Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Basem Al-Omari | -- | 3256 | 2024-02-27 13:42:24 | | | |
| 2 | Sirius Huang | Meta information modification | 3256 | 2024-02-28 02:19:59 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Mansour, S.; Alkhaaldi, S.M.I.; Sammanasunathan, A.F.; Ibrahim, S.; Farhat, J.; Al-Omari, B. Precision Nutrition in Obesity Management. Encyclopedia. Available online: https://encyclopedia.pub/entry/55546 (accessed on 14 January 2026).
Mansour S, Alkhaaldi SMI, Sammanasunathan AF, Ibrahim S, Farhat J, Al-Omari B. Precision Nutrition in Obesity Management. Encyclopedia. Available at: https://encyclopedia.pub/entry/55546. Accessed January 14, 2026.
Mansour, Samy, Saif M. I. Alkhaaldi, Ashwin F. Sammanasunathan, Saleh Ibrahim, Joviana Farhat, Basem Al-Omari. "Precision Nutrition in Obesity Management" Encyclopedia, https://encyclopedia.pub/entry/55546 (accessed January 14, 2026).
Mansour, S., Alkhaaldi, S.M.I., Sammanasunathan, A.F., Ibrahim, S., Farhat, J., & Al-Omari, B. (2024, February 27). Precision Nutrition in Obesity Management. In Encyclopedia. https://encyclopedia.pub/entry/55546
Mansour, Samy, et al. "Precision Nutrition in Obesity Management." Encyclopedia. Web. 27 February, 2024.
Copy Citation
Obesity is a complex metabolic disorder that is associated with several diseases. Precision nutrition (PN) has emerged as a tailored approach to provide individualised dietary recommendations.
precision nutrition
obesity
translational medicine
genetics
microbiome
lifestyle factors
1. Introduction
Obesity is a complex metabolic disorder that can present with cardiovascular diseases (CVD), type 2 diabetes mellitus (T2DM), non-alcoholic fatty liver disease (NAFLD), dyslipidaemias, and cancer [1][2][3]. According to the World Obesity Atlas 2023, 38% of the global population is currently either overweight or obese [4]. This progression of obesity has been primarily associated with the consumption of an unbalanced diet rich in fat and fructose for a long period while adopting a sedentary lifestyle [5][6]. Therefore, weight control is vital to prevent diseases. It is suggested that controlling weight is influenced by the sources and quality of food rather than the quantities consumed in the diet [7]. It is also suggested that the individual’s genetic and epigenetic interactions with dietary intake and physical activity are linked to an increased risk of developing obesity [8][9]. In case of intrinsic disruptions, a high intake of saturated fat or refined carbohydrates results in dysregulation of the central metabolic pathways and increased weight [10][11]. Additionally, the gut microbiota and its interactions with genes and diet can modify the risk of developing obesity [12].
The multifactorial nature of obesity has led to the ongoing application of precision public health approaches to enhance the understanding of the interplay between individuals’ intrinsic components and environmental factors [13]. These precision approaches have been implemented to ameliorate patients’ health and quality of life [14]. In recent years, precision nutrition (PN) was introduced as one of these approaches to provide customised dietary recommendations for individuals based on their genetic profile, microbiota, physical activity, and lifestyle [15][16]. In particular, PN has been applied in metabolic conditions such as obesity to provide individualised metabolic care and clinical nutrition [17]. PN focuses on biological biomarkers characterising each individual to apply more effective and personalised nutritional guidelines [18]. This enables patient subgroups to obtain customised nutritional instructions rather than general recommendations to improve their treatment outcomes [19][20]. In the long term, PN has been associated with a promising potential to extend patients’ health span and limit the burden of healthcare costs [21].
2. The Phenotypic and Genotypic Components of PN
In the last couple of years, multiple approaches have been designed to focus on nutrition and food science technology [1]. These advanced methodologies are based on understanding the individual variability in response to foods to provide personalised nutritional recommendations specific to patient subgroups [2]. PN is one of the promising methods that have been used for approaching the variation in individuals’ responses to diet, nutrients, metabolic activity, and treatment outcomes [3]. These variations have been linked primarily to the composition of the gut microbiome, genetics/metabolic profile, and social and lifestyle habits specific to each individual [4]. The gut microbiome has been classified as one of the factors that can predict individuals’ responses to diet and develop an appropriate model for PN [5]. Understanding the variation in genetic and metabolic profiles can also help in providing specific dietary advice for individuals or population subgroups in the form of PN [6]. PN can optimise the dietary response and health by considering the variations in individuals’ social status and lifestyle habits [7].
