinjiang, Northwest China). J. Arid. Environ. 2008, 72, 1843–1852.
- Prospero, J.M. Environmental characterization of global sources of atmospheric soil dust identified with the NIMBUS 7 Total Ozone Mapping Spectrometer (TOMS) absorbing aerosol product. Rev. Geophys. 2002, 40, 2-1–2-31.
- Gaston, C.J.; Pratt, K.A.; Suski, K.J.; May, N.W.; Gill, T.E.; Prather, K.A. Laboratory studies of the cloud droplet activation properties and corresponding chemistry of saline playa dust. Environ. Sci. Technol. 2017, 51, 1348–1356.
- Ramanathan, V.C.P.J.; Crutzen, P.J.; Kiehl, J.T.; Rosenfeld, D. Aerosols, climate, and the hydrological cycle. Science 2001, 294, 2119–2124.
- Lai, H.W.; Chen, H.W.; Kukulies, J.; Ou, T.; Chen, D. Regionalization of seasonal precipitation over the Tibetan Plateau and associated large-scale atmospheric systems. J. Clim. 2021, 34, 2635–2651.
- Chen, S.; Xue, L.; Yau, M.K. Impact of aerosols and turbulence on cloud droplet growth: An in-cloud seeding case study using a parcel–DNS (direct numerical simulation) approach. Atmos. Chem. Phys. 2020, 20, 10111–10124.
- Cziczo, D.J.; Froyd, K.D.; Hoose, C.; Jensen, E.J.; Diao, M.; Zondlo, M.A.; Murphy, D.M. Clarifying the dominant sources and mechanisms of cirrus cloud formation. Science 2013, 340, 1320–1324.
- Hoose, C.; Möhler, O. Heterogeneous ice nucleation on atmospheric aerosols: A review of results from laboratory experiments. Atmos. Chem. Phys. 2012, 12, 9817–9854.
- Intergovernmental Panel on Climate Change (IPCC). Climate Change—The Physical Science Basis; Working Group I Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2021.
- Helfer, F.; Lemckert, C.; Zhang, H. Impacts of climate change on temperature and evaporation from a large reservoir in Australia. J. Hydrol. 2012, 475, 365–378.
- Hussein, A.M.; Al-Zubaidi, A.; Naje, A.S.; Al-Ridah, Z.A.; Chabuck, A.; Ali, I.M. A statistical technique for modelling dissolved oxygen in salt lakes. Cogent. Eng. 2021, 8, 1875533.
- Obianyo, J.I. Effect of salinity on evaporation and the water cycle. Emerg. Sci. J. 2019, 3, 255–262.
- Irwandi, H.; Rosid, M.S.; Mart, T. The effects of ENSO, climate change and human activities on the water level of Lake Toba, Indonesia: A critical literature review. Geosci. Lett. 2021, 8, 21.
- Patti, B.; Fiorenti, F.; Fortibuoni, T.; Somarakis, S.; García-Lafuente, J. Editorial: Impacts of environmental variability related to climate change on biological resources in the Mediterranean. Front. Mar. Sci. 2022, 9, 1059424.
- Mojtahedi, A.; Dadashzadeh, M.; Azizkhani, M.; Mohammadian, A.; Almasi, R. Assessing climate and human activity effects on lake characteristics using spatio temporal satellite data and an emotional neural network. Environ. Earth Sci. 2022, 81, 61.
- Tusupova, K.; Peder Hjorth, A.; Morave, M. Drying lakes: A review on the applied restoration strategies and health conditions in contiguous areas. Water 2020, 12, 749.
- Izdebski, A.; Pickett, J.; Roberts, N.; Waliszewski, T. The environmental, archaeological and historical evidence for regional climatic changes and their societal impacts in the Eastern Mediterranean in Late Antiquity. Quat. Sci. Rev. 2015, 136, 189–208.
- Satgé, F.; Espinoza, R.; Zolá, R.; Roig, H.; Timouk, F.; Molina, J.; Garnier, J.; Calmant, S.; Seyler, F.; Bonnet, M.P. Role of climate variability and human activity on Poopó Lake droughts between 1990 and 2015 assessed using remote sensing data. Remote Sens. 2017, 9, 12.
- Farebrother, W.; Hesse, P.P.; Chang, H.C.; Jones, C. Dry lake beds as sources of dust in Australia during the Late Quarternary: A volumetric approach based on lake bed and deflated dune volumes. Quat. Sci. Rev. 2017, 161, 81–98.
- Hesam, A.-B.; Parisa, R.; Joseph, S.S.; Alberto, C.-R.; Mojtaba, A.A.; Armin, S. Is there a relationship between Lake Urmia saline lakebed emissions and wet deposition composition in the caucasus region? ACS Earth Space Chem. 2021, 5, 2970–2985.
- Gholampour, A.; Nabizadeh, R.; Hassanvand, M.S.; Taghipour, H.; Nazmara, S.; Mahvi, A.H. Characterization of saline dust emission resulted from Urmia Lake drying. J. Environ. Health. Sci. Eng. 2015, 28, 82.
