Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ana Rita Jesus | -- | 4421 | 2024-02-27 15:48:28 | | | |
| 2 | Jason Zhu | -3 word(s) | 4418 | 2024-02-28 06:58:05 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Domingues, L.; Duarte, A.R.C.; Jesus, A.R. Deep Eutectic Systems as Alternative Solvents. Encyclopedia. Available online: https://encyclopedia.pub/entry/55557 (accessed on 08 February 2026).
Domingues L, Duarte ARC, Jesus AR. Deep Eutectic Systems as Alternative Solvents. Encyclopedia. Available at: https://encyclopedia.pub/entry/55557. Accessed February 08, 2026.
Domingues, Luis, Ana Rita C. Duarte, Ana Rita Jesus. "Deep Eutectic Systems as Alternative Solvents" Encyclopedia, https://encyclopedia.pub/entry/55557 (accessed February 08, 2026).
Domingues, L., Duarte, A.R.C., & Jesus, A.R. (2024, February 27). Deep Eutectic Systems as Alternative Solvents. In Encyclopedia. https://encyclopedia.pub/entry/55557
Domingues, Luis, et al. "Deep Eutectic Systems as Alternative Solvents." Encyclopedia. Web. 27 February, 2024.
Copy Citation
Chemists in the medicinal chemistry field are constantly searching for alternatives towards more sustainable and eco-friendly processes for the design and synthesis of drug candidates. The pharmaceutical industry is one of the most polluting industries, having a high E-factor, which is driving the adoption of more sustainable processes not only for new drug candidates, but also in the production of well-established active pharmaceutical ingredients. Deep eutectic systems (DESs) have emerged as a greener alternative to ionic liquids, and their potential to substitute traditional organic solvents in drug discovery has raised interest among scientists.
green chemistry
deep eutectic systems
synthesis
1. Introduction
Deep Eutectic Solvents (DESs), i.e., a mixture of two or more compounds that suffer a significant depression in the melting point of each component, were first reported by Abbott et al. [1]. In 2003, they reported a system composed of choline chloride:urea (1:2), two solids at room temperature, with very high melting points (302 °C and 133 °C, respectively) and observed that the melting point of the mixture was −12 °C, meaning that it is liquid at room temperature, representing a significant decrease in their melting points. DESs are mainly formed through the establishment of intermolecular hydrogen bonds, usually between hydrogen bond donors and hydrogen bond acceptors. In terms of physicochemical properties, DESs have: (i) densities usually higher than water, except for hydrophobic systems, (ii) high viscosities at room temperature, (iii) poor conductivity at room temperature due to high viscosity, (iv) polarities dependent on the constituents, (v) different affinity to aqueous systems, depending on the composition, (vi) non-flammability, (vii) non-volatility, (viii) thermal stability, (ix) low toxicity, and (x) high biodegradability [2].
After Abbott’s first report, DESs have emerged mostly with the intention of not only replacing common volatile organic solvents, such as dichloromethane, methanol, chloroform, etc., in many different applications, but also as an alternative to ionic liquids [3].
When it comes to the preparation and discovery of active pharmaceutical ingredients (APIs), DESs have been reported as performing different roles, namely, as solvents or with a solvent/catalyst dual function. Because of this dual role, DESs will be referred to as deep eutectic systems rather than deep eutectic solvents.
Furthermore, DESs can also be used in biocatalytic transformations with pharmaceutical relevance. These topics will be further discussed in the following sections.
2. DESs as Alternative Solvents in Biocatalytic Processes in Drug Discovery
Biocatalysis has been defined as a chemical reaction in which enzymes, obtained from biological sources or whole cells, are employed to speed up the reaction [4].
In the pharmaceutical industry, biocatalysis has become crucial for the preparation of several APIs, mainly due to the sustainability and efficiency associated with these types of processes. Biocatalysts have the great advantage of conferring unparalleled selectivity during the synthesis of pharmaceuticals [5]. Furthermore, when optimized it can lead to robustness and scalable reactions. In the early days, hydrolase reactions were largely performed in the pharmaceutical industry [6], but others followed, including keto-reductases, transaminases, aldolases, and hydroxy nitrile lyases. Currently, researchers have been looking for other enzyme classes to be used in chemical processes, bridging the vast number of enzymes present in nature and the limited number of enzymes used in biocatalytic processes.
Although biocatalysis is seen as the future of the pharmaceutical industry, mainly because it requires water and phosphate buffers, thus completely avoiding the use of organic solvents, it presents a major drawback, which is related to the water solubility of raw materials [7]. Alternative solvents are being investigated, but such solvents should be non-toxic, biocompatible, biodegradable, and sustainable. It is also important that it should confer stability and maintain the enzyme activity. It has been reported that enzymes can remain stable and active in non-aqueous solvents, such as glycerol, but this is not applicable to all classes of enzymes [8][9][10]. Other alternatives have been searched, namely the use of deep eutectic systems (DESs) [11][12].
In the literature, it is possible to find several reports using DESs in biocatalysis, namely in reductions, oxidations, hydrolysis, esterification, and transesterifications, using isolated/immobilized enzymes or whole cells [12]. Although most reactions are reported per se, they can potentially be used in the preparation of pharmaceuticals. In the next sections, examples of biocatalytic processes for the preparation of bioactive molecules or pharmaceuticals, using DESs as an alternative reaction medium, will be summarized and discussed.
2.1. Enzymatic Reduction of Ketones and Aldehydes to Alcohols
The reduction of ketones and aldehydes into alcohols is quite useful to prepare APIs. At Hoechst AG in Germany, researchers use NaBH4 to reduce a ketone group to an alcohol in the preparation of an intermediate of HMG-CoA reductase inhibitors [13]. Another example, at Novartis, is the racemic synthesis of Fluvastatin, including the reduction of a ketone group into a racemic syn-1,3-diol through chemical methods [14]. However, Cicco et al. reported the first application of DESs on the asymmetric bioreduction of ketones using purified ketoreductases (KREDs) [15]. The authors showed that choline-based DESs (ChCl:Gly, 1:2 or ChCl:Sor, 1:1) were able to reach >99% conversion and up to a >99% enantiomeric excess of the corresponding alcohol, while in neat buffer, >99% conversion was also achieved but with far less stereoselectivity. In fact, they showed that the increase in the DESs % led to a gradual improvement on the stereoselectivity, reaching >99% ee using 80% (w/vw) of the DESs.