PN allows a better understanding of the inter-individual differences that are directly correlated to patients’ unique intrinsic factors, including the microbiome as well as the genetic and metabolic profile [8][9][10][11]. Other factors including patients’ health status, physical activity, and dietary pattern, and psychosocial and socioeconomic characteristics can also extrinsically affect the response to dietary behaviours [12][13][14][15], as shown in Figure 1 and explained in the following sections.
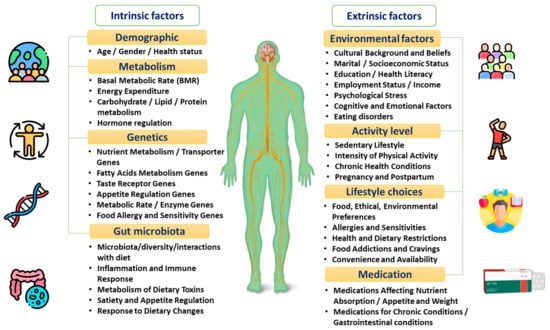
Figure 1. The factors affecting individuals’ dietary responses.
2.1. Gut Microbiota
The gut microbiota refers to the intestinal tract microorganisms responsible for the generation of metabolites, stabilisation of homeostasis, and maintenance of adequate immune responses [16][17]. The intestinal microbiota is also connected with the brain axis to allow the exchange of information across the hypothalamus, pituitary, and adrenal glands. This interconnection is responsible for activating the dual hunger–satiety circuit and the dopamine reward path, producing energy and acquiring food from the environment [18]. The axis between the brain and intestines can be influenced by the host’s genetic composition, level of stress, negative emotions, and diet type [19]. The microbiota can also regulate the pathogenesis, progression, and management of diseases [20][21]. The effectiveness of these functions relies on both the quantity and quality of the microbiota, as well as its metabolic potential [22]. This shows that the characteristics of the gut microbiota can significantly vary across individuals based on their genetic profile, lifestyle, and habits [23]. In practice, the gut microbiota is recognised as a key determinant in predicting how individuals respond to particular dietary components [24]. Consequently, the direct evaluation of host–microbiota interactions constitutes an advanced therapeutic tool during disease control and prevention stages [25]. This highlights the necessity of evaluating individuals’ microbiota to structure precision diets and interventions required for optimal health [26].
In daily life, the type of consumed diet has been shown to influence the microbiome’s composition. For example, diets rich in animal-based nutrients can stimulate the release of bile-resistant species, while plant-based foods are associated with a higher level of plant polysaccharide-fermenting species [27]. A limited 24 h consumption of carbohydrates can also decrease the production of bacteria responsible for destroying food fibres [28]. In obesity and weight gain cases, the microbiota plays a crucial role in monitoring energy use and the formation of gut metabolites [29]. Therefore, individuals eating an unhealthy diet and gaining weight for a prolonged period of more than ten years have limited intestinal microbiota diversity [30]. Other findings relate the disruption in the microbiota’s composition to some inherited and non-modifiable individual characteristics, such as ethnicity and geographical setting [31].
Another factor that can also interfere with the variety and stability of gut microbiota is the consumption of sugar substitutes [32]. It was found that the administration of sucralose for 12 weeks was associated with lower levels of anaerobes, bifidobacteria, lactobacilli, Bacteroides, clostridia, and total aerobic bacteria [33]. Prolonged sucralose consumption in mice caused a high release of bacterial pro-inflammatory genes and disruption in faecal metabolites [34]. In turn, the limited utilisation of emulsifiers as food additives showed a reduced microbial diversity [35]. The consumption of a fermentable oligosaccharides-, disaccharides-, monosaccharides-, and polyols (active b)-rich diet can also alter the gut microbiota [36]. This type of diet has been shown to minimise the risk of insulin resistance in healthy overweight and obese patients [37][38].
Animal and in vitro studies found that gluten-free bread intake lessens the microbiota dysbiosis usually occurring in gluten sensitivity or coeliac disease cases [39][40]. Following a four-week gluten-free diet (GFD), individuals presented with different metabolic profiles and subsequent changes in gut microbiota [41]. In healthy subjects, decreased levels of Bifidobacterium, Clostridium lituseburense, Faecalibacterium prausnitzii, and Lactobacillus, and higher Enterobacteriaceae and Escherichia coli counts following GFD, were reported [42]. There is some emphasis on the potential of a low-gluten diet to induce moderate changes in the intestinal microbiome, reduce fasting and postprandial hydrogen exhalation, and improve self-reported bloating in healthy individuals [43]. Despite its advantages, GFD can be associated with a higher risk of heart disease due to a possible reduction in whole grains’ consumption [44].
