Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Shunsuke Tanaka | -- | 3434 | 2024-02-27 14:49:07 | | | |
| 2 | Lindsay Dong | Meta information modification | 3434 | 2024-02-28 04:47:13 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Tanaka, S.; Fuku, K.; Ikenaga, N.; Sharaf, M.; Nakagawa, K. Metal–Organic Framework-Based Membranes for Gas Separation. Encyclopedia. Available online: https://encyclopedia.pub/entry/55552 (accessed on 27 February 2026).
Tanaka S, Fuku K, Ikenaga N, Sharaf M, Nakagawa K. Metal–Organic Framework-Based Membranes for Gas Separation. Encyclopedia. Available at: https://encyclopedia.pub/entry/55552. Accessed February 27, 2026.
Tanaka, Shunsuke, Kojiro Fuku, Naoki Ikenaga, Maha Sharaf, Keizo Nakagawa. "Metal–Organic Framework-Based Membranes for Gas Separation" Encyclopedia, https://encyclopedia.pub/entry/55552 (accessed February 27, 2026).
Tanaka, S., Fuku, K., Ikenaga, N., Sharaf, M., & Nakagawa, K. (2024, February 27). Metal–Organic Framework-Based Membranes for Gas Separation. In Encyclopedia. https://encyclopedia.pub/entry/55552
Tanaka, Shunsuke, et al. "Metal–Organic Framework-Based Membranes for Gas Separation." Encyclopedia. Web. 27 February, 2024.
Copy Citation
Metal–organic frameworks (MOFs) represent the largest class of materials among crystalline porous materials ever developed, and have attracted attention as core materials for separation technology. Their extremely uniform pore aperture and nearly unlimited structural and chemical characteristics have attracted great interest and promise for applying MOFs to adsorptive and membrane-based separations.
:metal–organic frameworks
polycrystalline membranes
membrane formation
1. Introduction
Research, development, and demonstration tests for the practical application of metal–organic frameworks (MOFs) are underway, involving companies and universities in various fields [1][2][3][4][5]. MOFs are porous materials consisting of coordination bonds between metal ions and multifunctional organic ligands. Unparalleled properties and functions (e.g., storage, adsorption, separation, catalytic, electromagnetic, and optical properties) can be exhibited by tuning their framework composition and pore structure. As companies begin to produce and market MOFs, products are being created that exploit their properties. Queen’s University Belfast start-ups, MOF Technologies and DECCO, have applied MOFs to a product that keeps fruit and vegetables fresh [6]. The role of MOFs is to store and release 1-methylcyclopropene, which inhibits the action of ethylene that ripens fruit and vegetables, as required. NuMat Technologies, a start-up company from Northwestern University, has commercialized a MOF as a gas cylinder that can store and safely transport toxic gases for the semiconductor industry [7]. Atomis, a start-up company from Kyoto University, is in the process of gaining approval for the commercial use of a MOF-based high-pressure gas container, CubiTan®. SyncMOF, a start-up company from Nagoya University, is in the process of commercializing a MOF-based gas separation system, MOFclean. Transaera, a start-up company from Massachusetts Institute of Technology, is in the process of commercializing dehumidifying air conditioning systems using MOFs. Svante and Crimeworks are also piloting the application of MOFs in direct air capture (DAC), which captures CO2 directly from the atmosphere. Thus, large-scale applications of MOFs are expected to expand.
To date, more than 14,000 unique MOFs, comprising more than 350 topologies, have been synthesized [8]. In addition, hundreds of thousands more have been computationally predicted [9]. The number of possible MOF structures may range from millions to billions [10]. When a new structure is proposed, it is important to properly characterize it in order to support its application and to understand its performance in the desired process. Different physical and chemical information can be obtained by different techniques, the choice of which depends on the type of material being studied and the equipment available. The available analytical techniques for MOFs include X-ray diffraction, X-ray photoelectron spectroscopy, X-ray energy-dispersive spectroscopy, thermogravimetry, differential thermal analysis, differential scanning calorimetry, Fourier transform infrared spectroscopy, Raman spectroscopy, scanning electron microscopy, transmission electron microscopy, nuclear magnetic resonance, and gas adsorption–desorption isotherm measurements. Of these, gas adsorption–desorption isotherm measurements are particularly essential for separation applications.
2. Characteristics of MOFs
2.1. Structural Flexibility
Some MOFs have flexible pore structures. It is known that the pore structure changes when gas is adsorbed. Some of these MOFs exhibit unique adsorption behavior in that they behave as nonporous materials under low-gas-pressure conditions and show no adsorption performance. On the other hand, when the gas pressure reaches a certain threshold pressure (so-called gate-opening pressure), they change to a porous structure, resulting in a rapid increase in adsorption. The gate-opening-type adsorption behavior, which is not observed in conventional porous materials, depends on the combination of metal ions and ligands constituting the framework. Various types of structural flexibility have been reported [11], for example, (1) changes in pore shape from a rhombic structure to a square structure and vice versa, (2) changes in the relative position of interpenetrating structures, (3) the stretching and shrinking of lattice layers, and (4) the rotation of ligands at the pore aperture. Furthermore, adsorption behavior has been reported to vary with crystal size and shape.
2.2. Structural Stability
The thermal and chemical stability of materials is one of the most important properties not only for membrane separation, but also for many industrial applications. Due to the instability of the metal–ligand coordination bond, the structure of many MOFs is degraded by moisture in the air. In order to prevent the collapse of the network structure due to the hydrolysis reactions of the metal–ligand coordination bonds or ligand substitution reactions, it is effective to have either a strong coordination bond that is thermodynamically stable or a kinetically stable structure using large steric hindrance. Basically, when the coordination environment with the ligand is the same, metal ions with higher valence and charge density form a more stable framework. This tendency is explained according to the HSAB theory and supported by many findings in MOF studies [12]. According to the HSAB theory, carboxylic acid ligands can be regarded as hard bases that form stable complexes with hard acid metal ions such as Al3+, Cr3+, Fe3+, Ti4+, and Zr4+.