Several authors have been reporting the use of alcohol dehydrogenases (ADHs) from horse liver (HLADH) to reduce ketones and aldehydes to alcohols. However, ADHs are cofactor-dependent, usually being combined with an in situ generation system, which requires a second enzymatic step mediated by glucose dehydrogenase or formate dehydrogenase. To overcome this need for a second reaction, Chanquia et al. have been exploring the use of smart co-substrates, which shift the reaction equilibrium, transforming the substrate into a thermodynamically and kinetically stable co-product [16][17]. As a model reaction, reduced cyclohexanone (CHO) to cyclohexanol (CHL), using a buffered solution of choline chloride:1,4-butanediol eutectic mixture as reaction medium (20% v/v), to decrease the viscosity and to provide optimal enzyme activity (Scheme 1A) was used. They were able to obtain a quantitative conversion of CHO to CHL, after 5 h. Afterwards, they reduced cinnamaldehyde to cinnamyl alcohol using the same conditions, with 25% conversion after 48 h (Scheme 1B).
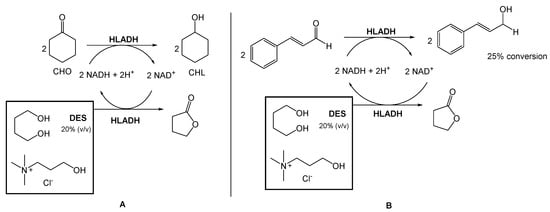
This conversion is highly relevant from an industrial point of view because the cinnamyl alcohol is an intermediate in the production of flunarizine, a selective calcium entry blocker with calmodulin binding properties and histamine H1 blocking activity, used to treat migraines, occlusive peripheral vascular disease, vertigo of central and peripheral origin, and as an adjuvant in the therapy of epilepsy.
In a different report, Bittner et al. investigated the use of ChCl:Gly (1:2), ChCl:EG (1:2), and EACl:Gly (2:3) for the reduction of cyclohexanone into cyclohexanol, also using HLADH (Scheme 2A) [18]. Contrarily to Chanquia’s work, the authors added 1,4-butanediol to the DES mixture. Their findings suggested that, in this case, the use of pure glycerol would be the best choice to achieve the maximum reactivity. However, the high viscosity of glycerol limits its applicability in these reactions. Therefore, the use of glycerol-based DESs provided less solvent viscosity and facilitated the manipulation of glycerol–water mixtures. Inspired by these results, the authors used a DES composed of choline chloride and glycerol, but with a higher glycerol content (1:9) to evaluate the reduction of cinnamaldehyde to cinnamyl alcohol (Scheme 2B). The use of this new DES allowed them to reach a 2.5-fold higher specific activity and a 40% conversion, instead of less than 7% when using a DES with a lower content of glycerol (ChCl:Gly (1:2)).
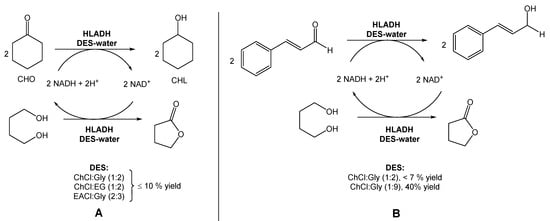
Scheme 2. (A) Alternative bioreduction of cyclohexanone (CHO) into cyclohexanol (CHL) and (B) alternative bioreduction of cinnamaldehyde using ChCl:Gly (1:2 vs. 1:9), reported by Bittner et al. [18].
A DES composed of choline chloride and glucose (1.5:1) was also employed for the bioreduction of ketones into alcohols, using different alcohol dehydrogenases (ADHs) (Scheme 3) [19]. The ratio selected between the two components was chosen so that glucose could be used for cofactor recycling. Furthermore, Mourelle-Insua et al. studied the combination of an aqueous buffer with the DES at up to 50% (v/v) [19]. The use of such high NADES concentration showed two main advantages. First, the presence of glucose enabled the nicotinamide cofactor recycling. Then, the authors were able to conduct the reaction in much higher substrate concentrations in comparison with the buffer system employing glucose/glucose dehydrogenase (GDH). This strategy allowed them to obtain the desired products with >99% conversion and >99% ee.
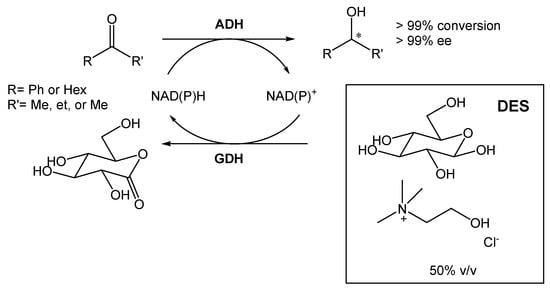
Scheme 3. Alcohol dehydrogenase-catalyzed bioreduction of ketone using ChCl:Glu (1.5:1), able to recycle the nicotinamide cofactor [NAD(P)H].
In another report, Vitale and co-workers [20] reported the use of ChCl:Gly (1:2) in 20 or 40% water (w/v), in the stereoselective enzymatic reduction of acetophenone derivatives with baker’s yeast RC, during the preparation of rivastigmine, an API used to manage and treat neurodegenerative diseases, such as dementia, in patients with Alzheimer’s and Parkinson disease (Scheme 4). From the several reaction conditions tested, it was observed that in a 40 w% aqueous solution of ChCl:Gly (2:1), it was possible to achieve the highest reaction yield. When water was used as the medium, the maximum yield was 27% after 120 h of reaction.
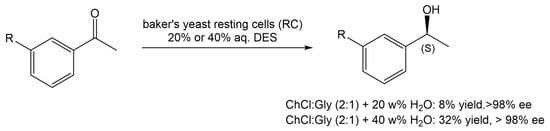
Scheme 4. Bioreduction of acetophenone derivatives in the presence of baker’s yeast and an aqueous solution of DES.
