Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maria-Anna Gatou | -- | 3423 | 2024-02-27 15:18:32 | | | |
| 2 | Lindsay Dong | Meta information modification | 3423 | 2024-02-28 04:53:36 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Gatou, M.; Skylla, E.; Dourou, P.; Pippa, N.; Gazouli, M.; Lagopati, N.; Pavlatou, E.A. Biomedical Applications of Magnesium Oxide Nanoparticles. Encyclopedia. Available online: https://encyclopedia.pub/entry/55554 (accessed on 28 February 2026).
Gatou M, Skylla E, Dourou P, Pippa N, Gazouli M, Lagopati N, et al. Biomedical Applications of Magnesium Oxide Nanoparticles. Encyclopedia. Available at: https://encyclopedia.pub/entry/55554. Accessed February 28, 2026.
Gatou, Maria-Anna, Eirini Skylla, Panagiota Dourou, Natassa Pippa, Maria Gazouli, Nefeli Lagopati, Evangelia A. Pavlatou. "Biomedical Applications of Magnesium Oxide Nanoparticles" Encyclopedia, https://encyclopedia.pub/entry/55554 (accessed February 28, 2026).
Gatou, M., Skylla, E., Dourou, P., Pippa, N., Gazouli, M., Lagopati, N., & Pavlatou, E.A. (2024, February 27). Biomedical Applications of Magnesium Oxide Nanoparticles. In Encyclopedia. https://encyclopedia.pub/entry/55554
Gatou, Maria-Anna, et al. "Biomedical Applications of Magnesium Oxide Nanoparticles." Encyclopedia. Web. 27 February, 2024.
Copy Citation
Magnesium oxide (MgO) nanoparticles have excellent biocompatibility, stability, and diverse biomedical uses, such as antimicrobial, antioxidant, anticancer, and antidiabetic properties, as well as tissue engineering, bioimaging, and drug delivery applications. Magnesium oxide nanoparticles demonstrate substantial biocompatibility and display significant antibacterial, antifungal, anticancer, and antioxidant properties.
magnesium oxide nanoparticles
MgO
Nanoparticles
1. Introduction
In recent times, nanotechnology has become a focal point in research owing to its diverse applications across scientific and technological domains. This field focuses on crafting nanoparticles (NPs) and harnessing their potential in areas like biomedicine, sensing, and catalysis [1]. The significance of NPs stems from their unique properties, such as small size, adaptable shapes, substantial surface area compared to volume, and outstanding magnetic, electronic, optical, and mechanical traits [2][3][4][5]. Yet, the distinctiveness of each nanoparticle primarily relies on the utilized synthesis method [6]. In general, nanoparticles have been synthesized using a variety physical, chemical, and green approaches [7][8][9][10][11][12].
Metal oxide nanoparticles represent a crucial category of nanomaterials extensively utilized today due to their distinct physical and chemical properties, finding applications across diverse fields like biosensing technology, tissue engineering, catalysis, food packaging, biomedicine, and environmental sciences [13]. The key members within the category of metal oxide nanoparticles include silicon dioxide (SiO2), ferric oxide (Fe2O3), copper oxide (CuO), zinc oxide (ZnO), titanium dioxide (TiO2), and magnesium oxide (MgO) [14]. Among these metal oxide nanoparticles, magnesium oxide (MgO) nanoparticles have gained considerable attention because of their exceptional biocompatibility, non-toxic nature, robust stability in abrupt conditions, and extensive applications, especially in biomedicine [15]. Furthermore, the United States Food and Drug Administration regards magnesium oxide as a safe material for human consumption [16]. MgO nanoparticles possess several advantageous physicochemical characteristics, such as enhanced ionic character, substantial specific surface area, distinctive crystal structures, as well as oxygen vacancies, enabling seamless interaction with various biological systems [17][18]. These nanoparticles have found widespread utility in diverse areas, including toxic waste remediation, paints, antiseptics, catalysis, superconductors, catalytic devices, semiconductors, additives in heavy fuel oils, refractory materials, adsorbents, reflective coatings, lithium-ion batteries, and more [19][20][21]. In the realm of biomedicine, magnesium oxide nanoparticles have been employed for stomach relief, heartburn alleviation, and bone regeneration [21][22], as well as for therapeutic applications, such as coated capsules, biological labeling, band-aids, blood collecting vessels, etc. [19]. Additionally, MgO nanoparticles have exhibited potential as antibacterial [21][22][23], fungicidal [24], anticancer [25][26], antioxidant [27], and antidiabetic [27] agents, as well as in applications such as tissue engineering [28][29], bioimaging [30], and drug delivery [31]. Hence, the pursuit of novel synthetic methods for producing magnesium oxide nanoparticles becomes imperative owing to their escalating usage in biomedicine.
2. Biomedical Applications of MgO Nanoparticles
2.1. Antibacterial Activity
In recent times, the rise of bacterial resistance to commonly used antibiotics has significantly impacted the effective treatment of bacterial infections. The United Nations General Assembly has highlighted antibiotic resistance as a critical global peril that humanity confronts [32]. Consequently, exploring alternative strategies to combat bacterial growth has become imperative, with nanoparticles emerging as a promising solution, due to their strong antibacterial properties. Among these nanoparticles, MgO nanoparticles have garnered attention owing to their remarkable effectiveness in combating bacteria. Studies have indicated the potent MgO nanoparticles’ antibacterial effects against various strains, such as E. coli [33], S. aureus [34], P. aeruginosa, A. baumannii [35], and P. carotovorum [36]. Additionally, a proposed antibacterial mechanism of MgO nanoparticles is depicted in Figure 1. More specifically, magnesium oxide nanoparticles have the ability to stimulate reactive oxygen species (ROS) within bacteria. This process leads to oxidative stress, resulting in significant impairment to their membrane lipids, proteins, and nucleic acids [37].
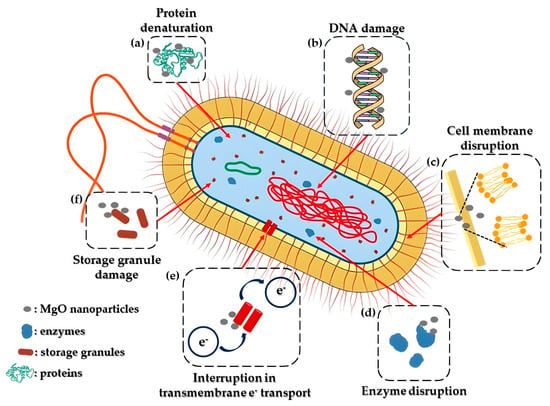
Figure 1. Representation of a feasible mechanism of MgO nanoparticles’ antibacterial activity. The entry of MgO nanoparticles into bacterial cells is facilitated by the disturbance of the bacterial cell membrane (c). Once inside the cytoplasm, these nanoparticles have the capacity to produce reactive oxygen species (ROS) or inflict direct harm on DNA and enzymes (b,d). This action results in protein denaturation and damage to the mitochondria (a,f). Additionally, they interfere with cellular memory and impede trans-tolerant electron transport (e). Consequently, the inflicted harm leads to the destruction of bacterial cells, prompting the release of their organelles and culminating in their eventual demise.