3. Hydrocarbon Adsorption on MOFs
3.1. Olefins and Paraffins
The first MOF investigated for potential application in olefin/paraffin separation was HKUST-1, which consists of a paddle-wheel Cu(II) dimer and 1,3,5-benzenetricarboxylate as building blocks. Wang et al. measured the adsorption isotherms of C2H4 and C2H6 on HKUST-1 at 295 K and showed that C2H4 is preferentially adsorbed [13]. Water molecules are coordinated to the metal site of HKUST-1 and dehydration forms coordinatively unsaturated open metal sites [14].
MOFs with open metal sites include frameworks of the MIL series, such as MIL-53, MIL-96, and MIL-100, and MOF-74. The MIL series, consisting of trivalent transition metals such as Fe(III), Cr(III), Al(III), and V(III), has been widely studied as a MOF for gas separation. Compared to divalent metals, trivalent transition metals have stronger bonds to ligands and can form more chemically stable structures [15]. However, the strong bonding between the metal and the ligand makes it difficult to synthesize MOFs with high crystallinity, and synthetic methods that satisfy the conditions for spontaneous self-assembly by reversible “weak bonding” are required.
The MIL series has trivalent metal sites with high electrophilicity and is excellent for the adsorption of electron-rich olefins. Yoon et al. reported that MIL-100(Fe) can be applied to C3H6/C3H8 separation [16]. Lee et al. reported that MIL-101(Cr), from which terephthalate anions were removed by treatment with NH4F solution, showed C2H4/C2H6 selectivity ~4 [17].
MOF-74 is a honeycomb structure composed of Mg(II), Mn(II), Ni(II), Co(II), Zn(II), Cu(II) or Fe(II), and 2,5-dihydroxyterephthalate as building blocks. Bao et al. first investigated Mg-MOF-74 for the separation of C2H4/C2H6 and C3H6/C3H8 [18].
Olefin-selective adsorption using open metal sites of MOFs is enhanced by increasing the charge density of coordinatively unsaturated open metal sites. However, these MOFs exhibit very high enthalpies of adsorption (>tens of kJ/mol) and suffer a significant energy penalty in adsorbent regeneration. Furthermore, such MOFs may decrease their adsorption capacity in the presence of water.
Most MOFs without open metal sites do not show the selective adsorption of olefins/paraffins, with the notable exception of NOTT-300, which is composed of [AlO4(OH)2] and biphenyl-3,3′,5,5′-tetracarboxylate as building blocks. NOTT-300 exhibits a very high C2H4/C2H6 selectivity of 48.7, while its low enthalpy of adsorption, approximately 16 kJ/mol. The energy penalty for regeneration is also reduced [19].
The use of adsorbents that selectively adsorb paraffins saves energy by eliminating the adsorption–desorption cycle required for olefin recovery. However, C2H6 has a smaller quadrupole moment and larger dynamic molecular size than C2H4, making selective adsorption generally more difficult. On the other hand, the selective adsorption of C2H6 has been reported in several MOFs. ZIF-7, composed of Zn(II) and benzimidazolate, has been reported to adsorb C2H6 (and C3H8 compared to C3H6) at lower pressures than C2H4, although there is no large difference in saturation adsorption capacity for olefins and paraffins [20][21].
MAF-49, which is composed of Zn(II) and the triazole ligand bis(5-amino-1H-1,2,4-triazol-3-yl)methane and has one-dimensional zigzag channels, is also known to preferentially adsorb C2H6 [22]. The enthalpy of C2H6 adsorption by MAF-49 (60 kJ/mol) is higher than that of C2H4 (48 kJ/mol), and it preferentially adsorbs C2H6 in the low-pressure region, where C-H⋯N hydrogen bonds and electrostatic interactions occur between electronegative nitrogen atoms and C2H6
3.2. Other Hydrocarbons
The separation of 1,3-butadiene from C4 hydrocarbon mixtures is essential to produce synthetic rubber. However, C4 isomers have close boiling points, and some components form azeotropic mixtures. Kishida et al. discussed the possibility of separating 1,3-butadiene from C4 hydrocarbons by a MOF [23]. The synthesized MOF is called SD-65 and has an interpenetrating structure in which Zn(II) is coordinated to two components: 5-nitroisophthalate and 1,2-di(4-pyridyl)ethylene. SD-65 adsorbed almost no n-C4H10, i-C4H10, 1-butene, isobutene, trans-2-butene, or cis-2-butene (adsorption capacity ~2.5 cm3/g at approximately 1 bar), while it adsorbed 40 cm3/g of 1,3-butadiene. The pore structure remains closed until the pressure of 1,3-butadiene is about 0.6 bar, at which point the pore structure rapidly transitions to an open pore structure and butadiene is adsorbed.
The separation of linear/branched hydrocarbons using MOFs has also been studied. Pan et al. reported that a MOF composed of paddlewheel Cu(II) dimer and 4,4′-(hexafluoroisopropylidene)bis-(benzoic acid) adsorbs C3H8, C3H6, and n-C4H10, while i-C4H10, n-pentane, i-pentane, n-Hexane, and 3-methylpentane are not adsorbed [24]. Peralta et al. reported the separation of linear/branched hydrocarbons by ZIF-8 [25]. ZIF-8 adsorbs n-hexane and 3-methylpentane, but not 2,2-dimethylbutane.