2.2. Bioreduction of Imines to Amines
About 40% of pharmaceutical drugs contain a chiral amine group. These can be obtained through the hydrogenation of imines, enamines, and their derivatives; but most of these reactions require expensive precious metal catalysts [21]. Because of this limitation, several sustainable biocatalytic routes are being used in the industry for the preparation of chiral amines that include lipases [22], transaminases [23], ammonia lyases [24], amine dehydrogenases [25], and amine oxidases [26]. In 2010, imine reductases (IREDs) were first described for the reduction of the imine 2-methyl-1-pyrroline into (R) or (S)-2-methylpyrrolidine [27]. Since then, interest on this enzyme family has grown [28]. These enzymes are NADPH-dependent enzymes able to successfully produce primary, secondary, and tertiary amines from cyclic imines.
Recently, Arnodo et al. reported the use of IREDs in DESs for the reduction of cyclic imines into amines (Scheme 5) [29]. They started by using a choline chloride:glycerol DES (1:2) using the IRED-44 enzyme. They observed that when the reaction was carried out in pure DES, which has a lower viscosity when compared with other DESs, it was not possible to recover the desired product, even when using a high concentration of the substrate. Therefore, they attempted to use a 50% (v/v) DES solution in phosphate buffer (PB), which is the optimal amount of DES used in other studies using other reductase enzymes, such as KREDs. This change in the protocol led to the recovery of the product in good yield in both PB and DES/PB mixtures, even at low concentrations of the substrate.
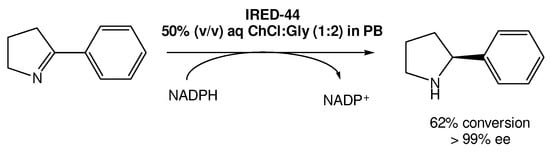
Scheme 5. Imine enzymatic reduction in a 50% (v/v) DES/PB mixture in the presence of IRED-44.
However, the authors observed that once using higher concentrations (100 mM) of the substrate, the enzymes became ineffective, without product formation. But, using the DES/PB mixtures as solvents they were able to not only recover the product, but also improve the reaction yield, reaching a maximum of 62% yield with >99% ee. Nevertheless, even when using substrate concentrations higher than 100 mM it was possible to reach 49–51% yields, which does not represent a significant decrease in its efficiency.
Overall, another important and relevant outcome from this initial study was that it was possible to work at higher concentrations without the need to increase the solvent volume.
Wang et al. designed a new DES composed of choline chloride and glutathione, and utilized it for the asymmetric reductions of 3,5-bis(trifluorometyl) acetophenone (3,5-BTAP), an important intermediate in the preparation of (R)-[3,5-Bis(trifluoromethyl)phenyl]ethanol ((R)-BTPE), which is a building block in the synthesis of antiemetic drugs used to prevent chemotherapy-induced side effects, such as aprepitant and fosaprepitant (Scheme 6) [30]. the authors reported the use of whole cells of Trichoderma asperellum ZJPH0810. Similarly to other studies using DESs in biocatalytic processes, also in this report the authors were able to significantly increase the concentration of the substrate (up to a 2-fold increase). Comparing the reaction in the absence or presence of the DES in the solvent, the yield increased from 53.1% to 70.8%. The authors even compared the effect of replacing the DES with the [BMIM][PF6] ionic liquid. In terms of substrate concentration, the use of a DES-based medium and an IL-based medium allowed the use of higher concentrations (70 mM—IL vs. 100 mM—DES). In terms of conversion yield, the presence of ChCl:GSH afforded higher values (87.6%) than using the [BMIM][PF6] ionic liquid (82.5%). Later, they tested the same DES in other conversions, namely the reduction of ethyl acetoacetate (EAA) and 5-(4-fluorophenyl)-5-oxopentanoic acid (FPOPA) (Scheme 6). In the first case, the conversion of EAA in EHB resulted in similar results (83.8% yield and >99 ee%) when the reaction was performed in a DES-based medium and water, although faster. In the second case, the biocatalytic reduction of FPOPA in the presence of the DES resulted in significantly higher reaction yields than in water, after the same reaction time (83.9% vs. 65.9%). Both reactions were carried out with the same substrate concentration and reaction time.
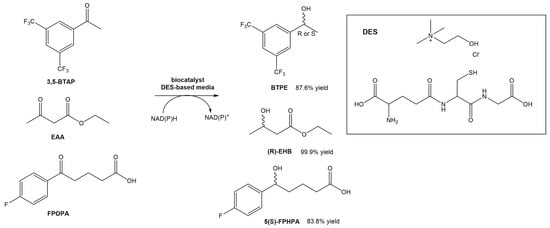
Scheme 6. Bioreduction of 3,5-BTAP, EAA and FPOPA in a DES-based medium.
2.3. Oxidation
Many bioactive molecules are prepared through oxidated building blocks, hence the high relevance of bio-oxidations. Enzymes employed in this type of reaction have a metal core (either iron or copper) that interacts with oxygen atoms, promoting their transfer to the substrates.
Laccase, a copper-containing enzyme, is one of the most used enzymes in bio-oxidations. It catalyzes the single-electron oxidation of organic and inorganic compounds through the reduction of molecular oxygen in water [31].
To understand the applicability of DESs as solvents or cosolvents in laccase-catalyzed reactions, Toledo et al. investigated the effect of 16 aqueous choline- and betaine-based DES solutions on laccase activity, under different conditions [32]. The preliminary results showed that the presence of ChCl in the media deactivated the enzyme, especially if the DES is used at a high concentration (50 w%). They further confirmed the negative effect of ChCl by increasing the ChCl ratio in DESs (1:2 vs. 2:1), supporting the fact that the Cl ion is responsible for the deactivation of the enzyme, as reported in the literature [33]. Replacing the ChCl with cholinium-based salts was investigated, namely, ChDHP (choline dihydrogen phosphate) and ChDHC (choline dihydrogen citrate), and significant improvements were observed. Regarding the betaine-based DES aqueous solutions they were able to maintain, or even increase by 20%, the laccase activity when compared to the buffer control.