Makhluf and colleagues [38] demonstrated the antibacterial properties of powdered magnesium oxide prepared through a microwave-assisted synthetic approach, applying it to combat both Staphylococcus aureus and Escherichia coli cultures. The most substantial antibacterial effect was observed when subjecting both bacterial strains to 8 nm magnesium oxide nanoparticles. Following exposure to MgO for 60 min, less than one fifth of both cultures survived. Subsequently, after 4 h of treatment, the survival rates decreased significantly to less than 5% for Staphylococcus aureus and a mere 0.1% for Escherichia coli. In contrast, using 23 nm MgO resulted in a reduction in bacterial counts to approximately 40% for S. aureus and 35% for E. coli, comparatively less effective than the 8 nm particles.
Mechanism of MgO Nanoparticles’ Bactericidal Activity
The effectiveness of MgO nanoparticles in eradicating bacteria largely relies on reactive oxygen species (ROS) production. This reliance is specifically associated with factors such as the particle’s surface characteristics, polarity, crystal size, increased oxygen defects, morphology, the ability of molecules to chemically diffuse, as well as the release of Mg2+ ions. The bactericidal process involving magnesium oxide nanoparticles entails sterilization through particle release, a multifaceted mechanism, and absorption. The crystals’ size and their extensive surface area may contribute significantly to their potent antibacterial properties. Superoxide radicals, formed through reactions between H2O2 and ROS, inflict damage on cellular proteins and DNA, leading to cell death [39].
The mechanism underlying the antimicrobial efficacy of magnesium oxide nanostructures can be elucidated as follows. Primarily, a crucial antibacterial process involves a light-driven catalytic mechanism. Specifically, the creation of ROS on the nanoparticle surface, in the presence of light, initiates oxidative stress on microbial cells, ultimately leading to cellular demise. ROS comprises relatively low levels of toxic radicals like the superoxide anion radical (O2−), reactive hydroxyl radical (·OH), and a mild oxidizing agent, hydrogen peroxide (H2O2). A subsequent series of reactions also takes place, where the superoxide anion radical (•O2−) combines with hydrogen ions, forming the •HO2− radical, which then reacts with hydrogen ions to produce H2O2. This compound can interact with DNA, cellular proteins, and cell membranes, ultimately resulting in microbial death. The generation of a substantial amount of ROS hinges on the creation of smaller crystalline structures with increased specific surface areas and a corresponding rise in surficial defects.
2.2. Antifungal Activity
Apart from showcasing antibacterial effects, MgO nanoparticles have demonstrated significant antifungal capabilities against various pathogenic fungal strains (Figure 2). Fungi constitute natural pathogens as they present plenty of similarities with the host cell, inhibiting antifungal compounds’ growth [40]. Sierra-Fernandez and co-researchers [41] studied the antifungal activity of Zn-doped magnesium oxide nanoparticles synthesized through a facile sol-gel approach and compared it with that of pure ZnO and MgO nanoparticles. The as-mentioned nanoparticles presented enhanced antifungal efficiency compared to that of pure magnesium oxide or zinc oxide nanoparticles, restraining the growth of fungi Aspergillus niger, Penicillium oxalicum, Paraconiothyrium sp., and Pestalotiopsis maculans.

Figure 2. Diagrammatic representation of the way that MgO nanoparticles operate as an antifungal agent: Initially, the nanoparticles engage with fungal cell membranes through electrostatic interactions, leading to the disruption of both the membranes and the glucan matrix. Following this, they initiate the production of ROS and the release of Mg2+. Subsequently, they interfere with mitochondria by inducing DNA damage, subsequently impeding protein synthesis, disrupting proteins, leading to intracellular leakage, and ultimately resulting in the demise of fungal cells.
Moreover, De la Rosa-García and co-workers [42] evaluated the antifungal activity (towards strains of C. gloeosporioides) of pure ZnO and MgO, as well as ZnO/MgO and ZnO/Mg(OH)2 composites fabricated under different synthetic approaches (co-precipitation and hydrothermal). According to the acquired results, all tested nanoparticles at the tested concentrations significantly restrained conidia’s germination and led to the structural damage of the fungal cells, verifying that the as-mentioned nanoparticles could constitute promising fungicidal agents against C. gloeosporioides.
In addition, Castillo and his team [24] demonstrated through in vitro experiments that magnesium oxide nanoparticles, characterized by a diameter equal to 12 nm, presented fungistatic efficiency towards three filamentous fungal strains (T. reesei, A. niger, and C. cladosporioides), at concentrations ranging from 3 to 12 mg/mL.
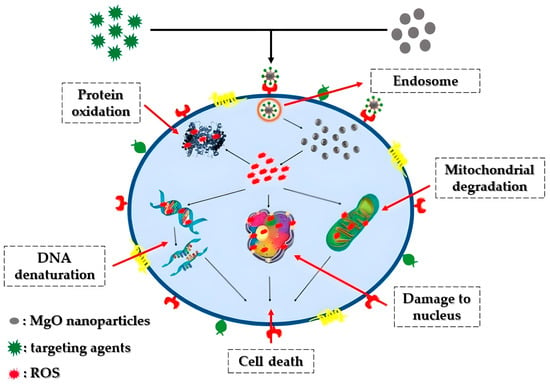
2.3. Anticancer Activity
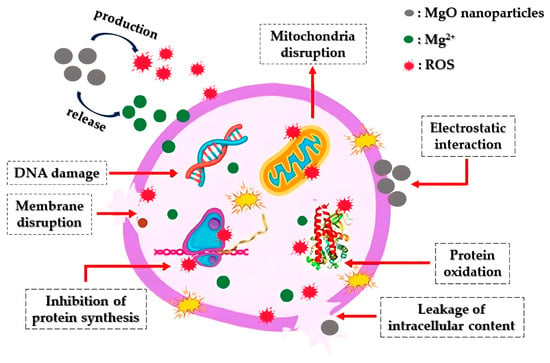
Cancer stands as one of the most lethal and intricate diseases known to date. The unregulated growth of cancerous cells detrimentally impacts neighboring healthy cells, leading to fatality [43]. Various treatments, including surgery, radiation therapy, and chemotherapy, have been proposed for combating cancer [44]. Nonetheless, the aforementioned approaches may harm normal cells, resulting in numerous side effects and potential disease recurrence [45]. Recently, there has been a rising focus on the development of nanoparticle-based nanomaterials, notably magnesium oxide nanoparticles, renowned for their potent anti-cancer properties. Figure 3 portrays a hypothetical anti-cancer mechanism associated with magnesium oxide nanoparticles. The advancement of synthesis methods and techniques has significantly propelled the application of magnesium oxide nanoparticles in anti-cancer therapy.