The MIL series, including MIL-47 and MIL-53, has also been studied for xylene isomer separation [26][27][28][29][30]. MIL-47 and MIL-53 have the same crystal topology consisting of [MO4(OH)2] and phthalic acid. MIL-47, which is composed of V(III), has a rigid structure, whereas MIL-53, which is composed of Al(III), Cr(III), and Fe(III), shows a unique flexibility called the breathing effect. The p-xylene/m-xylene separation by MIL-47 showed a selectivity of 2.9. On the other hand, MIL-53(Al) could not separate p-xylene and m-xylene.
UiO-66, composed of zirconium and terephthalic acid, is well known for its excellent chemical and thermal stability. UiO-66 preferentially adsorbs branched hydrocarbons (2,2-dimethylbutane and 2,3-dimethylbutane) over linear hydrocarbons (n-hexane) [31]. This unique adsorption behavior is attributed to the 6–7 Å triangular lattice of the channel pores of UiO-66, which is believed to be responsible for the preferential adsorption of o-xylene over p-xylene.
High-purity C2H2 is an important raw material for the production of a variety of valuable chemicals. C2H2 production inevitably involves trace or large amounts of CO2 (1–50%), making C2H2/CO2 separation extremely important in the petrochemical industry. Since the realization of the first example of MOFs for C2H2 adsorption by Matsuda et al. [32], MOFs showing a highly selective separation of C2H2/CO2 through powerful strategies of pore tuning and pore functionalization have been actively investigated.
4. CO2 Capture and H2 Purification
Since global CO2 emissions from energy conversion, such as power generation, account for more than 40% of total global CO2 emissions, the decarbonization of energy conversion is crucial to reducing emissions [33]. CO2 separation and capture processes in the power-generation sector can be classified into pre-combustion, post-combustion, and oxy-fuel combustion [34]. The most mature technology for capturing CO2 after combustion is chemical absorption using monoethanolamine (MEA). However, the energy cost of CO2 separation and capture is high, even for power plants that use the captured CO2 for enhanced oil recovery (EOR) [35]. Carbon pricing through “carbon taxes” and “emissions trading” has been introduced as a measure to reduce CO2 emissions.
Membrane gas separation was commercialized in the late 1970s for hydrogen separation and has since been applied to carbon dioxide separation from natural gas, biogas, and landfill gas, air separation (nitrogen-enriched gas and oxygen-enriched gas production), and air dehumidification. However, membrane separation as a CO2 separation and recovery technology for CO2 Capture, Utilization and Storage (CCUS) has only been studied up to bench scale with a few exceptions.
The global energy crisis caused by increasing energy consumption calls for sustainable alternative energy sources. Although H2 is the most promising energy source with high energy density and CO2-free emissions, more than 90% of H2 is produced by the steam reforming process of natural gas using fossil fuel hydrocarbons, which emit CO2 [36]. The produced H2 is expected to be used as energy in fuel cell vehicles (FCVs), FC buses, and power generation; however, to date it has been used in large-scale processes in various industries, including petrochemicals, electronics, metallurgy, steelmaking, pharmaceuticals, and the production of raw material chemicals.Membrane technology is the most promising alternative as it is lower cost, consumes less energy, and is easy to operate continuously compared to other conventional methods such as fractional/cryogenic distillation and pressure/temperature swing adsorption. To date, intensive research has been conducted for H2 separation and recovery using conventional materials, ranging from organic polymers to inorganic materials such as palladium-based metals, silica, zeolites, and carbon. Polymers such as silicone rubber, cellulose acetate, polysulfone, and polyimide have mainly been used as membrane materials. Recently, porous membranes with sub-nanometer-sized pores have been extensively studied, with silica and zeolite membranes receiving much attention. Mixed-matrix membranes (MMMs), in which MOFs are mixed with polymer matrix as filler, have also been actively studied. Pre-combustion is primarily intended for use in integrated gasification combined cycles (IGCCs), a process in which coal and natural gas are partially oxidized to produce natural gas vapor. Fuel gas is purified by separating and recovering CO2 from synthesis gas (consisting primarily of H2 and CO) produced by the partial oxidation of coal and natural gas, or by the steam reforming of natural gas to produce H2 and CO2 by reacting CO with aqueous gas shift. Since high-pressure gas is the separation target (mainly CO2/H2) in pre-combustion, equipment such as vacuum pumps is not required, saving energy and cost. However, the separation membrane must be durable under high temperature and high pressure. In addition, since H2 has a smaller molecular size than CO2, H2-selective permeation membranes have mainly been studied. On the other hand, post-conversion targets the separation of combustion exhaust gas generated from boilers in power plants at relatively low pressure, which requires the installation of vacuum pumps and compressors, making it difficult to achieve significant energy conservation and cost reduction compared to existing technologies.
5. MOF-Based Membranes
5.1. Types of Membranes
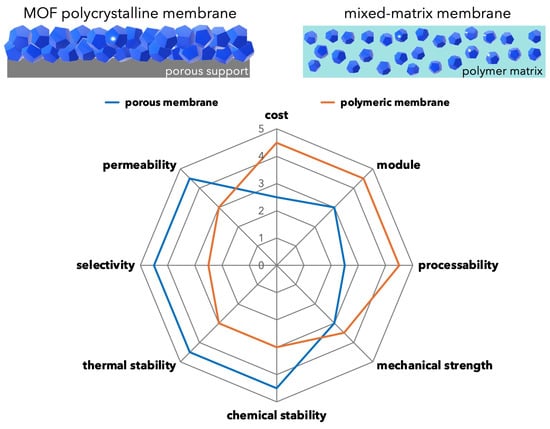
Separation membranes based on MOF can be broadly classified into two categories. One is a polycrystalline membrane composed of MOF alone, and the other is a mixed-matrix membrane (MMM) in which MOF is mixed with a polymer membrane as a filler. Similar to porous inorganic membranes such as silica and zeolite membranes, MOF polycrystalline membranes are often formed on porous ceramic supports to ensure the mechanical strength of the membrane. MOFs are often compared and discussed with zeolites because of their similarities with zeolites in terms of crystalline porous structure. The MMM, on the other hand, is a strategy to improve membrane performance by synergistically combining the excellent processability of polymers with the porous properties of MOF fillers (Figure 1).