Oligomeric derivatives of flavonoids are promising molecules for pharmaceutical applications due to their strong antioxidant, anticarcinogenic, and antiviral properties. Considering this, Yaropolov and co-workers [34] investigated the laccase-catalyzed oxidative polymerization of dihydroquercetin (DHQ), a natural flavonoid, in the presence of a (2,2,6,6-tetramethylpiperidin-1-yl)oxyl radical (TEMPO), and using a DES-derived buffer as the medium (Scheme 7). The authors evaluated the activity of laccase in the pure DES and observed that it resulted in the complete inactivation of the enzyme. But, as previously showed and reported by several authors, the oxidoreductase activity is maintained using DES-buffered solutions [32][35][36][37]. Bearing this in mind, the reaction using a 60% (v/v) aqueous system Bet:Gly (1:2) and 0.61% of TEMPO was investigated. A high loading of DHQ was reached (>17 mM) and the enzyme remained stable for 12 h. This reaction afforded a product with 58% yield and a molecular weight of 1800 g/mol, with an average length of six monomers. The results showed that the eutectic system Bet:Gly (1:2) can be used for the laccase-catalyzed polymerization of dihydroquercetin, thus fulfilling the green chemistry parameters.
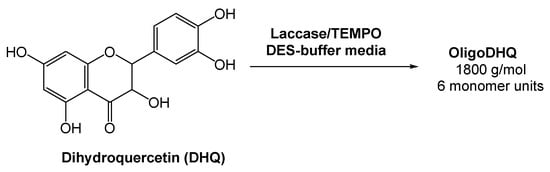
Scheme 7. Laccase-catalyzed oxidative polymerization of dihydroquercetin.
A different approach, using whole cell oxidations is also common to produce bioactive molecules. Prednisone acetate (PA) is a steroid with anti-inflammatory and anti-allergy properties, and it can be obtained from cortisone acetate (CA) through a Δ1,2-dehydrogenation reaction using Arthrobacter simplex cells (Scheme 8). Lu et al. investigated this biotransformation using choline-based DESs as alternative solvents. From the three DESs tested, ChCl:Gly, ChCl:EG, and ChCl:U, the last one showed the best performance, allowing for a 93% conversion [38]. The authors also tested the recyclability of this medium and, interestingly, only a slight decrease to 81% conversion after five cycles, was observed. More importantly, the conversion was more efficient using a DES-based aqueous medium (93%) than without the DES (72%), demonstrating their high relevance in biohydrogenation reactions.
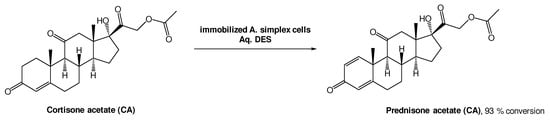
Scheme 8. Δ1,2-dehydrogenation bioreaction using Arthrobacter simplex cells in aqueous solutions of DESs.
In 2020, Mao et al. proposed and studied the combination of the fungus Penicillium raistrickii and DESa in the industrial process for the 15α-hydroxylation of D-ethylgonendione (Scheme 9), which is a key intermediate in the synthesis of gestodene (15α-hydroxyl-D-ethylgonendione), a progestin used in menopausal hormonal therapy [38].
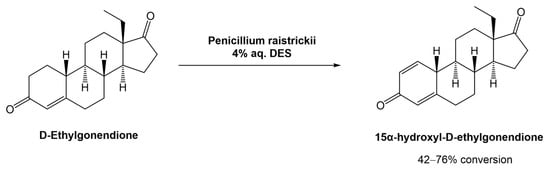
Scheme 9. Biocatalytic 15α-hydroxylation of D-ethylgonendione in aqueous solutions of DESs.
16 choline-based DESs were used. It was observed that the composition of the DESs had a great impact on the success of the biotransformation. For example, when ChCl was combined with organic acids (lactic acid, oxalic acid, malonic acid, citric acid, and malic acid), there was no recovery of the desired product, mostly linked to the acidity of the medium which can inactivate the fungus. Comparing the combination of ChCl with sugars (glucose, fructose, sucrose, or xylose), urea, or polyols (ethylene glycol, glycerol, or sorbitol), the results showed that the polyols afforded the product in higher yields (>50%), with ChCl:Gly (1:2) being the best system (76% conversion). Although the ChCl:sugar systems also showed high efficiency in the biotransformation (42–46%), their viscosity hindered the product isolation. The authors compared the efficiency of the DESs against the physical mixture (ChCl + Gly) and the individual components alone in the reaction media, in which the DESs worked significantly better.
The concentration of the DES in the media was also optimized and 4% (v/v) afforded the best results, which can be justified by the high viscosity when higher concentrations of DESs were used. Furthermore, they also compared the reaction time against conversion rate with and without the DES. The substrate conversion in DES-based media reached 82% after 72 h, while the control reaction, i.e., without DES, was only 33% after the same time. Additionally, the authors showed that the DES ChCl:Gly (1:2) is highly biocompatible with the fungus used in these transformations.
2.4. Hydrolysis
Hydrolases, another common enzyme family used in the preparation of relevant molecules, catalyze bond cleavage using water, and the transformation does not require the use of cofactors.
The gisennoside compound K (CK) is well absorbed in the body and is the main compound responsible for ginseng’s bioactivity, including antitumoral bioactivity. Since this compound is rarely found in natural sources, researchers have been searching for an easy way to prepare it, and enzymatic strategies have aroused their interest. The methods for CK preparation can be found in the literature [39][40][41]. However, the poor solubility of some intermediates can hinder the outcome. The use of organic solvents could solve this solubility problem, but it is against the green chemistry principles. DESs emerged as a potential solution for solubility and enzyme stability problems, and since there were no enzymatic processes reported for the ginsenoside conversion, Han et al. studied the use of these alternative solvents in CK biosynthesis (Scheme 10) [42]. They combined choline chloride with different hydrogen bond donors such as glycerol, ethylene glycol, and urea. After some studies, they concluded that the performance of the enzymatic reaction was significantly higher in polyols-based DESs. In terms of substrate solubility, the substrate was much more soluble in the DES–aqueous medium than in acetate buffer. The reaction carried out with 30% (v/v) ChCl:EG (2:1) reached the maximum conversion (89.1%), while in acetate buffer it was only possible to reach 29.1% conversion.