Figure 3. Representation of the potential anticancer mechanism involving MgO nanoparticles: Through electrostatic interactions with the cell surface, MgO nanoparticles gain entry into the cell via the intracellular pathway. Once inside, these nanoparticles prompt the formation of reactive oxygen species (ROS) within the cells, resulting in DNA damage, protein oxidation, and mitochondrial impairment, ultimately culminating in cell death.
Behzadi and his team [46] reported that the tested magnesium oxide nanoparticles presented selective cytotoxicity against the K562 cell line, thus rendering them as a novel anticancer agent. Their study’s data revealed that MgO nanoparticle-mediated apoptosis was initiated through reactive oxygen species generation within the cancer cells.
Additionally, magnesium oxide nanoparticles synthesized through a simple sol-gel method and further modified with polyethylene glycol were successfully fabricated by Alfaro and his team [47] to be utilized as carrier for the anticancer drug 2-Methoxyestradiol for advancing its clinical utilization. According to their research’s results, the as-developed nanoparticles significantly reduced the viability of a prostate cancer cell line (LNCap), rendering the as-mentioned nanocomposite appropriate as a drug delivery system towards anticancer prostate therapy.
2.4. Antioxidant Activity
For a considerable duration, researchers have established that free radicals negatively impact human health, potentially leading to various illnesses including heart disease, arteriosclerosis, tumors, diabetes, and aging [48]. Consequently, the focus on understanding antioxidants has expanded to counteract the free radicals’ detrimental effects. However, despite their effectiveness, potent antioxidant agents also bring along several adverse effects. For instance, Edaravone, acknowledged as a free radical scavenger beneficial in preventing lipid oxidation and treating ischemic stroke, mitigates nerve cell damage. Yet, when utilized clinically, Edaravone induces numerous side effects, including liver and kidney toxicity, which can impact human health [49]. Hence, exploring antioxidant capabilities via enhanced magnesium oxide nanoparticles presents a possible solution to mitigate several associated drawbacks.
Podder and colleagues [50] explored the antioxidant activity of three nano-MgO structures (i.e., nanoparticles, nanoplates, and nanorods). They reported the effective production of superoxide anions (•O2−) and hydroxyl radicals (•OH) at increased concentrations (>500 μg/mL) and the scavenging of •O2− at lower concentrations (40 μg/mL) for all examined nanostructures. More specifically, it was observed that magnesium oxide nanorods produce the most increased levels of superoxide anions, while magnesium oxide nanoparticles possessed the most enhanced ability (60%) to scavenge superoxide anions. Lastly, the researchers also reported a 100% scavenging ability of the nitrogen-centered free radical (DPPH) by magnesium oxide nanoplates, given their significantly enhanced specific surface area (342.2 m2/g).
Magnesium oxide nanoparticles (42 nm) were successfully developed utilizing geranium leaf extract by Mylarappa et al. [51]. The antioxidant characteristics of the synthesized nanoparticles were evaluated using the DPPH method. Based on the obtained results, MgO nanoparticles displayed significant efficacy in scavenging free radicals, as demonstrated by their DPPH scavenging activities.
2.5. MgO-Based Biosensors towards Diabetes Detection and Treatment
Diabetes comprises a collection of severe and enduring metabolic disorders associated with elevated blood glucose levels, contributing to increased rates of premature morbidity. In spite of advancements in life’s quality, there hasn’t been a decline in diabetes prevalence; instead, it continues to strain global healthcare systems [52]. Often diagnosed after irreversible organ damage due to prolonged hyperglycemia, diabetes stands among the most pressing global health challenges [52], alongside cancer, chronic respiratory issues, and cardiovascular diseases, causing approximately five million deaths annually in developed nations. Notably, cardiovascular disease (50%) and kidney failure (10–20%) account for the majority of these fatalities. Diabetes also leads to complications like blindness, lower limb amputations, and severe outcomes in viral infections, such as COVID-19 [53]. The primary diabetes types include insulin-dependent or juvenile diabetes (T1DM-Type 1 Diabetes Mellitus), non-insulin-dependent (T2DM-Type 2 Diabetes Mellitus), and gestational diabetes [54].
The initial and crucial aspect of managing diabetes involves diagnosis. Presently, traditional methods such as assessing fasting plasma glucose (FPG) levels, conducting oral glucose tolerance tests (OGTT), and measuring hemoglobin A1c (HbA1c) levels [55][56] are employed for diabetes diagnosis. However, the aforementioned methods are often uncomfortable and painful for patients, due to blood withdrawal, leading to potential neglect of therapy. Additionally, periodic measurements might not capture significant fluctuations in glucose levels between testing intervals. Moreover, variations in measured values can occur due to factors like timing of testing, age, and an individual’s physiological state. These approaches are also unsuitable for continuous monitoring due to their laborious nature, prolonged diagnosis duration, increased blood withdrawal, and complex blood processing [57]. Notably, clinical signs of detrimental diabetes symptoms, like hyperglycemia, are usually observed only after the disease has progressed, hindering early intervention. To mitigate these complications, it is crucial to develop diagnostic tools that are more affordable, rapid, and widely accessible [58].
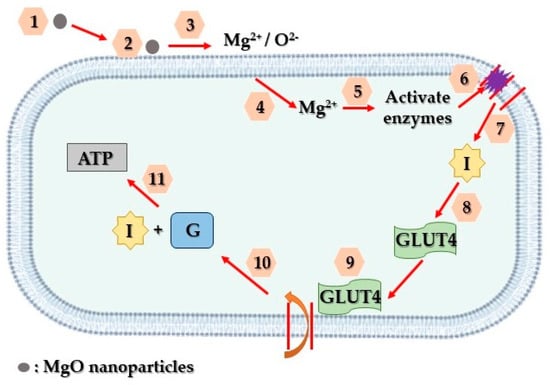
Addressing these challenges, various nanotechnologies focusing on diverse biomarkers have emerged, aiming to enable early and non-invasive diabetes detection. Analyzing specific biomarkers serves as an indicator for multiple diseases [59]. Lately, there has been a notable emphasis on advancing research related to diabetes treatment through the utilization of nanoparticles. Magnesium oxide nanoparticles, in particular, have gained substantial attention among various nanoparticles and are extensively employed in biomedical studies, particularly in diabetes treatment, through a suggested mechanism depicted in Figure 4.