Figure 1. Schematic illustration of polycrystalline membrane and MMM. Radar chart showing the advantages and disadvantages of porous and polymeric membranes. The ideal vision for MMM is to improve membrane performance by combining the advantages of porous and polymeric materials.
MOF polycrystalline membranes exhibit high separation performance by selecting the optimum structure for the separation target because the only membrane permeation pathway for gas molecules is through the pores of the MOF. However, nonselective permeation often occurs due to the formation of grain boundaries between crystals, pinholes, and intracrystalline defects. In order to fabricate membranes with dense grain boundaries, polycrystalline membranes are generally prepared by using seed crystals via the secondary growth method [37][38][39][40].
MMM is a membrane in which MOF fillers are dispersed in a polymer matrix. The dispersion state of the polymer and filler greatly affects the performance of the membrane [41]. MMMs may be prepared on supports, but they differ from MOF polycrystalline membranes in that the processability of polymers can be used to fabricate freestanding membranes.
5.2. MOF Membrane-Preparation Method and Points to Consider
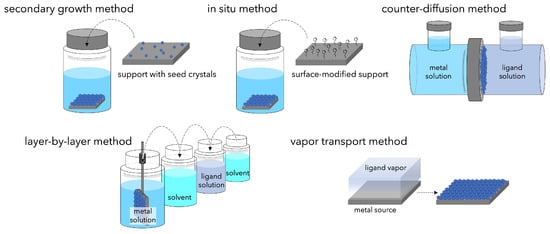
If MOFs can be thinned so that there are no voids between crystals, they can be applied as separation membranes. However, fabricating polycrystalline membranes is not so easy. It must be noted that cracks, pinholes, and intra-crystal defects between crystals cause non-selective permeation, and that large areas must be achieved with thin membranes. Various methods have been proposed for preparing MOF membranes (Figure 2).

Figure 2. Schematic of the methods developed for synthesis of continuous MOF membranes.
To fabricate continuous polycrystalline membranes on a support, a dense heterogeneous nucleation field must exist on the support surface. The secondary growth method is often used, in which pre-prepared seed crystals are loaded on the support surface and grown to form continuous films. Seeding techniques such as dip coating [42], slip coating [43], and rubbing [44] are used, followed by solvothermal or hydrothermal synthesis.
To address the issue of adhesion between membrane and support, modification of the support surface with compounds that bind the MOF crystals and the support has been used [45][46][47][48]. These compounds have one end that can coordinate with the nodes constituting the MOF and the other end that can covalently bond with the support. The functional groups immobilized on the support cause the heterogeneous nucleation of MOFs and promote crystal growth, resulting in continuous MOF membranes with a high degree of crystallinity and relatively thin membrane thickness. The chemical modification method is also effective when using polymers as supports in addition to ceramic supports [49][50].
5.3. Olefin/Paraffin Separation
MOFs have potential for a wide range of separation targets due to their excellent pore structure and composition, as well as the diversity of their synthesis and membrane production methods. Although MOFs appear promising for olefin/paraffin separation, only a few MOF membranes are currently available. While they have been demonstrated to be effective for the separation of C3H6/C3H8, few have been reported to be able to efficiently separate C2H4/C2H6 [51][52][53][54][55][56].
ZIF-8, which is composed of Zn(II) and 2-methylimidazolate as building blocks and has an SOD structure, has been the most studied MOF for C3H6/C3H8 separation. The effective pore size of ZIF-8 is 4.0–4.2 Å, but even 1,2,4-trimethylbenzene of approximately 7.6 Å enters the pores [57], suggesting a lack of sharp molecular sieving. Indeed, the selectivity of CO2/CH4 separation by the ZIF-8 membrane is only about 5 [58]. On the other hand, the structural flexibility of ZIF-8 works effectively in C3H6/C3H8 separation, showing a sharp cut-off between C3H6 and C3H8 molecular sizes.
5.4. Other Hydrocarbon Separations
Eum et al. applied the IMMP method, which produced ZIF-8 membranes on polyamide-imide hollow fibers, to carbon hollow fibers to produce ZIF-90 membranes [59]. In general, polymer supports have poor chemical resistance and swell when exposed to organic compounds. In contrast, ZIF-90 membranes fabricated on chemically inert carbon hollow fibers exhibited high chemical resistance. ZIF-90, which is composed of Zn(II) and 2-imidazolecarboxaldehyde, has the same crystal topology as ZIF-8, and its crystallographic pore size (3.5 Å) is not much different from that of ZIF-8. On the other hand, its effective pore size (5.0 Å) is larger than ZIF-8 due to its structural flexibility. The ZIF-90 membrane showed n-C4H10/i-C4H10 selectivity of 12 and n-C4H10 permeability of 6.0 × 10−8 mol m−2 Pa−1 s−1, indicating its potential for the separation of butane isomers.