This may suggest the impact of dietary habits on gut microbiota and confirm the crucial role of the microbiome as a major contributing factor to PN (Figure 2). Therefore, the variation between individuals and populations can affect the overall response to diet in terms of meals’ digestion, nutritional benefits, and personalisation.
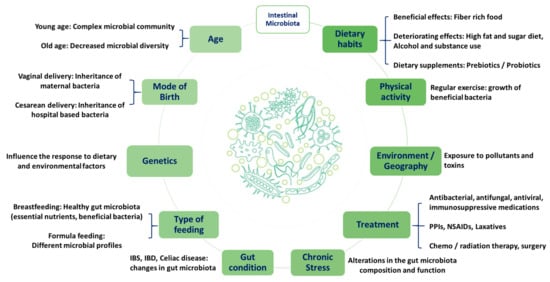
Figure 2. Factors affecting individuals’ intestinal microbiota. Proton pump inhibitors (PPIs), nonsteroidal anti-inflammatory drugs (NSAIDs), irritable bowel syndrome (IBS), and irritable bowel disease (IBD).
2.2. Genetics and Metabolic Profile
The genetic makeup is another important factor that may contribute to the inter-individual variation in dietary behaviours [45]. Some of the recent advances in the field of genomics assisted researchers in better understanding the role of genetic variant sites and functions in the development of chronic conditions. This also contributed to predicting the risk of chronic diseases and personalising their prevention and treatment plans [46]. Accordingly, individuals may present with genetic variations, known as polymorphisms, which can lead to differences in the metabolic processing of nutrients within the same population [47]. For example, the presence of a single-nucleotide polymorphism (SNP) in intron 1 of the cytochrome P450 enzyme CYP1A2 gene was linked to a variation in caffeine metabolism [48][49]. This may account for the high inter-individual variability found in caffeine intrinsic concentrations. In addition, individuals with the CC genotype of a SNP were more likely to gain weight when eating a high-saturated-fat diet (around 10% higher BMI), whereas those with the TT genotype were not associated with this complication [50][51].
The mutual mapping of the individuals’ genetic and metabolic profiles constitutes an important tool for assessing the body’s response to different nutrients and tailoring a personalised diet [52]. For example, a protein- and fibre-rich diet may benefit individuals suffering from low-insulin sensitivity, while a high monounsaturated fatty acids-based diet may benefit patients with insulin resistance [53]. Interestingly, when measuring the metabolic response to high and low glycaemic index meals, it was found that certain subjects had a variance in their glucose and insulin responses to the same standardised index meals [54]. This further strengthens the importance of taking into consideration the inter-individual alterations in metabolic profile while tailoring the diet to produce better health outcomes. In parallel, biological components, including proteins, metabolites, microbiota, and epigenetic markers, helped in understanding the possible association between the individuals’ physiological mechanisms and their susceptibility to chronic diseases [55].
2.3. Psychosocial and Socioeconomic Status
Obesity has been correlated with an increased risk of psychosocial burden [56]. In particular, obese individuals can suffer from mood, self-esteem, and body image-related issues [57]. It was suggested that depressive symptoms are associated with altered eating behaviours and increased food and beverage caloric intake [58]. This is likely due to the food’s ability to activate brain reward circuits that lead to the release of dopamine [59]. Patients with eating disorders also reported difficulties in controlling the frequency of their eating, portion sizes, or extreme eating behaviour [60]. This can be further associated with disordered eating leading to uncontrolled weight gain. In such cases, the psychosocial status of individuals should be considered as a crucial factor when tailoring a specific nutritional plan [61]. The implementation of a long-term weight loss plan should also be considered to allow a gradual improvement in an individual’s psychological distress [62].