Figure 4. Schematic representation of the way that MgO nanoparticles contribute to diabetes treatment. Firstly, MgO nanoparticles adhere to the surface of affected cells (1, 2, 3). Subsequently, they discharge Mg2+ and O2− ions, initiating the activation of internal enzymes (4, 5). These enzymes, in turn, facilitate the reversal of insulin resistance and facilitate the entry of glucose transporter 4 into the cell’s plasma membrane (6, 7, 8). Ultimately, this glucose transporter enables the absorption of glucose into the cells, where insulin functions to decrease glucose levels and generate ATP (9, 10, 11).
2.6. Tissue Engineering Applications
2.6.1. Bone Tissue Engineering
Enhanced biomaterials aimed at restoring bone integrity are necessary to address the escalating number of individuals grappling with deteriorating or damaged bones [60][61][62][63]. Presently, existing biomaterial solutions for this purpose involve invasive procedures that introduce permanent materials, potentially leading to prolonged issues within the body. Although progress has been made in bone defect regeneration using injectable cements and various scaffold materials, significant enhancements are still needed [64][65][66]. The ideal biomaterials for bone tissue regeneration should harmonize mechanically with surrounding tissue, lessening stress and strain discrepancies, while also possessing suitable chemical compositions and surface features that foster bone cell adhesion, growth, movement, and the production of proteins forming the extracellular matrix.
Furthermore, Suryavanshi and co-researchers [67] evaluated the suitability of electrospun polycaprolactone polymer composites loaded with magnesium oxide nanoparticles as scaffolds for bone-soft tissue engineering. Magnesium oxide nanoparticles were synthesized using a hydroxide precipitation sol-gel process. The nanocomposites exhibited significantly improved mechanical properties compared to the pure polymer samples, due to the even dispersion of MgO nanoparticles throughout the polymer fibers. In immersion tests, the nanocomposite scaffolds displayed notable bioactivity by developing a surface hydroxyapatite layer by the third day of incubation. The electrospun polymer mats loaded with magnesium oxide nanoparticles demonstrated enhanced in vitro biological performance with osteoblast-like MG-63 cells, showing increased adhesion, proliferation, and enhanced differentiation marker activity.
2.6.2. Skin Tissue Regeneration
The skin, being the body’s largest vital organ, serves as a protective barrier against the external environment. While skin tissue possesses self-regenerating abilities, these capabilities significantly diminish in cases of full-thickness injuries, necessitating skin grafts or dressings [68]. The process of cutaneous wound healing, essential for repairing damaged skin tissue, involves several intricate stages: hemostasis, inflammation, proliferation, and remodeling [69]. Hemostasis, occurring immediately after injury, involves platelet aggregation and blood clotting. The inflammatory stage involves the presence of neutrophils and macrophages releasing cytokines at the wound site. During the proliferative phase, fibroblast differentiation leads to the initiation of re-epithelialization through the synthesis of the extracellular matrix. The final stage involves collagen synthesis and myofibroblast activity, facilitating tissue remodeling [70][71]. These stages progress sequentially within a specific timeframe for complete healing.
Globally, various wound dressings have been developed to address epidermal damage. Traditional materials like bandages, cotton wool, lint, and gauze were historically utilized to absorb wound exudates, maintaining dryness to prevent bacterial infection [71][72]. Given the complexities of wound healing, an ideal wound dressing should possess exceptional biocompatibility to enhance tissue regeneration [73]. It should also enable gas exchange, shield the wound from microbial infections, absorb excess fluids without leakage, and be non-adherent and comfortable [74]. As a result, novel materials meeting the aforementioned characteristics need to be developed. Amongst them, MgO-based nanomaterials have gained considerable researchers’ attention within the last decade, given the fact that magnesium oxide is considered to be biologically safe, capable of biodegradation, cost-effective, and environmentally friendly, holding significant promise for various biomedical applications [75].
The primary impediment in the healing process of diabetic wounds is insufficient angiogenesis. Based on existing scientific reports, electrospun nanofiber membranes have demonstrated potential as wound dressings. To effectively address diabetic wounds, it is crucial for electrospun membranes to stimulate wound angiogenesis. Current strategies predominantly focus on employing pro-angiogenic growth factors to augment the angiogenic properties of these membranes. However, integrating growth factors into electrospun nanofibers and sustaining their activity long-term pose technical challenges. Taking the aforementioned into consideration, Liu and co-researchers [29] introduced an electrospun membrane comprising polycaprolactone, gelatin, and magnesium oxide nanoparticles, releasing Mg2+ ions to further promote angiogenesis. The as-prepared membranes encouraged human umbilical vein endothelial cell proliferation and enhanced vascular endothelial growth factor production in vitro. Implantation studies in a rat model reveal that the MgO-included membrane facilitated the early formation of robust blood vessels within a week post surgery, fostering enriched capillary networks within the degrading membrane over time.
2.7. Bioimaging Applications
Extensive research focuses on fluorescent nanoparticles to enable real-time bioimaging and tracking of biological processes at the nanoscale. These nanoparticles hold promise for advancing diagnostic tools and targeted drug release therapies. Metal oxide nanoparticles [76][77][78] have gained attention as contrast agents in bioimaging, due to their room-temperature single-photon emission [76][78], customizable optical properties [79], and low toxicity. However, challenges persist in their application, such as low quantum efficiency and brightness [77][78], propensity for agglomeration in cell culture media [77], and dose-dependent cytotoxicity [80].
For effective in vitro experiments, a fluorescent marker must absorb light above 500 nm and emit light beyond 600 nm to mitigate cell autofluorescence [81]. In contrast, for in vivo experiments, emission in the near-infrared (NIR) range, between 700 and 900 nm, is crucial as it penetrates tissue over centimeters, unlike visible light, which travels mere microns [82]. Magnesium oxide nanoparticles apart from being biocompatible and biodegradable as previously mentioned, are also intrinsically fluorescent [83].
Taking the aforementioned into account, Rasheed and Sandhyarani [84] conducted the synthesis of luminescent nanocrystals of magnesium oxide by introducing a very low amount of Cr3+ as a dopant. The production of chromium-doped magnesium oxide nanocrystals involved the use of magnesium nitrate as the base material and chromic nitrate as the doping agent.
Additionally, in a study by Khalid and co-researchers [83], the inherent and enduring fluorescent characteristics of magnesium oxide nanoparticles derived from naturally present chromium Cr3+ and vanadium V2+ ions were detailed. These properties encompassed a fluorescence spectrum spanning from the visible to the near-infrared range, enabling their potential utilization for real-time monitoring of live cells derived from both normal and cancerous tissues.