5.5. CO2 Separation
Commercially available PolyactiveTM [60] and Pebax® [61] membranes are PEO-based block copolymers with high CO2 permselectivity. PEO-based membranes exhibit a high CO2/N2 permselectivity of over 40 due to the high affinity of ether oxygen for CO2. However, the CO2 permeability is not high enough to be of practical use. Due to the relatively low CO2 permeability, when PEO membranes are used for CO2 capture from flue gases emitted from coal-fired power plants, the membrane thickness should be reduced to less than 200 nm to achieve a CO2 permeance of more than 1000 GPU, which is required for practical use [62]. MOF-based membrane research targeting CO2 separation has been actively investigated [63]. HKUST-1, MIL-53, MIL-100, and MIL-101 are candidates for combustion flue gas, natural gas purification, and hydrogen purification due to their higher CO2 adsorption capacity than typical zeolites [64][65][66][67][68][69][70][71][72][73][74][75][76].
6. Conclusions
The development of membrane separation using MOFs has been active due to the rapid increase in the number of studies on MOFs, from synthesis and structural design to application. Relatively long-term durability tests have also been conducted at the laboratory level. Although various MOF-based membranes have been fabricated, a common issue is how to achieve thin membrane formation without generating defects such as pinholes, cracks, and grain boundaries. To this end, it is important to understand the formation mechanism of MOFs based on complexation reactions between metal ions and ligands, and to develop the elementary processes of membrane formation, which can control nucleation and crystal growth.
References
- Li, J.R.; Kuppler, R.J.; Zhou, H.C. Selective gas adsorption and separation in metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504.
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 974–986.
- Rubio-Martinez, M.; Avci-Camur, C.; Thornton, A.W.; Imaz, I.; Maspoch, D.; Hill, M.R. New synthetic routes towards MOF production at scale. Chem. Soc. Rev. 2017, 46, 3453–3480.
- Dai, S.; Tissot, A.; Serre, C. Metal-organic frameworks: From ambient green synthesis to applications. Bull. Chem. Soc. Jpn. 2021, 94, 2623–2636.
- Zulkifli, Z.I.; Lim, K.L.; Teh, L.P. Metal-organic frameworks (MOFs) and their applications in CO2 adsorption and conversion. ChemistrySelect 2022, 7, e202200572.
- Petit, C. Present and future of MOF research in the field of adsorption and molecular separation. Curr. Opin. Chem. Eng. 2018, 20, 132–142.
- Editorial article. Frameworks for commercial success. Nat. Chem. 2016, 8, 987.
- Chung, Y.G.; Haldoupis, E.; Bucior, B.J.; Haranczyk, M.; Lee, S.; Zhang, H.D.; Vogiatzis, K.D.; Milisavljevic, M.; Ling, S.L.; Camp, J.S.; et al. Advances, updates, and analytics for the computation-ready, experimental metal-organic framework database: CoRE MOF 2019. J. Chem. Eng. Data 2019, 64, 5985–5998.
- Anderson, R.; Gómez-Gualdrón, D.A. Increasing topological diversity during computational “synthesis” of porous crystals: How and why. CrystEngComm 2019, 21, 1653–1665.
- Bobbitt, N.S.; Shi, K.H.; Bucior, B.J.; Chen, H.Y.; Tracy-Amoroso, N.; Li, Z.; Sun, Y.Z.S.; Merlin, J.H.; Siepmann, J.I.; Siderius, D.W.; et al. MOFX-DB: An online database of computational adsorption data for nanoporous materials. J. Chem. Eng. Data 2023, 68, 483–498.
- Schneemann, A.; Bon, V.; Schwedler, I.; Senkovska, I.; Kaskel, S.; Fischer, R.A. Flexible metal–organic frameworks. Chem. Soc. Rev. 2014, 43, 6062–6096.
- Devic, T.; Serre, C. High valence 3p and transition metal based MOFs. Chem. Soc. Rev. 2014, 43, 6097–6115.
- Wang, Q.M.; Shen, D.M.; Bulow, M.; Lau, M.L.; Deng, S.G.; Fitch, F.R.; Lemcoff, N.O.; Semanscin, J. Metallo-organic molecular sieve for gas separation and purification. Microporous Mesoporous Mater. 2002, 55, 217–230.
- Lin, K.S.; Adhikari, A.K.; Ku, C.N.; Chiang, C.L.; Kuo, H. Synthesis and characterization of porous HKUST-1 metal organic frameworks for hydrogen storage. Int. J. Hydrogen Energy 2012, 37, 13865–13871.
- Abednatanzi, S.; Gohari, D.P.; Depauw, H.; Coudert, F.X.; Vrielinck, H.; Van Der Voort, P.; Leus, K. Mixed-metal metal–organic frameworks. Chem. Soc. Rev. 2019, 48, 2535–2565.
- Yoon, J.W.; Seo, Y.K.; Hwang, Y.K.; Chang, J.S.; Leclerc, H.; Wuttke, S.; Bazin, P.; Vimont, A.; Daturi, M.; Bloch, E. Controlled reducibility of a metal–organic framework with coordinatively unsaturated sites for preferential gas sorption. Angew. Chem. Int. Ed. 2010, 49, 5949–5952.
- Lee, S.J.; Yoon, J.W.; Seo, Y.K.; Kim, M.B.; Lee, S.K.; Lee, U.H.; Hwang, Y.K.; Bae, Y.S.; Chang, J.S. Effect of purification conditions on gas storage and separations in a chromium-based metal-organic framework MIL-101. Microporous Mesoporous Mater. 2014, 193, 160–165.
- Bao, Z.; Alnemrat, S.; Yu, L.; Vasiliev, I.; Ren, Q.; Lu, X.; Deng, S. Adsorption of ethane, ethylene, propane, and propylene on a magnesium-based metal–organic framework. Langmuir 2011, 27, 13554–13562.
- Yang, S.; Ramirez-Cuesta, A.J.; Newby, R.; Garcia-Sakai, V.; Manuel, P.; Callear, S.K.; Campbell, S.I.; Tang, C.C.; Schroder, M. Supramolecular binding and separation of hydrocarbons within a functionalized porous metal–organic framework. Nat. Chem. 2014, 7, 121–129.