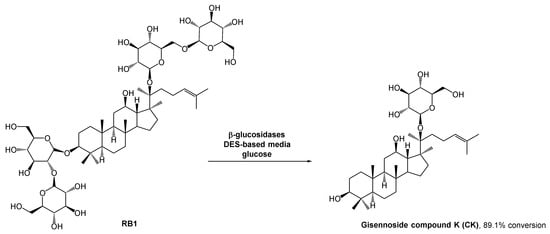
Scheme 10. β-Glucosidase catalyzed hydrolysis of RB1 to afford ginennoside compound K (CK) in the presence of a DES-aqueous medium.
As expected, higher DES concentrations lead to lower yields, resulting from the inactivation of the enzyme. the authors also observed that when using high concentrations of the substrate, the reaction yield decreased. Therefore, a fed-batch system was developed in which the substrate was continuously fed into the reaction vessel and the product was continuously removed. This strategy mitigated the inhibition caused by the high viscosity of the initial mixture and the accumulation of CK, and might be a sustainable solution for the large production of CK, thus allowing to increase the conversion yield up to 89.1%%, 29% higher than using only traditional aqueous media.
Polyphenols, such as quercetin, have several biological activities, hence they are desired target molecules for both the food and pharmaceutical industries [43][44][45] Most flavonoids are found in flowers, leaves, and fruits, mainly in glycosides and aglycones forms.
In industrial processes, quercetin is usually prepared via hydrolysis from rutin, which is found in high content in Sophora japonica. Rutin is usually extracted using traditional organic solvents. However, many authors have already reported the use of DESs for the extraction of rutin from Sophora japonica [46][47][48][49][50]. All of them found that DESs are much more efficient for rutin extraction than common organic solvents, such as ethanol and methanol. Based on these studies, Zang and co-workers [51] studied the conversion of rutin, extracted with DESs, in quercetin with the degrading enzyme (RDE) obtained from germinated tartary buckwheat in situ (Scheme 11).
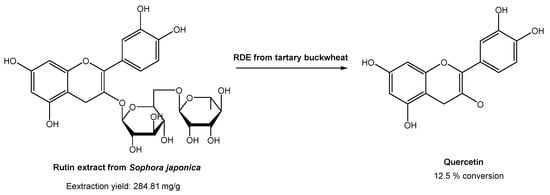
Scheme 11. Quercetin preparation from rutin extracted from Sophora japonica, in a DES-aqueous medium, using degrading enzyme (RDE) from germinated tartary buckwheat.
Briefly, several DESs were used to extract rutin, but the most promising ones were ChCl:Gly and EG:Ala. Later, they studied the effect of water in the extraction process. In the case of ChCl, the increase in water content up to 20% increased the rutin recovery efficiency. On the other hand, the increase in water content in EG:Ala resulted in a decrease in the extraction efficiency. After selecting the best system, the activity of degrading enzyme in DESs was evaluated to assess its applicability of rutin conversion into quercetin. Using ChCl:Gly with 20% water, the best catalytic activities were obtained. After the optimization of the catalytic reaction conditions (pH, time, substrate concentration, enzyme amount, and temperature), it was possible to reach only 12.5% conversion. Although the traditional process reached higher yields (50% yield), the methodology proposed by the authors is far more sustainable and environmentally friendly, since the study involved sustainable and eco-friendly solvents for both substrate extraction and catalytic reaction, and even the enzyme was isolated from a plant.
2.5. Esterification and Transesterifications
Esterification and transesterification are among the most common organic transformations in industry, including the pharmaceutical industry. Lipases are probably the mostly used enzymes in these transformations [52][53][54][55][56]. In drug discovery, esterification can play an important role, especially in the design of prodrugs, commonly used to improve the pharmacokinetic and pharmacodynamic properties of a drug [57].
For example, dihydromyricetin (DMY), a flavonol derivative showing promising bioactivities that include antioxidant, anti-inflammatory, analgesic, and others. However, the corresponding acetate presents an improved liposolubility and even an improved antioxidant activity. The chemical synthesis of DMY acetate presents several problems, namely low yield, low regioselectivity, and harsh reaction conditions, and it requires time-consuming purification steps. To overcome all these problems, enzymatic synthesis has been considered, but solvent incompatibility also represents an issue. Cao et al. explored the acylation of DMY using Aspergillus niger lipase (ANL) which was immobilized in magnetic nanoparticles (ANL@PD-MNPs) using a DES as the reaction medium. They used DESs (ChCl:Gly, ChCl:Xyl, ChCl:U) combined with DMSO, which was already successfully used in these types of reactions [58][59]. The substrate was significantly more soluble (1.73-fold) in DESs than in DMSO alone; however, the reaction did not occur in the pure DES. Therefore, the authors screened different volume fractions of DES/DMSO and concluded that 1:3 (v/v) was the optimal value, allowing a 91.3% conversion against 18.8% with 3:1 (v/v). In DMSO alone, the conversion rate is reported as 79.3% [60], hence a significant improvement by the combination of DMSO with DES.
The authors also evaluated the recyclability of ANL@PD-MNPs in ChCl:Gly/DMSO (1:3, v/v). The enzyme retained its catalytic activity (>90%) up to 5 cycles, although after 10 cycles, its activity still allowed a 56.7% conversion.
In a different study, conducted by Liu and co-workers, 1-caffeoylglycerol (1-CG) was obtained via a transesterification from methyl caffeate (MC) and glycerin, using Novozym 435 in several solvents, including DMSO, acetone, and DES media. Using a batch reactor, the maximum reaction yield, 90.63%, was obtained using 10% (v/v) ChCl:U in glycerol, at 75 °C and 10 h. As an alternative, using a microreactor at 65 °C for 150 min, the reaction yield was significantly improved, up to 96.44%. The authors observed that the temperature played a significant role in this high yield, most likely due to the drastic decrease in the viscosity of the reaction media.
The recyclability of Novozym 435 was also evaluated, due to its relevance for industrial applications. A significant decrease in reaction yield was observed after only 16 cycles, although after 20 runs the yield remained higher than 50%, showing that when the reaction is carried out in the microreactor, the catalytic activity and regioselectivity of the enzyme is maintained.
2.6. Other Transformations
The amine and amide chemical groups are widely spread among active compounds, including peptides and oligo peptides.