2.8. Drug Delivery Applications
Nanotechnology offers promising avenues in drug delivery, especially for combating terminal illnesses such as cancer [85][86][87]. Previous studies have explored the utilization of nanostructures to administer drugs [88][89], and nanoparticles have shown potential in targeting specific cell genes, particularly those in tumor cells. Nanostructures possess advantageous qualities, including a significant volume-to-surface ratio, customizable surface properties, and multifunctionality, making them appealing for drug delivery applications [90][91][92].
Sabbagh and Muhamad [93] employed acrylamide-based hydrogel systems for drug delivery, specifically for the release of Acyclovir from magnesium oxide nanocomposite hydrogel. Acyclovir was incorporated into the polymer through a soaking process, enabling the hydrogel system for use in vaginal drug delivery and subsequent release. An assessment of the chemical and physical properties of the reinforced hydrogels provided an analysis of the polymer’s morphological structure, swelling behavior, gel formation, and physical attributes. The drug release behavior in different mediums, PBS and SVF aqueous solutions, was examined, and the quantity of the released drug was determined using HPLC.
References
- Singh, T.A.; Das, J.; Sil, P.C. Zinc oxide nanoparticles: A comprehensive review on its synthesis, anticancer and drug delivery applications as well as health risks. Adv. Colloid Interface Sci. 2020, 286, 102317.
- Thakur, N.; Thakur, S.; Chatterjee, S.; Das, J.; Sil, P.C. Nanoparticles as smart carriers for enhanced cancer immunotherapy. Front. Chem. 2020, 8, 1217.
- Thakur, N.; Manna, P.; Das, J. Synthesis and biomedical applications of nanoceria, a redox active nanoparticle. J. Nanobiotechnol. 2019, 17, 84.
- Tejwan, N.; Saha, S.K.; Das, J. Multifaceted applications of green carbon dots synthesized from renewable sources. Adv. Colloid Interface Sci. 2020, 275, 102046.
- Thakur, N.; Das, J.; Sil, R.C. Emerging role of redox-active nanoceria in cancer therapeutics via oxidative stress. In Handbook of Oxidative Stress in Cancer: Therapeutic Aspects; Chakraborti, S., Ed.; Springer: Singapore, 2021; pp. 1–23.
- Rana, A.; Yadav, K.; Jagadevan, S. A comprehensive review on green synthesis of nature-inspired metal nanoparticles: Mechanism, application and toxicity. J. Clean. Prod. 2020, 272, 122880.
- Rafique, M.; Sadaf, I.; Rafique, M.S.; Tahir, M.B. A review on green synthesis of silver nanoparticles and their applications. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1272–1291.
- Bandeira, M.; Giovanela, M.; Roesch-Ely, Μ.; Devine, D.M.; da Silva Crespo, J. Green synthesis of zinc oxide nanoparticles: A review of the synthesis methodology and mechanism of formation. Sustain. Chem. Pharm. 2020, 15, 100223.
- Yildiz, Y.; Okyay, T.O.; Sen, B.; Gezer, B.; Kuzu, S.; Savk, A.; Demir, E.; Dasdelen, Z.; Sert, H.; Sen, F. Highly monodisperse Pt/Rh nanoparticles confined in the graphene oxide for highly efficient and reusable sorbents for methylene blue removal from aqueous solutions. ChemistrySelect 2017, 2, 697–701.
- Göksu, H.; Çelik, B.; Yıldız, Y.; Şen, F.; Kılbaş, B. Superior monodisperse CNT-supported CoPd (CoPd@CNT) nanoparticles for selective reduction of nitro compounds to primary amines with NaBH4 in aqueous medium. ChemistrySelect 2016, 1, 2366–2372.
- Sen, B.; Şavk, A.; Sen, F. Highly efficient monodisperse Pt nanoparticles confined in the carbon black hybrid material for hydrogen liberation. J. Colloid Interface Sci. 2018, 520, 112–118.
- Şen, B.; Aygün, A.; Şavk, A.; Akocak, S.; Şen, F. Bimetallic palladium-iridium alloy nanoparticles as highly efficient and stable catalyst for the hydrogen evolution reaction. Int. J. Hydrogen Energy 2018, 43, 20183–20191.
- Amina, M.; Al Musayeib, N.M.; Alarfaj, N.A.; El-Tohamy, M.F.; Oraby, H.F.; Al Hamoud, G.A.; Bukhari, S.I.; Moubayed, N.M.S. Biogenic green synthesis of MgO nanoparticles using Saussurea costus biomasses for a comprehensive detection of their antimicrobial, cytotoxicity against MCF-7 breast cancer cells and photocatalysis potentials. PLoS ONE 2020, 15, e0237567.
- Danish, M.S.S.; Bhattacharya, A.; Stepanova, D.; Mikhaylov, A.; Grilli, M.L.; Khosravy, M.; Senjyu, T. A Systematic Review of Metal Oxide Applications for Energy and Environmental Sustainability. Metals 2020, 10, 1604.
- Abdel-Aziz, M.M.; Emam, T.M.; Elsherbiny, E.A. Bioactivity of magnesium oxide nanoparticles synthesized from cell filtrate of endobacterium Burkholderia rinojensis against Fusarium oxysporum. Mater. Sci. Eng. C 2020, 109, 110617.
- Aničić, N.; Vukomanović, M.; Koklič, T.; Suvorov, D. Fewer defects in the surface slows the hydrolysis rate, decreases the ROS generation potential, and improves the non-ROS antimicrobial activity of MgO. Small 2018, 14, 1800205.
- Anand, K.V.; Anugraga, A.R.; Kannan, M.; Singaravelu, G.; Govindaraju, K. Bio-engineered magnesium oxide nanoparticles as nano-priming agent for enhancing seed germination and seedling vigour of green gram (Vigna radiata L.). Mater. Lett. 2020, 271, 127792.
- Verma, S.K.; Nisha, K.; Panda, P.K.; Patel, P.; Kumari, P.; Mallick, M.A.; Sarkar, B.; Das, B. Green synthesized MgO nanoparticles infer biocompatibility by reducing in vivo molecular nanotoxicity in embryonic zebrafish through arginine interaction elicited apoptosis. Sci. Total Environ. 2020, 713, 136521.
- Khan, A.; Shabir, D.; Ahmad, P.; Khandaker, M.U.; Faruque, M.R.I.; Din, I.U. Biosynthesis and antibacterial activity of MgO-NPs produced from Camellia-sinensis leaves extract. Mater. Res. Express 2020, 8, 15402.