- Gücüyener, C.; van den Bergh, J.; Gascon, J.; Kapteijn, F. Ethane/ethene separation turned on its head: Selective ethane adsorption on the metal−organic framework ZIF-7 through a gate-opening mechanism. J. Am. Chem. Soc. 2010, 132, 17704–17706.
- Van den Bergh, J.; Gucuyener, C.; Pidko, E.A.; Hensen, E.J.M.; Gascon, J.; Kapteijn, F. Understanding the anomalous alkane selectivity of ZIF-7 in the separation of light alkane/alkene mixtures. Chem. Eur. J. 2011, 17, 8832–8840.
- Liao, P.Q.; Zhang, W.X.; Zhang, J.P.; Chen, X.M. Efficient purification of ethene by an ethane-trapping metal-organic framework. Nat. Commun. 2015, 6, 8697.
- Kishida, K.; Okumura, Y.; Watanabe, Y.; Mukoyoshi, M.; Bracco, S.; Comotti, A.; Sozzani, P.; Horike, S.; Kitagawa, S. Recognition of 1,3-butadiene by a porous coordination polymer. Angew. Chem. Int. Ed. 2016, 55, 13784–13788.
- Pan, L.; Olson, D.H.; Ciemnolonski, L.R.; Heddy, R.; Li, J. Separation of hydrocarbons with a microporous metal–organic framework. Angew. Chem. Int. Ed. 2006, 45, 616–619.
- Peralta, D.; Chaplais, G.; Simon-Masseron, A.; Barthelet, K.; Pirngruber, G.D. Separation of C6 paraffins using zeolitic imidazolate frameworks: Comparison with zeolite 5A. Ind. Eng. Chem. Res. 2012, 51, 4692–4702.
- Alaerts, L.; Kirschhock, C.E.; Maes, M.; van der Veen, M.A.; Finsy, V.; Depla, A.; Martens, J.A.; Baron, G.V.; Jacobs, P.A.; Denayer, J.F.M.; et al. Selective Adsorption and separation of xylene isomers and ethylbenzene with the microporous vanadium(IV) terephthalate MIL-47. Angew. Chem. Int. Ed. 2007, 46, 4293–4297.
- Alaerts, L.; Maes, M.; Jacobs, P.A.; Denayer, J.F.M.; De Vos, D.E. Activation of the metal-organic framework MIL-47 for selective adsorption of xylenes and other difunctionalized aromatics. Phys. Chem. Chem. Phys. 2008, 10, 2979–2985.
- Alaerts, L.; Maes, M.; Giebeler, L.; Jacobs, P.A.; Martens, J.A.; Denayer, J.F.M.; Kirschhock, C.E.A.; De Vos, D.E. Selective adsorption and separation of ortho-substituted alkylaromatics with the microporous aluminum terephthalate MIL-53. J. Am. Chem. Soc. 2008, 130, 14170–14178.
- Finsy, V.; Verelst, H.; Alaerts, L.; De Vos, D.; Jacobs, P.A.; Baron, G.V.; Denayer, J.F.M. Pore-filling-dependent selectivity effects in the vapor-phase separation of xylene isomers on the metal−organic framework MIL-47. J. Am. Chem. Soc. 2008, 130, 7110–7118.
- Finsy, V.; Christine, E.A.; Kirschhock, C.E.; Vedts, G.; Maes, M.; Alaerts, L.; De Vos, D.E.; Baron, G.V.; Denayer, J.F.M. Framework breathing in the vapour-phase adsorption and separation of xylene isomers with the metal–organic framework MIL-53. Chem. Eur. J. 2009, 15, 7724–7731.
- Bárcia, P.S.; Guimarães, D.; Mendes, P.A.P.; Silva, J.A.C.; Guillerm, V.; Chevreau, H.; Serre, C.; Rodrigues, A.E. Reverse shape selectivity in the adsorption of hexane and xylene isomers in MOF UiO-66. Microporous Mesoporous Mater. 2011, 139, 67–73.
- Matsuda, R.; Kitaura, R.; Kitagawa, S.; Kubota, Y.; Belosludov, R.V.; Kobayashi, T.C.; Sakamoto, H.; Chiba, T.; Takata, M.; Kawazoe, Y.; et al. Highly controlled acetylene accommodation in a metal-organic microporous material. Nature 2005, 435, 238–241.
- Masson-Delmotte, V.; Zhai, P.; Pörtner, H.O.; Roberts, D.; Skea, J.; Shukla, P.R.; Pirani, A.; Moufouma-Okia, W.; Péan, C.; Pidcock, R.; et al. Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change. World Meteorological Organization Technical Document: Geneva, Switzerland, 2018. Available online: http://www.ipcc.ch/report/sr15/ (accessed on 25 January 2024).
- Siegelman, R.L.; Kim, E.J.; Long, J.R. Porous materials for carbon dioxide separations. Nat. Mater. 2021, 20, 1060–1072.
- Schlissel, D.W.D.; Wamsted, D. Holy Grail of Carbon Capture Continues to Elude Coal Industry. Inst. Energy Econ. Financ. Anal. 2018. Available online: https://ieefa.org/resources/holy-grail-carbon-capture-continues-elude-coal-industry (accessed on 25 January 2024).
- Panda, P.K.; Sahoo, B.; Ramakrishna, S. Hydrogen production, purification, storage, transportation, and their applications: A review. Energy Technol. 2023, 11, 2201434.