Cefaclor, a cephalosporin second-generation antibiotic, has been prepared using biocatalysis through the immobilization of penicillin acylase in magnetic nanocrystalline cellulose [61]. But the substrate 7-amino-3-deacetoxycephalosporanic acid (7-ACCA) presented a low solubility in aqueous buffer, limiting the reaction rate and leading to undesirable by-products. The use of traditional organic solvents, such as DMSO, ethanol, acetonitrile, acetone, etc., could easily solve this solubility issue. However, their incompatibility with enzymatic systems limits further development.
As mentioned before, DESs emerged to solve problems like this, and Wu et al. investigated the preparation of cefaclor using penicillin acylate (PA) in immobilized magnetic nanocrystalline cellulose (MNCC) and DESs as reaction media (Scheme 12) [62]. They combined choline chloride with compounds such as organic acids (citric acid, malic acid, oxalic acid, p-toluene sulfonic acid, and tartaric acid), polyols (butyl glycol, EG, glycerol, propylene glycol, and xylitol), and others (urea and imidazole). When the reaction was screened using the selected DES, ChCl:EG (1:2) in aqueous buffer (30% v/v) afforded the highest yield (ca. 89%), slightly higher than in water. Although this was already a good result, the authors noticed that the increase in DES:buffer lead to the improvement of the reaction outcome, reaching the maximum yield (91.2%) at 70% (v/v) concentration of DES. Considering the side reaction, namely hydrolysis, the authors verified that it was possible to increase the synthesis to hydrolysis (S/H) ratio from 1.24, in water, to 1.8 in DES–buffer mixtures.
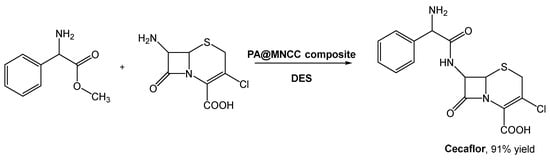
Scheme 12. Biosynthesis of Cecaflor using DESa as solvents and penicillin acylate in immobilized magnetic nanocrystalline cellulose (PA@MNCC).
References
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147.
- Omar, K.A.; Sadeghi, R. Physicochemical properties of deep eutectic solvents: A review. J. Mol. Liq. 2022, 360, 119524.
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural deep eutectic solvents—Solvents for the 21st century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071.
- Paul, P.E.V.; Sangeetha, V.; Deepika, R.G. Chapter 9—Emerging Trends in the Industrial Production of Chemical Products by Microorganisms. In Recent Developments in Applied Microbiology and Biochemistry; Buddolla, V., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 107–125.
- Lewis, R.D.; France, S.P.; Martinez, C.A. Emerging Technologies for Biocatalysis in the Pharmaceutical Industry. ACS Catal. 2023, 13, 5571–5577.
- Hauer, B. Embracing Nature’s Catalysts: A Viewpoint on the Future of Biocatalysis. ACS Catal. 2020, 10, 8418–8427.
- Hollmann, F.; Fernandez-Lafuente, R. Grand Challenges in Biocatalysis. Front. Catal. 2021, 1, 633893.
- Castro, G.R.; Knubovets, T. Homogeneous biocatalysis in organic solvents and water-organic mixtures. Crit. Rev. Biotechnol. 2003, 23, 195–231.
- Holtmann, D.; Hollmann, F. Is water the best solvent for biocatalysis? Mol. Catal. 2022, 517, 112035.
- Wang, M.; Sheng, Y.; Cui, H.; Li, A.; Li, X.; Huang, H. The Role of Glycerol in Preserving Proteins Needs to Be Reconsidered. ACS Sustain. Chem. Eng. 2022, 10, 15175–15185.
- Jesus, A.R.; Paiva, A.; Duarte, A.R.C. Current developments and future perspectives on biotechnology applications of natural deep eutectic systems. Curr. Opin. Green. Sustain. Chem. 2023, 39, 100731.
- Arnodo, D.; Maffeis, E.; Marra, F.; Nejrotti, S.; Prandi, C. Combination of Enzymes and Deep Eutectic Solvents as Powerful Toolbox for Organic Synthesis. Molecules 2023, 28, 100731.
- Beck, G.; Jendralla, H.; Kesseler, K.D. Practical Large Scale Synthesis of tert-Butyl (3R,5S)-6-Hydroxy-3,5-O-isopropylidene-3,5-dihydroxyhexanoate: Essential Building Block for HMG-CoA Reductase Inhibitors. Synthesis 1995, 1995, 1014–1018.
- Fuenfschilling, P.C.; Hoehn, P.; Mutz, J.-P. An Improved Manufacturing Process for Fluvastatin. Org. Process Res. Dev. 2007, 11, 13–18.
- Cicco, L.; Ríos-Lombardía, N.; Rodríguez-Álvarez, M.J.; Morís, F.; Perna, F.M.; Capriati, V.; García-Álvarez, J.; González-Sabín, J. Programming cascade reactions interfacing biocatalysis with transition-metal catalysis in Deep Eutectic Solvents as biorenewable reaction media. Green Chem. 2018, 20, 3468–3475.
- Chanquia, S.N.; Huang, L.; García Liñares, G.; de María, P.; Kara, S. Deep Eutectic Solvents as Smart Cosubstrate in Alcohol Dehydrogenase-Catalyzed Reductions. Catalysts 2020, 10, 1013.
- Huang, L.; Bittner, J.P.; de María, P.; Jakobtorweihen, S.; Kara, S. Modeling Alcohol Dehydrogenase Catalysis in Deep Eutectic Solvent/Water Mixtures. ChemBioChem. 2020, 21, 811–817.
- Bittner, J.P.; Zhang, N.; Huang, L.; de María, P.; Jakobtorweihen, S.; Kara, S. Impact of deep eutectic solvents (DESs) and individual DES components on alcohol dehydrogenase catalysis: Connecting experimental data and molecular dynamics simulations. Green Chem. 2022, 24, 1120–1131.
- Mourelle-Insua, Á.; Lavandera, I.; Gotor-Fernández, V. A designer natural deep eutectic solvent to recycle the cofactor in alcohol dehydrogenase-catalysed processes. Green Chem. 2019, 21, 2946–2951.