- Essien, E.R.; Atasie, V.N.; Okeafor, A.O.; Nwude, D.O. Biogenic synthesis of magnesium oxide nanoparticles using Manihot esculenta (Crantz) leaf extract. Int. Nano Lett. 2020, 10, 43–48.
- Tang, Z.X.; Lv, B.F. MgO nanoparticles as antibacterial agent: Preparation and activity. Braz. J. Chem. Eng. 2014, 31, 591–601.
- El-Sayyad, G.S.; Mosallam, F.M.; El-Batal, A.I. One-pot green synthesis of magnesium oxide nanoparticles using Penicillium chrysogenum melanin pigment and gamma rays with antimicrobial activity against multidrug-resistant microbes. Adv. Powder Technol. 2018, 29, 2616–2625.
- Maji, J.; Pandey, S.; Basu, S. Synthesis and evaluation of antibacterial properties of magnesium oxide nanoparticles. Bull. Mater. Sci. 2020, 43, 25.
- Castillo, I.F.; Guillén, E.G.; Jesús, M.; Silva, F.; Mitchell, S.G. Preventing fungal growth on heritage paper with antifungal and cellulase inhibiting magnesium oxide nanoparticles. J. Mater. Chem. B 2019, 7, 6412–6419.
- Di, D.R.; He, Z.Z.; Sun, Z.Q.; Liu, J. A new nano-cryosurgical modality for tumor treatment using biodegradable MgO nanoparticles. Nanomedicine 2012, 8, 1233–1241.
- Krishnamoorthy, K.; Moon, J.Y.; Hyun, H.B.; Cho, S.K.; Kim, S.J. Mechanistic investigation on the toxicity of MgO nanoparticles toward cancer cells. J. Mater. Chem. 2012, 22, 24610–24617.
- Moeini-Nodeh, S.; Rahimifard, M.; Baeeri, M.; Abdollahi, M. Functional improvement in rats’ pancreatic islets using magnesium oxide nanoparticles through antiapoptotic and antioxidant pathways. Biol. Trace Elem. Res. 2017, 175, 146–155.
- Derakhshankhah, H.; Nekounam, H.; Izadi, Z.; Allahyari, Z.; Samari, M.; Feizi, M.; Samadian, H. Fabrication of electroactive nanocomposite based on carbon nanofibers/magnesium oxide nanoparticles for bone tissue engineering. J. Drug Deliv. Sci. Technol. 2023, 89, 105082.
- Liu, M.; Wang, R.; Liu, J.; Zhang, W.; Liu, Z.; Lou, X.; Nie, H.; Wang, H.; Mo, X.; Abd-Elhamid, A.I.; et al. Incorporation of magnesium oxide nanoparticles into electrospun membranes improves pro-angiogenic activity and promotes diabetic wound healing. Biomater. Adv. 2022, 133, 112609.
- Li, J.; Khalid, A.; Verma, R.; Abraham, A.; Qazi, F.; Dong, X.; Liang, G.; Tomljenovic-Hanic, S. Silk Fibroin Coated Magnesium Oxide Nanospheres: A Biocompatible and Biodegradable Tool for Noninvasive Bioimaging Applications. Nanomaterials 2021, 11, 695.
- El-Sawy, N.M.; Raafat, A.I.; Badawy, N.A.; Mohamed, A.M. Radiation development of pH-responsive (xanthan-acrylic acid)/MgO nanocomposite hydrogels for controlled delivery of methotrexate anticancer drug. Int. J. Biol. Macromol. 2020, 142, 254–264.
- Sagar, S.; Kaistha, S.; Das, A.J.; Kumar, R. Bacteriophage: A new hope for the control of antibiotic-resistant bacteria. In Antibiotic Resistant Bacteria: A Challenge to Modern Medicine; Springer: Singapore, 2019; pp. 153–164.
- Younis, I.Y.; El-Hawary, S.S.; Eldahshan, O.A.; Abdel-Aziz, M.M.; Ali, Z.Y. Green Synthesis of Magnesium Nanoparticles Mediated from Rosa Floribunda Charisma Extract and Its Antioxidant, Antiaging and Antibiofilm Activities. Sci. Rep. 2021, 11, 16868.
- Das, B.; Moumita, S.; Ghosh, S.; Khan, M.I.; Indira, D.; Jayabalan, R.; Tripathy, S.K.; Mishra, A.; Balasubramanian, P. Biosynthesis of magnesium oxide (MgO) nanoflakes by using leaf extract of Bauhinia purpurea and evaluation of its antibacterial property against Staphylococcus aureus. Mater. Sci. Eng. C 2018, 91, 436–444.
- Ananda, A.; Ramakrishnappa, T.; Archana, S.; Reddy Yadav, L.S.; Shilpa, B.M.; Nagaraju, G.; Jayanna, B.K. Green synthesis of MgO nanoparticles using Phyllanthus emblica for Evans blue degradation and antibacterial activity. Mater. Today Proc. 2021, 49, 801–810.
- Sharma, S.K.; Khan, A.U.; Khan, M.; Gupta, M.; Gehlot, A.; Park, S.; Alam, M. Biosynthesis of MgO nanoparticles using Annona squamosa seeds and its catalytic activity and antibacterial screening. Micro Nano Lett. 2020, 15, 30–34.
- Cai, L.; Chen, J.; Liu, Z.; Wang, H.; Yang, H.; Ding, W. Magnesium oxide nanoparticles: Effective agricultural antibacterial agent against Ralstonia solanacearum. Front. Microbiol. 2018, 9, 790.
- Makhluf, S.; Dror, R.; Nitzan, Y.; Abramovich, Y.; Jelinek, R.; Gedanken, A. Microwave-assisted synthesis of nanocrystalline MgO and its use as a bacteriocide. Adv. Funct. Mater. 2005, 15, 1708–1715.
- Nejati, M.; Rostami, M.; Mirzaei, H.; Rahimi-Nasrabadi, M.; Vosoughifar, M.; Sobhani Nasab, A.; Ganjali, M.R. Green methods for the preparation of MgO nanomaterials and their drug delivery, anti-cancer and anti-bacterial potentials: A review. Inorg. Chem. Commun. 2022, 136, 109107.
- Rodrigues, M.L.; Nosanchuk, J.D. Fungal diseases as neglected pathogens: A wake-up call to public health officials. PLoS Negl. Trop. Dis. 2020, 14, e0007964.
- Sierra-Fernandez, A.; De la Rosa-García, S.C.; Gomez-Villalba, L.S.; Gomez-Cornelio, S.; Rabanal, M.E.; Fort, R.; Quintana, P. Synthesis, photocatalytic, and antifungal properties of MgO, ZnO, and Zn/Mg oxide nanoparticles for the protection of calcareous stone heritage. ACS Appl. Mater. Interfaces 2017, 9, 24873–24886.