- Hermes, S.; Schroder, F.; Chelmowski, R.; Wöll, C.; Fischer, R.A. Selective nucleation and growth of metal−organic open framework thin films on patterned COOH/CF3-terminated self-assembled monolayers on Au(111). J. Am. Chem. Soc. 2005, 127, 13744–13745.
- Arnold, M.; Kortunov, P.; Jones, D.J.; Nedellec, Y.; Kärger, J.; Caro, J. Oriented crystallisation on supports and anisotropic mass transport of the metal-organic framework manganese formate. Eur. J. Inorg. Chem. 2007, 2007, 60–64.
- Scherb, C.; Schodel, A.; Bein, T. Directing the structure of metal–organic frameworks by oriented surface growth on an organic monolayer. Angew. Chem. Int. Ed. 2008, 47, 5777–5779.
- Gascon, J.; Aguado, S.; Kapteijn, F. Manufacture of dense coatings of Cu3(BTC)2 (HKUST-1) on α-alumina. Microporous Mesoporous Mater. 2008, 113, 132–138.
- Lin, R.J.; Hernandez, B.V.; Ge, L.; Zhu, Z.H. Metal organic framework based mixed matrix membranes: An overview on filler/polymer interfaces. J. Mater. Chem. A 2018, 6, 293–312.
- Liu, Y.; Zeng, G.; Pan, Y.; Lai, Z. Synthesis of highly c-oriented ZIF-69 membranes by secondary growth and their gas permeation properties. J. Membr. Sci. 2011, 379, 46–51.
- Zheng, B.; Pan, Y.; Lai, Z.; Huang, K.W. Molecular dynamics simulations on gate opening in ZIF-8: Identification of factors for ethane and propane separation. Langmuir 2013, 29, 8865–8872.
- Venna, S.R.; Carreon, M.A. Highly permeable zeolite imidazolate framework-8 membranes for CO2/CH4 separation. J. Am. Chem. Soc. 2010, 132, 76–78.
- Huang, A.; Bux, H.; Steinbach, F.; Caro, J. Molecular-sieve membrane with hydrogen permselectivity: ZIF-22 in LTA topology prepared with 3-aminopropyltriethoxysilane as covalent linker. Angew. Chem. Int. Ed. 2010, 49, 4958–4961.
- McCarthy, M.C.; Guerrero, V.V.; Barnett, G.V.; Jeong, H.K. Synthesis of zeolitic imidazolate framework films and membranes with controlled microstructures. Langmuir 2010, 26, 14636–14641.
- Tanaka, S.; Shimada, T.; Fujita, K.; Miyake, Y.; Kida, K.; Yogo, K.; Denayer, J.F.M.; Sugita, M.; Takewaki, T. Seeding-free aqueous synthesis of zeolitic imidazolate framework-8 membranes: How to trigger preferential heterogeneous nucleation and membrane growth in aqueous rapid reaction solution. J. Membr. Sci. 2014, 472, 29–38.
- Tanaka, S.; Okubo, K.; Kida, K.; Sugita, M.; Takewaki, T. Grain size control of ZIF-8 membranes by seeding-free aqueous synthesis and their performances in propylene/propane separation. J. Membr. Sci. 2017, 544, 306–311.
- Li, W.; Yang, Z.; Zhang, G.; Fan, Z.; Meng, Q.; Shen, C.; Gao, C. Stiff metal–organic framework–polyacrylonitrile hollow fiber composite membranes with high gas permeability. J. Mater. Chem. A 2014, 2, 2110–2118.
- Li, W.; Meng, Q.; Zhang, C.; Zhang, G. Metal–organic framework/PVDF composite membranes with high H2 permselectivity synthesized by ammoniation. Chem. Eur. J. 2015, 21, 7224–7230.
- Wei, R.C.; Liu, X.W.; Zhou, Z.Y.; Chen, C.L.; Yuan, Y.Y.; Li, Z.; Li, X.; Dong, X.L.; Lu, D.W.; Han, Y.; et al. Carbon nanotube supported oriented metal organic framework membrane for effective ethylene/ethane separation. Sci. Adv. 2022, 8, eabm6741.
- Bux, H.; Chmelik, C.; Krishna, R.; Caro, J. Ethene/ethane separation by the MOF membrane ZIF-8: Molecular correlation of permeation, adsorption, diffusion. J. Membr. Sci. 2011, 369, 284–289.
- Sánchez, E.P.V.; Gliemann, H.; Haas-Santo, K.; Ding, W.J.; Hansjosten, E.; Wohlgemuth, J.; Woll, C.; Dittmeyer, R. α-Al2O3-supported ZIF-8 SURMOF membranes: Diffusion mechanism of ethene/ethane mixtures and gas separation performance. J. Membr. Sci. 2019, 594, 117421.
- Sun, Y.W.; Ji, T.T.; Gao, Y.L.; Yan, J.H.; He, Y.F.; Xu, G.L.; Yan, F.Y.; Bian, Q.; Liu, Y. Freezing contra-diffusion: A new protocol for synthesizing Co-gallate MOF membranes toward superior ethylene/ethane separation performance. ACS Mater. Lett. 2023, 5, 558–564.
- Yang, K.; Ban, Y.J.; Yang, W.S. Layered MOF membranes modified with ionic liquid/AgBF4 composite for olefin/paraffin separation. J. Membr. Sci. 2021, 639, 119771.
- Wang, J.Y.; Wang, Y.; Liu, Y.T.; Wu, H.; Zhao, M.G.; Ren, Y.X.; Pu, Y.C.A.; Li, W.P.; Wang, S.Y.; Song, S.Q.; et al. Ultrathin ZIF-8 membrane through inhibited Ostwald ripening for high-flux C3H6/C3H8 separation. Adv. Funct. Mater. 2022, 32, 2208064.