Socioeconomic status can also be directly correlated with psychological status [63], as individuals with a high socioeconomic status (SES) seem to follow healthier food habits [64]. Individuals with a low SES are prone to a poorer health status, as they are unable to comply with the required nutritional recommendations or dietary guidelines; in turn, they are more likely to experience unhealthy conditions [59][63]. Recent evidence suggested that a higher percentage of low SES households had unhealthy eating habits, such as consumption of fast foods, while high SES households had healthier eating patterns [65]. This could be due to the lack of purchasing power in those with a low SES, which can lead to the consumption of cheaper and lower-quality ingredients, causing nutritional deficiencies [66]. This confirms the importance of SES consideration when tailoring an individualised diet to improve the nutritive quality. Moreover, both social inequity and diet quality, in conjunction with healthy dietary behaviours, constitute a crucial and active public health concern.
3. PN Use in the Management of Obesity
Obesity is a complex non-communicable disease, which is influenced by both environmental and hereditary factors, representing a relevant target for PN [67]. Recently, studies have shown significant correlations between individuals’ intrinsic components and the extrinsic factors that can directly affect their lifestyle and habits [67]. In this case, PN can help in simultaneously evaluating individuals’ genes, metabolic markers, microbial species, environmental elements (sociodemographic and physical activity), and obesity phenotypic traits (body weight, body mass index, waist circumference, and central and regional adiposity).
3.1. Genetic Basis of Obesity
The progression of obesity has been correlated with multiple genetic factors that can affect the interaction of macronutrients with the individual’s genotype [68][69]. Recently, advanced human genome sequencing and applied population genetics studies have been used as a main tool for detecting the gene variants associated with obesity and its related traits [69]. SNPs have been known as one of the main types of genetic variants that are associated with obesity [70]. For example, insulin-like growth factor, dioxygenase enzyme, melanocortin receptor, and apolipoprotein present SNPs associated with an increased risk of obesity [1][2][16][17][18][71]. In parallel, the conduction of a Genome-Wide Association Study (GWAS) helped in detecting more than 140 obesity-related SNPs [72], while another one revealed about 300 SNPs in total [73]. Despite their discovery, the impact of these SNPs on BMI is still modestly rated, and further investigations are needed for evaluating the influence of genetics on the development of obesity [70][73]. This cannot be relevant to Prader-Willy syndrome cases or genes related to leptin and melanocortin signalling, which have a higher influence on BMI [74].
In some cases, genes are integrated with carbohydrate and lipid metabolism [75], and in others, genes are responsible for activating the proteins of carbohydrates and lipid taste receptors [76]. Some of the genes participate in encoding the lipid transporters of proteins or digestive enzymes present in starch and milk [77], and some other genes are responsible for using and storing energy, food reward, and gut regulatory processes [78][79]. For example, the intake of dietary fats and carbohydrates was found to be associated with the SNP “rs1761667” and “rs35874116” on the cluster of differentiation 36 (CD36) protein and taste 1 receptor member 2 (TAS1R2) gene, respectively [18]. Moreover, “rs1799883” SNP of the fatty acid binding protein 2 (FABP2) gene was found to be associated with hypertriglyceridemia, while “rs9939609” SNP of the fat mass and obesity-associated (FTO) gene was correlated with an increased risk of body fat accumulation [18]. In parallel, the “rs1800497” SNP of the dopamine receptor D2 (DRD2) gene was seen to interlink with the brain–gut microbiota axis and stimulate dysbiosis, negative emotions, and obesity [18]. Despite their unsatisfactory effects, some genetic variations, such as a high AMY1 copy number, were seen to protect the body against obesity [80].
It is worth mentioning that epigenetic changes linked to external factors can change genetic activity and express the obese phenotype [81][82]. These epigenetic modifications were mainly documented following the practice of a dietary plan, physical activity, and surgeries [13]. Therefore, advanced gene-based technologies have been implemented to personalise dietary recommendations based on individuals’ genetic profiles [83]. Nutrigenomics studies reported how individuals’ genetic variations influence their responses to nutrients and how diet, in turn, affects gene expression [84]. Limited data supported the superiority of this technology regarding weight loss results in comparison to standardised care [85][86]. Currently, the increased application of pharmacogenomics to evaluate therapeutic responses to pharmaceutical compounds is associated with a marked clinical relevance [87]. This advanced technique allows the mapping of genetic variants that can influence the response to weight loss treatment [81]. However, pharmacogenomics application is still limited due to financial issues. In recent studies, the discovery of next-generation probiotics has been linked to several health benefits [88]. The clinical use of these probiotics is also limited due to several reasons, such as safety, efficacy, and cost [89][90][91][92]. In some cases, the transplantation of faecal matter has been initialised for the treatment of obesity and other metabolic disorders. Its clinical application remains limited due to the variation in the obtained findings [93].