- De la Rosa-García, S.C.; Martínez-Torres, P.; Gómez-Cornelio, S.; Corral-Aguado, M.A.; Quintana, P.; Gómez-Ortíz, N.M. Antifungal activity of ZnO and MgO nanomaterials and their mixtures against Colletotrichum gloeosporioides strains from tropical fruit. J. Nanomater. 2018, 2018, 3498527.
- Al-Fahdawi, M.Q.; Rasedee, A.; Al-Doghachi, F.A.; Rosli, R.; Taufiq-Yap, Y.H.; Al-Qubaisi, M.S. Anticancer palladium-doped magnesia nanoparticles: Synthesis, characterization, and in vitro study. Nanomedicine 2020, 15, 547–561.
- van der Valk, M.J.M.; Marijnen, C.A.M.; van Etten, B.; Dijkstra, E.A.; Hilling, D.E.; Kranenbarg, E.M.-K.; Putter, H.; Roodvoets, A.G.H.; Bahadoer, R.R.; Fokstuen, T.; et al. Compliance and tolerability of short-course radiotherapy followed by preoperative chemotherapy and surgery for high-risk rectal cancer—Results of the international randomized RAPIDO-trial. Radiother. Oncol. 2020, 147, 75–83.
- Hussain, Y.; Islam, L.; Khan, H.; Filosa, R.; Aschner, M.; Javed, S. Curcumin-isplatin chemotherapy: A novel strategy in promoting chemotherapy efficacy and reducing side effects. Phyther. Res. 2021, 35, 6514–6529.
- Behzadi, E.; Sarsharzadeh, R.; Nouri, M.; Attar, F.; Akhtari, K.; Shahpasand, K.; Falahati, M. Albumin binding and anticancer effect of magnesium oxide nanoparticles. Int. J. Nanomed. 2018, 14, 257–270.
- Alfaro, A.; León, A.; Guajardo-Correa, E.; Reúquen, P.; Torres, F.; Mery, M.; Segura, R.; Zapata, P.A.; Orihuela, P.A. MgO nanoparticles coated with polyethylene glycol as carrier for 2-Methoxyestradiol anticancer drug. PLoS ONE 2019, 14, e0214900.
- Kainama, H.; Fatmawati, S.; Santoso, M.; Papilaya, P.M.; Ersam, T. The relationship of free radical scavenging and total phenolic and flavonoid contents of Garcinia lasoar PAM. Pharm. Chem. J. 2020, 53, 1151–1157.
- Li, C.-W.; Li, L.-L.; Chen, S.; Zhang, J.-X.; Lu, W.-L. Antioxidant nanotherapies for the treatment of inflammatory diseases. Front. Bioeng. Biotechnol. 2020, 8, 200.
- Podder, S.; Chanda, D.; Mukhopadhyay, A.K.; De, A.; Das, B.; Samanta, A.; Hardy, J.G.; Ghosh, C.K. Effect of morphology and concentration on crossover between antioxidant and pro-oxidant activity of MgO nanostructures. Inorg. Chem. 2018, 57, 12727–12739.
- Mylarappa, M.; Rekha, S.; Kantharaju, S.; Chandruvasan, S.; Shravana, K. Synthesis and characterization of ZnO and MgO nanoparticles through green approach and their antioxidant properties. ECS Trans. 2022, 107, 689.
- American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018, 41 (Suppl. S1), S13–S27.
- Szunerits, S.; Melinte, S.; Barras, A.; Pagneux, Q.; Voronova, A.; Abderrahmani, A.; Boukherroub, R. The impact of chemical engineering and technological advances on managing diabetes: Present and future concepts. Chem. Soc. Rev. 2021, 50, 2102–2146.
- Debele, T.A.; Park, Y. Application of Nanoparticles: Diagnosis, Therapeutics, and Delivery of Insulin/Anti-Diabetic Drugs to Enhance the Therapeutic Efficacy of Diabetes Mellitus. Life 2022, 12, 2078.
- Lemmerman, L.R.; Das, D.; Higuita-Castro, N.; Mirmira, R.G.; Gallego-Perez, D. Nanomedicine-Based Strategies for Diabetes: Diagnostics, Monitoring, and Treatment. Trends Endocrinol. Metab. 2021, 31, 448–458.
- Lagopati, N.; Pavlatou, E.A. Nanotechnology in Diabetes Management. Interv. Obes. Diabetes 2021, 5, 419–424.
- Tang, L.; Chang, S.J.; Chen, C.-J.; Liu, J.-T. Non-Invasive Blood Glucose Monitoring Technology: A Review. Sensors 2020, 20, 6925.
- Lagopati, N.; Valamvanos, T.-F.; Proutsou, V.; Karachalios, K.; Pippa, N.; Gatou, M.-A.; Vagena, I.-A.; Cela, S.; Pavlatou, E.A.; Gazouli, M.; et al. The Role of Nano-Sensors in Breath Analysis for Early and Non-Invasive Disease Diagnosis. Chemosensors 2023, 11, 317.
- Rydosz, A. Nanosensors for exhaled breath monitoring as a possible tool for noninvasive diabetes detection. In Nanosensors for Smart Cities; Kumar Singh, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 467–481.
- Navarro, M.; Michiardi, A.; Castano, O.; Planell, J.A. Biomaterials in orthopaedics. J. R. Soc. Interface 2008, 5, 1137–1158.
- Deng, M.; James, R.; Laurencin, C.T.; Kumbar, S.G. Nanostructured polymeric scaffolds for orthopaedic regenerative engineering. IEEE Trans. NanoBiosci. 2012, 11, 3–14.
- Smith, I.O.; Liu, X.H.; Smith, L.A.; Ma, P.X. Nano-structured polymer scaffolds for tissue engineering and regenerative medicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009, 1, 226–236.
- Laurencin, C.T.; Ambrosio, A.M.A.; Borden, M.D.; Cooper Jr, J.A. Tissue engineering: Orthopedic applications. Ann. Rev. Biomed. Eng. 1999, 1, 19–46.
- Tien, Y.C.; Chih, T.T.; Lin, J.H.; Ju, C.P.; Lin, S.D. Augmentation of tendon-bone healing by the use of calcium-phosphate cement. J. Bone Jt. Surg. Br. 2004, 86, 1072–1076.
- Huangfu, X.; Zhao, J. Tendon-bone healing enhancement using injectable tricalcium phosphate in a dog anterior cruciate ligament reconstruction model. Arthroscopy 2007, 23, 455–462.
- Dvir, T.; Timko, B.P.; Kohane, D.S.; Langer, R. Nanotechnological strategies for engineering complex tissues. Nat. Nanotechnol. 2011, 6, 13–22.