- Vitale, P.; Perna, F.M.; Agrimi, G.; Pisano, I.; Mirizzi, F.; Capobianco, R.V.; Capriati, V. Whole-Cell Biocatalyst for Chemoenzymatic Total Synthesis of Rivastigmine. Catalysts 2018, 8, 55.
- Qian, D.; Bera, S.; Hu, X. Chiral Alkyl Amine Synthesis via Catalytic Enantioselective Hydroalkylation of Enecarbamates. J. Am. Chem. Soc. 2021, 143, 1959–1967.
- De Miranda, A.S.; Miranda, L.S.M.; de Souza, R.O.M.A. Lipases: Valuable catalysts for dynamic kinetic resolutions. Biotechnol. Adv. 2015, 33, 372–393.
- Guo, F.; Berglund, P. Transaminase biocatalysis: Optimization and application. Green Chem. 2017, 19, 333–360.
- Parmeggiani, F.; Weise, N.J.; Ahmed, S.T.; Turner, N.J. Synthetic and Therapeutic Applications of Ammonia-lyases and Aminomutases. Chem. Rev. 2018, 118, 73–118.
- Knaus, T.; Böhmer, W.; Mutti, F.G. Amine dehydrogenases: Efficient biocatalysts for the reductive amination of carbonyl compounds. Green Chem. 2017, 19, 453–463.
- Batista, V.F.; Galman, J.L.; Pinto, D.C.G.A.; Silva, A.M.S.; Turner, N.J. Monoamine Oxidase: Tunable Activity for Amine Resolution and Functionalization. ACS Catal. 2018, 8, 11889–11907.
- Mitsukura, K.; Suzuki, M.; Tada, K.; Yoshida, T.; Nagasawa, T. Asymmetric synthesis of chiral cyclic amine from cyclic imine by bacterial whole-cell catalyst of enantioselective imine reductase. Org. Biomol. Chem. 2010, 8, 4533–4535.
- Cárdenas-Fernández, M.; Roddan, R.; Carter, E.M.; Hailes, H.C.; Ward, J.M. The Discovery of Imine Reductases and their Utilisation for the Synthesis of Tetrahydroisoquinolines. ChemCatChem. 2023, 15, e202201126.
- Arnodo, D.; De Nardi, F.; Parisotto, S.; De Nardo, E.; Canana, S.; Salvatico, F.; De Marchi, E.; Scarpi, D.; Blangetti, M.; Occhiato, E.G.; et al. Asymmetric Reduction of Cyclic Imines by Imine Reductase Enzymes in Non-Conventional Solvents. ChemSusChem 2023, e202301243.
- Li, J.; Wang, P.; He, Y.-S.; Zhu, Z.-R.; Huang, J. Toward Designing a Novel Oligopeptide-Based Deep Eutectic Solvent: Applied in Biocatalytic Reduction. ACS Sustain. Chem. Eng. 2019, 7, 1318–1326.
- Ducros, V.; Brzozowski, A.M.; Wilson, K.S.; Brown, S.H.; Østergaard, P.; Schneider, P.; Yaver, D.S.; Pedersen, A.H.; Davies, G.J. Crystal structure of the type-2 Cu depleted laccase from Coprinus cinereus at 2.2 Å resolution. Nat. Struct. Biol. 1998, 5, 310–316.
- Toledo, M.L.; Pereira, M.M.; Freire, M.G.; Silva, J.P.A.; Coutinho, J.A.P.; Tavares, A.P.M. Laccase Activation in Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2019, 7, 11806–11814.
- Zhao, H.; Baker, G.A.; Holmes, S. Protease activation in glycerol-based deep eutectic solvents. J. Mol. Catal. B Enzym. 2011, 72, 163–167.
- Khlupova, M.; Vasil’eva, I.; Shumakovich, G.; Zaitseva, E.; Chertkov, V.; Shestakova, A.; Morozova, O.; Yaropolov, A. Enzymatic Polymerization of Dihydroquercetin (Taxifolin) in Betaine-Based Deep Eutectic Solvent and Product Characterization. Catalysts 2021, 11, 639.
- Khodaverdian, S.; Dabirmanesh, B.; Heydari, A.; Dashtban-Moghadam, E.; Khajeh, K.; Ghazi, F. Activity, stability and structure of laccase in betaine based natural deep eutectic solvents. Int. J. Biol. Macromol. 2018, 107, 2574–2579.
- Delorme, A.E.; Andanson, J.-M.; Verney, V. Improving laccase thermostability with aqueous natural deep eutectic solvents. Int. J. Biol. Macromol. 2020, 163, 919–926.
- Wu, B.-P.; Wen, Q.; Xu, H.; Yang, Z. Insights into the impact of deep eutectic solvents on horseradish peroxidase: Activity, stability and structure. J. Mol. Catal. B Enzym. 2014, 101, 101–107.
- Mao, S.; Yu, L.; Ji, S.; Liu, X.; Lu, F. Evaluation of deep eutectic solvents as co-solvent for steroids 1-en-dehydrogenation biotransformation by Arthrobacter simplex. J. Chem. Technol. Biotechnol. 2016, 91, 1099–1104.
- Park, C.-S.; Yoo, M.-H.; Noh, K.-H.; Oh, D.-K. Biotransformation of ginsenosides by hydrolyzing the sugar moieties of ginsenosides using microbial glycosidases. Appl. Microbiol. Biotechnol. 2010, 87, 9–19.
- Bae, E.A.; Park, S.Y.; Kim, D.H. Constitutive β-Gluccosidases Hydrolyzing Ginsenoside Rb1 and Rb2 from Human Intestinal Bacteria. Biol. Pharm. Bull. 2000, 23, 1481–1485.
- Yoo, M.-H.; Yeom, S.-J.; Park, C.-S.; Lee, K.-W.; Oh, D.-K. Production of aglycon protopanaxadiol via compound K by a thermostable β-glycosidase from Pyrococcus furiosus. Appl. Microbiol. Biotechnol. 2011, 89, 1019–1028.
- Han, X.; Li, W.; Duan, Z.; Ma, X.; Fan, D. Biocatalytic production of compound K in a deep eutectic solvent based on choline chloride using a substrate fed-batch strategy. Bioresour. Technol. 2020, 305, 123039.