3.2. Weight Management
The consumption of specific types of food can help prevent the development of obesity [94]. According to epidemiological research, consuming dairy products and vegetarian protein sources can protect against obesity, unlike consuming large amounts of meat, which is correlated with a greater risk of weight gain [95][96][97]. This was supported by a Chinese study, which viewed that consuming large quantities of fatty food can increase the chance of weight gain and obesity [98]. Therefore, poor diet quality was strongly correlated with a greater risk of weight gain despite gender differences [99]. The increased caloric density of high-fat foods also promotes low-satiety effects, especially when consumed in large quantities [100]. This can emphasise the need for a more passive focus on selecting the appropriate intervention for dietary self-monitoring adherence [101].
In practice, the majority of obese women valued the use of weight management services and advice, despite the limited practical discussions and application of these approaches [102]. The use of individualised information while providing nutritional advice helped in sustaining changes in healthy dietary behaviours [103]. A UK-based study showed that applying PN advice through mobile applications was beneficial in improving diet quality and individuals’ engagement in dietary habits, in comparison to the general population [104]. These findings highlighted the potential of PN to improve individuals’ adherence to dietary habits as well as improve weight and glucose management for a long period [105][106][107]. In the long term, the benefits of PN use were associated with a decrease in total fat intake, in addition to compliance with nutritional advice [108].
In daily life, limited physical activity due to a sedentary lifestyle, high screen time, processed meats, physical education, and transportation constitute a direct risk factor for obesity [109]. For example, individuals exercising minimal physical effort have a greater chance of alleviating the risk of type 2 diabetes by 26% compared to unenergetic ones [110]. When applied to physical activity, PN is still inconsistently modifying behavioural changes, such as the ones documented in dietary patterns and diet quality [111][112][113]. The value of personalising dietary advice for modifying physical activity levels was not found to be superior to the conventional guidelines when the physical activity was objectively assessed [114][115]. In some cases, PN was seen to significantly enhance individuals’ exercise frequency when they were informed about their genetic testing results [116].
In summary, the obtained findings can illustrate crucial inputs about PN application in practice and its impact on changing individuals’ diet quality and physical activity, highlighting the need for further research.
3.3. Intestinal Bacterial Flora
The different responses to nutritional recommendations can be regulated by the composition of the gut microbiome for each individual [117][118]. It has been evidenced that the intestinal microbiota is intrinsically affected by the overall health, including obesity risk [119]. Physiologically, the gut microbiota utilises energy from the diet and interacts with the host genes that regulate the expansion and storage of energy [120]. Therefore, live bacteria (probiotics), nondigestible or limited digestible food constituents, such as oligosaccharides (prebiotics), or both (synbiotics), or even faecal transplants have been used as an emerging tool to restore the intestinal microbiota and treat or prevent obesity [121][122][123][124]. This may suggest the key role of the gut microbiota while applying the PN criteria to facilitate weight loss in obese individuals [125]. A human study reported that obese individuals had more Firmicutes and nearly 90% fewer Bacteroidetes than lean individuals [126]. It was observed that a healthy weight and good metabolic health were seen in patients with bacteria of the genus Oscillospira, while Collinsella aerofaciens bacteria were more frequently documented in obese individuals [127]. Other anatomical and physiological changes can also occur in the gut microbiota following the performance of bariatric surgery. Despite its documented benefits regarding weight loss and glycaemic control, a recent study verified the alterations in microbial diversity and composition three months following bariatric surgery [128]. More specifically, a high level of Proteobacteria and Bacteroidetes and a low Firmicutes concentration were reported [129]. In the long term, microbiota species were still maintained, indicating that bariatric surgery could achieve a fast and prolonged modification in the patient’s gut microbiota [129]. The transplantation of faecal microbiota has been reported to reduce body fat accumulation two weeks post-procedure in germ-free mice who had their Roux-en-Y Gastric Bypass (RYGB) or sleeve gastrectomy (SG; 46% and 26%, respectively) [128]. Thus, the inter-individual changes in gut microbiota should always be considered when structuring a nutritional plan, since it can directly influence metabolism, resulting in either weight loss or gain [130]. This was supported by a study validating that individuals’ gut microbiota can be used to design personalised diets for glucose homeostasis [131].
References
- Khoo, C.S.; Knorr, D. Grand Challenges in Nutrition and Food Science Technology. Front. Nutr. 2014, 1, 4.
- Head, R.J.; Buckley, J.D. Human Variation in Response to Food and Nutrients. Nutr. Rev. 2020, 78, 49–52.