- Zhang, K.; Lively, R.P.; Zhang, C.; Chance, R.R.; Koros, W.J.; Sholl, D.S.; Nair, S. Exploring the framework hydrophobicity and flexibility of ZIF-8: From biofuel recovery to hydrocarbon separations. J. Phys. Chem. Lett. 2013, 4, 3618–3622.
- Bux, H.; Chmelik, C.; van Baten, J.M.; Krishna, R.; Caro, J. Novel MOF-membrane for molecular sieving predicted by IR-diffusion studies and molecular modeling. Adv. Mater. 2010, 22, 4741–4743.
- Eum, K.; Ma, C.; Koh, D.Y.; Rashidi, F.; Li, Z.; Jones, C.W.; Lively, R.P.; Nair, S. Zeolitic imidazolate framework membranes supported on macroporous carbon hollow fibers by fluidic processing techniques. Adv. Mater. Interfaces 2017, 4, 1700080.
- Rahman, M.M.; Abetz, C.; Shishatskiy, S.; Martin, J.; Müller, A.J.; Abetz, V. CO2 selective PolyActive membrane: Thermal transitions and gas permeance as a function of thickness. ACS Appl. Mater. Interfaces 2018, 10, 26733–26744.
- Embaye, A.S.; Martínez-Izquierdo, L.; Malankowska, M.; Téllez, C.; Coronas, J. Poly(ether-block-amide) copolymer membranes in CO2 separation applications. Energy Fuels 2021, 35, 17085–17102.
- Merkel, T.C.; Lin, H.Q.; Wei, X.T.; Baker, R. Power plant post-combustion carbon dioxide capture: An opportunity for membranes. J. Membr. Sci. 2010, 359, 126–139.
- Venna, S.R.; Carreon, M.A. Metal organic framework membranes for carbon dioxide separation. Chem. Eng. Sci. 2015, 124, 3–19.
- Llewellyn, P.L.; Bourrelly, S.; Serre, C.; Vimont, A.; Daturi, M.; Hamon, L.; De Weireld, G.; Chang, J.S.; Hong, D.Y.; Hwang, Y.K. High uptakes of CO2 and CH4 in mesoporous metal—Organic frameworks MIL-100 and MIL-101. Langmuir 2008, 24, 7245–7250.
- Dunne, J.; Myers, A.L. Adsorption of gas mixtures in micropores: Effect of difference in size of adsorbate molecules. Chem. Eng. Sci. 1994, 49, 2941–2951.
- Pakseresht, S.; Kazemeini, M.; Akbarnejad, M.M. Equilibrium isotherms for CO, CO2, CH4 and C2H4 on the 5A molecular sieve by a simple volumetric apparatus. Sep. Purif. Technol. 2002, 28, 53–60.
- Maghsoudi, H.; Soltanieh, M.; Bozorgzadeh, H.; Mohamadalizadeh, A. Adsorption isotherms and ideal selectivities of hydrogen sulfide and carbon dioxide over methane for the Si-CHA zeolite: Comparison of carbon dioxide and methane adsorption with the all-silica DD3R zeolite. Adsorption 2013, 19, 1045–1053.
- Calleja, G.; Pau, J.; Calles, J.A. Pure and multicomponent adsorption equilibrium of carbon dioxide, ethylene, and propane on ZSM-5 zeolites with different Si/Al ratios. J. Chem. Eng. Data 1998, 43, 994–1003.
- Venna, S.R.; Carreon, M.A. Synthesis of SAPO-34 crystals in the presence of crystal growth inhibitors. J. Phys. Chem. B 2008, 112, 16261–16265.
- Lin, J.B.; Nguyen, T.T.T.; Vaidhyanathan, R.; Burner, J.; Taylor, J.M.; Durekova, H.; Akhtar, F.; Mah, R.K.; Ghaffari-Nik, O.; Marx, S.; et al. A scalable metal-organic framework as a durable physisorbent for carbon dioxide capture. Science 2021, 374, 1464–1469.
- Millward, A.R.; Yaghi, O.M. Metal−organic frameworks with exceptionally high capacity for storage of carbon dioxide at room temperature. J. Am. Chem. Soc. 2005, 127, 17998–17999.
- Bourrelly, S.; Llewellyn, P.L.; Serre, C.; Millange, F.; Loiseau, T.; Férey, G. Different adsorption behaviors of methane and carbon dioxide in the isotypic nanoporous metal terephthalates MIL-53 and MIL-47. J. Am. Chem. Soc. 2005, 127, 13519–13521.
- Liu, J.; Wang, Y.; Benin, A.I.; Jakubczak, P.; Willis, R.R.; LeVan, M.D. CO2/H2O adsorption equilibrium and rates on metal−organic frameworks: HKUST-1 and Ni/DOBDC. Langmuir 2010, 26, 14301–14307.
- Mason, J.A.; Sumida, K.; Herm, Z.R.; Krishna, R.; Long, J.R. Evaluating metal–organic frameworks for post-combustion carbon dioxide capture via temperature swing adsorption. Energy Environ. Sci. 2011, 4, 3030–3040.
- Shekhah, O.; Belmabkhout, Y.; Chen, Z.; Guillerm, V.; Cairns, A.; Adil, K.; Eddaoudi, M. Made-to-order metal-organic frameworks for trace carbon dioxide removal and air capture. Nat. Commun. 2014, 5, 4228.
- Venna, S.R.; Zhu, M.Q.; Li, S.G.; Carreon, M.A. Knudsen diffusion through ZIF-8 membranes synthesized by secondary seeded growth. J. Porous Mater. 2014, 21, 235–240.
More
Information
Subjects:
Chemistry, Applied
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
5.3K
Revisions:
2 times
(View History)
Update Date:
28 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