- Suryavanshi, A.; Khanna, K.; Sindhu, K.R.; Bellare, J.; Srivastava, R. Magnesium oxide nanoparticle-loaded polycaprolactone composite electrospun fiber scaffolds for bone-soft tissue engineering applications: In-vitro and in-vivo evaluation. Biomed. Mater. 2017, 12, 055011.
- MacNeil, S. Biomaterials for tissue engineering of skin. Mater. Today 2008, 11, 26–35.
- Broughton, G.; Janis, J.E.; Attinger, C.E. The basic science of wound healing. Plast. Reconstr. Surg. 2006, 117, 12S–34S.
- Diegelmann, R.F.; Evans, M.C. Wound healing: An overview of acute, fibrotic and delayed healing. Front. Biosci. 2004, 1, 283–289.
- Gosain, A.; DiPietro, L.A. Aging and wound healing. World J. Surg. 2004, 28, 321–326.
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.; Eccleston, G.M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008, 97, 2892–2923.
- Jiang, Q.; Zhou, W.; Wang, J.; Tang, R.; Zhang, D.; Wang, X. Hypromellose succinate-crosslinked chitosan hydrogel films for potential wound dressing. Int. J. Biol. Macromol. 2016, 91, 85–91.
- Wen, Y.; Oh, J.K. Recent strategies to develop polysaccharide-based nanomaterials for biomedical applications. Macromol. Rapid Commun. 2014, 35, 1819–1832.
- Nishchay, V.; Krishna, P.; Amit, K.S.; Amit, B. Design of magnesium oxide nanoparticle incorporated carboxy methyl cellulose/poly vinyl alcohol composite film with novel composition for skin tissue engineering. Mater. Technol. 2022, 37, 706–716.
- Davis, M.E.; Chen, Z.G.; Shin, D.M. Nanoparticle therapeutics: An emerging treatment modality for cancer. Nat. Rev. Drug Discov. 2008, 7, 771–782.
- Petros, R.A.; DeSimone, J.M. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010, 9, 615–627.
- Wolfbeis, O.S. An overview of nanoparticles commonly used in fluorescent bioimaging. Chem. Soc. Rev. 2015, 44, 4743–4768.
- Monici, M. Cell and tissue autofluorescence research and diagnostic applications. Biotechnol. Annu. Rev. 2005, 11, 227–256.
- Vahrmeijer, A.L.; Hutteman, M.; van der Vorst, J.R.; van de Velde, C.J.; Frangioni, J.V. Image-guided cancer surgery using near-infrared fluorescence. Nat. Rev. Clin. Oncol. 2013, 10, 507–518.
- Dempsey, G.T.; Vaughan, J.C.; Chen, K.H.; Bates, M.; Zhuang, X. Evaluation of fluorophores for optimal performance in localization-based super-resolution imaging. Nat. Methods 2011, 8, 1027–1036.
- Kairdolf, B.A.; Smith, A.M.; Stokes, T.H.; Wang, M.D.; Young, A.D.; Nie, S. Semiconductor quantum dots for bioimaging and biodiagnostic applications. Annu. Rev. Anal. Chem. 2013, 6, 143–162.
- Khalid, A.; Norello, R.; Abraham, A.N.; Tetienne, J.-P.; Karle, T.J.; Lui, E.W.C.; Xia, K.; Tran, P.A.; O’Connor, A.J.; Mann, B.G.; et al. Biocompatible and Biodegradable Magnesium Oxide Nanoparticles with In Vitro Photostable Near-Infrared Emission: Short-Term Fluorescent Markers. Nanomaterials 2019, 9, 1360.
- Rasheed, P.A.; Sandhyarani, N. Synthesis of Luminescent MgO Nanocrystals and Their Application in Bioimaging. Adv. Sci. Eng. Med. 2014, 6, 283–289.
- Zhao, Q.; Liu, J.; Zhu, W.; Sun, C.; Di, D.; Zhang, Y.; Wang, P.; Wang, Z.; Wang, S. Dual-stimuli responsive hyaluronic acid-conjugated mesoporous silica for targeted delivery to CD44-overexpressing cancer cells. Acta Biomater. 2015, 23, 147–156.
- Rostami, M.; Nasab, A.S.; Fasihi-Ramandi, M.; Badiei, A.; Ganjali, M.R.; Nasrabadi, M.R.; Ahmadi, F. Cur-loaded magnetic ZnFe2O4@mZnO-Ox-pg-C3N4 composites as dual pH-and ultrasound responsive nano-carriers for controlled and targeted cancer chemotherapy. Mater. Chem. Phys. 2021, 271, 124863.
- Zamani, M.; Rostami, M.; Aghajanzadeh, M.; Manjili, H.K.; Rostamizadeh, K.; Danafar, H. Mesoporous titanium dioxide@ zinc oxide–graphene oxide nanocarriers for colon-specific drug delivery. J. Mater. Sci. 2018, 53, 1634–1645.
- Toledo, L.; Racine, L.; Pérez, V.; Henríquez, J.P.; Auzely-Velty, R.; Urbano, B.F. Physical nanocomposite hydrogels filled with low concentrations of TiO2 nanoparticles: Swelling, networks parameters and cell retention studies. Mater. Sci. Eng. C 2018, 92, 769–778.
- Rostami, M.; Aghajanzadeh, M.; Zamani, M.; Manjili, H.K.; Danafar, H. Sono-chemical synthesis and characterization of Fe3O4@mTiO2-GO nanocarriers for dual-targeted colon drug delivery. Res. Chem. Intermed. 2018, 44, 1889–1904.
- Mendes, R.G.; Bachmatiuk, A.; Büchner, B.; Cuniberti, G.; Rümmeli, M.H. Carbon nanostructures as multi-functional drug delivery platforms. J. Mater. Chem. B 2013, 1, 401–428.
- Jana, A.; Nguyen, K.T.; Li, X.; Zhu, P.; Tan, N.S.; Ågren, H.; Zhao, Y. Perylene-derived single-component organic nanoparticles with tunable emission: Efficient anticancer drug carriers with real-time monitoring of drug release. ACS Nano 2014, 8, 5939–5952.
- Danhier, F.; Feron, O.; Préat, V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control. Release 2010, 148, 135–146.
- Sabbagh, F.; Muhamad, I.I. Acrylamide-based hydrogel drug delivery systems: Release of Acyclovir from MgO nanocomposite hydrogel. J. Taiwan Inst. Chem. Eng. 2017, 72, 182–193.
More
Information
Subjects:
Nanoscience & Nanotechnology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.8K
Revisions:
2 times
(View History)
Update Date:
28 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