- Li, F.; Sun, X.-Y.; Li, X.-W.; Yang, T.; Qi, L.-W. Enrichment and separation of quercetin-3-O-β-d-glucuronide from lotus leaves (nelumbo nucifera gaertn.) and evaluation of its anti-inflammatory effect. J. Chromatogr. B. 2017, 1040, 186–191.
- De Oliveira, M.R.; Nabavi, S.M.; Braidy, N.; Setzer, W.N.; Ahmed, T.; Nabavi, S.F. Quercetin and the mitochondria: A mechanistic view. Biotechnol. Adv. 2016, 34, 532–549.
- Roy, A.; Khan, A.; Ahmad, I.; Alghamdi, S.; Rajab, B.S.; Babalghith, A.O.; Alshahrani, M.Y.; Islam, S.; Islam, R. Flavonoids a Bioactive Compound from Medicinal Plants and Its Therapeutic Applications. Ullah, R.; editor. Biomed. Res. Int. 2022, 2022, 5445291.
- Le, N.T.; Nguyen, T.P.D.; Ho, D.V.; Phung, H.T.; Nguyen, H.T. Green solvents-based rutin extraction from Sophora japonica L. J. Appl. Res. Med. Aromat. Plants. 2023, 36, 100508.
- Zhao, B.-Y.; Xu, P.; Yang, F.-X.; Wu, H.; Zong, M.-H.; Lou, W.-Y. Biocompatible Deep Eutectic Solvents Based on Choline Chloride: Characterization and Application to the Extraction of Rutin from Sophora japonica. ACS Sustain. Chem. Eng. 2015, 3, 2746–2755.
- Peng, F.; Xu, P.; Zhao, B.-Y.; Zong, M.-H.; Lou, W.-Y. The application of deep eutectic solvent on the extraction and in vitro antioxidant activity of rutin from Sophora japonica bud. J. Food Sci. Technol. 2018, 55, 2326–2333.
- Nam, M.W.; Zhao, J.; Lee, M.S.; Jeong, J.H.; Lee, J. Enhanced extraction of bioactive natural products using tailor-made deep eutectic solvents: Application to flavonoid extraction from Flos sophorae. Green Chem. 2015, 17, 1718–1727.
- Jakovljević, M.; Vladić, J.; Vidović, S.; Pastor, K.; Jokić, S.; Molnar, M.; Jerković, I. Application of Deep Eutectic Solvents for the Extraction of Rutin and Rosmarinic Acid from Satureja montana L. and Evaluation of the Extracts Antiradical Activity. Plants 2020, 9, 153.
- Zang, Y.-Y.; Yang, X.; Chen, Z.-G.; Wu, T. One-pot preparation of quercetin using natural deep eutectic solvents. Process Biochem. 2020, 89, 193–198.
- de Souza, R.O.M.A.; Antunes, O.A.C.; Kroutil, W.; Kappe, C.O. Kinetic Resolution of rac-1-Phenylethanol with Immobilized Lipases: A Critical Comparison of Microwave and Conventional Heating Protocols. J. Org. Chem. 2009, 74, 6157–6162.
- Chen, C.-C.; Reddy, P.M.; Devi, C.S.; Chang, P.-C.; Ho, Y.-P. Study of microwave effects on the lipase-catalyzed hydrolysis. Enzyme Microb. Technol. 2016, 82, 164–172.
- Shi, Y.; Li, J.; Chu, Y.-H. Enzyme-catalyzed regioselective synthesis of sucrose-based esters. J. Chem. Technol. Biotechnol. 2011, 86, 1457–1468.
- Magrone, P.; Cavallo, F.; Panzeri, W.; Passarella, D.; Riva, S. Exploiting enzymatic regioselectivity: A facile methodology for the synthesis of polyhydroxylated hybrid compounds. Org. Biomol. Chem. 2010, 8, 5583–5590.
- Izquierdo, D.F.; Bernal, J.M.; Burguete, M.I.; García-Verdugo, E.; Lozano, P.; Luis, S.V. An efficient microwave-assisted enzymatic resolution of alcohols using a lipase immobilised on supported ionic liquid-like phases (SILLPs). RSC Adv. 2013, 3, 13123–13126.
- Zhang, N.; Domínguez de María, P.; Kara, S. Biocatalysis for the Synthesis of Active Pharmaceutical Ingredients in Deep Eutectic Solvents: State-of-the-Art and Prospects. Catalysts 2024, 14, 84.
- Cao, S.-L.; Deng, X.; Xu, P.; Huang, Z.-X.; Zhou, J.; Li, X.-H.; Zong, M.-H.; Wenyong, L. Highly Efficient Enzymatic Acylation of Dihydromyricetin by the Immobilized Lipase with Deep Eutectic Solvents as Cosolvent. J. Agric. Food Chem. 2017, 65, 2084–2088.
- Ge, J.; Lu, D.; Wang, J.; Liu, Z. Lipase Nanogel Catalyzed Transesterification in Anhydrous Dimethyl Sulfoxide. Biomacromolecules 2009, 10, 1612–1618.
- Deng, X.; Cao, S.; Li, N.; Wu, H.; Smith, T.J.; Zong, M.; Lou, W. A magnetic biocatalyst based on mussel-inspired polydopamine and its acylation of dihydromyricetin. Chin. J. Catal. 2016, 37, 584–595.
- Huang, Z.-X.; Cao, S.-L.; Xu, P.; Wu, H.; Zong, M.-H.; Lou, W.-Y. Preparation of a novel nanobiocatalyst by immobilizing penicillin acylase onto magnetic nanocrystalline cellulose and its use for efficient synthesis of cefaclor. Chem. Eng. J. 2018, 346, 361–368.
- Wu, X.; Xiong, J.; Huang, Z.; Cao, S.; Zong, M.; Lou, W. Improving biocatalysis of cefaclor with penicillin acylase immobilized on magnetic nanocrystalline cellulose in deep eutectic solvent based co-solvent. Bioresour. Technol. 2019, 288, 121548.
More
Information
Subjects:
Chemistry, Organic; Chemistry, Medicinal
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Entry Collection:
Biopharmaceuticals Technology
Revisions:
2 times
(View History)
Update Date:
28 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