- Radhi, M.; Box, M.A.; Box, G.P.; Mitchell, R.M.; Cohen, D.D.; Stelcer, E.; Keywood, M.D. Size-resolved mass and chemical properties of dust aerosols from Australia’s Lake Eyre Basin. Atmos. Environ. 2010, 44, 3519–3528.
- Liu, X.Q.; Shen, J.; Wang, S.M.; Yang, X.D.; Tong, G.B.; Zhang, E.L. A 16000-year pollen record of Qinghai Lake and its paleoclimate and paleoenvironment. Chin. Sci. Bull. 2002, 47, 1931–1937.
- Xu, H.; Ai, L.; Tan, L.C.; An, Z.S. Stable isotopes in bulk carbonates and organic matter in recent sediments of Lake Qinghai and their climatic implications. Chem. Geol. 2006, 235, 262–275.
- Henderson, A.C.G.; Holmes, J.A.; Zhang, J.W.; Leng, M.J.; Carvalho, L.R. A carbon- and oxygen-isotope record of recent environmental change from Qinghai Lake, NE Tibetan Plateau. Chin. Sci. Bull. 2003, 48, 1463–1468.
- Zhang, N.; Cao, J.; Liu, S.; Zhao, Z.; Xu, H.; Xiao, S. Chemical composition and sources of PM2.5 and TSP collected at Qinghai Lake. Atmos. Res. 2014, 138, 213–222.
- Geng, H.; Hwang, H.; Liu, X.; Dong, S.; Ro, C.U. Investigation of aged aerosols in size-resolved Asian dust storm particles transported from Beijing, China, to Incheon, Korea, using low-Z particle EPMA. Atmos. Chem. Phys. 2014, 14, 3307–3323.
- Yoo, H.; Wu, L.; Geng, H.; Ro, C.-U. Physicochemical and temporal characteristics of individual atmospheric aerosol particles in urban Seoul during KORUS-AQ campaign: Insights from single-particle analysis. Atmos. Chem. Phys. 2024, 24, 853–867.
- Ghale, Y.A.G.; Tayanc, M.; Unal, A. Dried bottom of Urmia Lake as a new source of dust in northwestern Iran: Understanding the impacts on local and regional air quality. Atmos. Environ. 2021, 262, 118635.
- Alizadeh, F.; Hamzehpour, N.; Mola, A.; Abasiyan, S.; Rahmati, M.T. Wind erodibility in the newly emerged surfaces of Urmia Playa Lake and adjacent agricultural lands and its determining factors. Catena 2020, 194, 10467.
- World Health Organization. WHO Global Air Quality Guidelines. 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK574594/ (accessed on 5 February 2024).
- Backer, L.C.; Carmichael, W.; Kirkpatrick, B. Recreational exposure to low concentrations of microsystins during an algal bloom in a small lake. Mar. Drugs 2008, 6, 389–406.
- Glantz, M.H. Creeping Environmental Problems and Sustainable Development in the Aral Sea Basin; Cambridge University Press: Cambridge, UK, 1999.
- Lim, C.C.; Yoon, J.; Reynolds, K.; Geral, L.B.; Ault, A.P.; Heo, S.; Bell, M.L. Harmful algal bloom aerosols and human health. Ebio Med. 2023, 93, 104604.
- Litvinenko, L.I.; Kozlov, A.V.; Kovalenko, A.I.; Bauer, D.S. Salinity of water as a factor to determine the development of the brine shrimp Artemia populations in Siberian lakes. Hydrobiologia 2007, 576, 95–101.
- Asselman, J.; Acker, E.V.; Rijcke, M.D.; Tilleman, L.; Nieuwerbugh, F.V.; Mees, J.; Jansen, C.R. Positive human health effects of sea spray aerosol: Molecular evidence from exposed lung cell lines. bioRxiv 2018, 397141.
- Er, W.; Hou, T.; Bao, Z. Research of clinical efficacy and safety of salt rock aerosol in the treatment of occupational pneumoconiosis. Xinjiang Med. J. 2019, 49, 804–806. (In Chinese)
- Chen, C.; Zhang, Q.; Luo, W.; Liu, Z.; Xu, H.; Wang, Y.; Chen, G.; Cuo, X.; Ming, Y.; Zhang, X.; et al. Effect of rock salt aerosol therapy on quality of life of patients with pneumoconiosis: A multicenter, randomized, double-blind clinical trial. Park. J. Pharm. Sci. 2022, 35, 441–445.
- Hu, X.; Li, L. Short-term and long-term efficacy evaluation of bronchodilators combined with rock salt aerosol in patients with pneumoconiosis complicated with COPD. J. Hunan Normal Univ. Med. Sci. 2021, 18, 149–152. (In Chinese)
- Wang, S.; Zhao, X.; Xu, Q.; Li, X.; Zhang, J.; Hao, X.; Guo, L.; Liu, H. The effect of rock salt aerosol on the prevention of silicosis in rats. Chin. Occup. Med. 2020, 47, 147–153.