- Stover, P.J.; King, J.C. More Nutrition Precision, Better Decisions for the Health of Our Nation. J. Nutr. 2020, 150, 3058–3060.
- Chen, L.; Zhernakova, D.V.; Kurilshikov, A.; Andreu-Sánchez, S.; Wang, D.; Augustijn, H.E.; Vich Vila, A.; Weersma, R.K.; Medema, M.H.; Netea, M.G.; et al. Influence of the Microbiome, Diet and Genetics on Inter-Individual Variation in the Human Plasma Metabolome. Nat. Med. 2022, 28, 2333–2343.
- Hughes, R.L.; Kable, M.E.; Marco, M.; Keim, N.L. The Role of the Gut Microbiome in Predicting Response to Diet and the Development of Precision Nutrition Models. Part II: Results. Adv. Nutr. 2019, 10, 979–998.
- Simopoulos, A.P.; Serhan, C.N.; Bazinet, R.P. The Need for Precision Nutrition, Genetic Variation and Resolution in COVID-19 Patients. Mol. Aspects Med. 2021, 77, 100943.
- Antwi, J. Precision Nutrition to Improve Risk Factors of Obesity and Type 2 Diabetes. Curr. Nutr. Rep. 2023, 12, 679–694.
- Ordovas, J.M.; Corella, D. Nutritional Genomics. Annu. Rev. Genom. Hum. Genet. 2004, 5, 71–118.
- Ordovas, J.M. Genotype-Phenotype Associations: Modulation by Diet and Obesity. Obesity 2008, 16, S40–S46.
- Celis-Morales, C.; Marsaux, C.F.; Livingstone, K.M.; Navas-Carretero, S.; San-Cristobal, R.; Fallaize, R.; Macready, A.L.; O’Donovan, C.; Woolhead, C.; Forster, H.; et al. Can Genetic-Based Advice Help You Lose Weight? Findings from the Food4Me European Randomized Controlled Trial. Am. J. Clin. Nutr. 2017, 105, 1204–1213.
- O’Donovan, C.B.; Walsh, M.C.; Gibney, M.J.; Brennan, L.; Gibney, E.R. Knowing Your Genes: Does This Impact Behaviour Change? Proc. Nutr. Soc. 2017, 76, 182–191.
- Gibney, E.R. Personalised Nutrition—Phenotypic and Genetic Variation in Response to Dietary Intervention. Proc. Nutr. Soc. 2020, 79, 236–245.
- Kirwan, L.; Walsh, M.C.; Celis-Morales, C.; Marsaux, C.F.M.; Livingstone, K.M.; Navas-Carretero, S.; Fallaize, R.; O’Donovan, C.B.; Woolhead, C.; Forster, H.; et al. Phenotypic Factors Influencing the Variation in Response of Circulating Cholesterol Level to Personalised Dietary Advice in the Food4Me Study. Br. J. Nutr. 2016, 116, 2011–2019.
- Shyam, S.; Lee, K.X.; Tan, A.S.W.; Khoo, T.A.; Harikrishnan, S.; Lalani, S.A.; Ramadas, A. Effect of Personalized Nutrition on Dietary, Physical Activity, and Health Outcomes: A Systematic Review of Randomized Trials. Nutrients 2022, 14, 4104.
- Rankin, A.; Bunting, B.P.; Poínhos, R.; van der Lans, I.A.; Fischer, A.R.; Kuznesof, S.; Almeida, M.; Markovina, J.; Frewer, L.J.; Stewart-Knox, B.J. Food Choice Motives, Attitude towards and Intention to Adopt Personalised Nutrition. Public Health Nutr. 2018, 21, 2606–2616.
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut Microbiota Functions: Metabolism of Nutrients and Other Food Components. Eur. J. Nutr. 2018, 57, 1–24.
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Reddy, D.N. Role of the Normal Gut Microbiota. World J. Gastroenterol. 2015, 21, 8787–8803.
- Panduro, A.; Roman, S.; Garcia Milan, R.; Torres-Reyes, L.; Aldaco, K. Chapter 10. Personalized Nutrition to Treat and Prevent Obesity and Diabetes. In Nutritional Signaling Pathway Activities in Obesity and Diabetes; ResearchGate GmbH: Berlin, Germany, 2020; pp. 272–294. ISBN 978-1-78801-557-8.
- Panduro, A.; Rivera-Iñiguez, I.; Sepulveda-Villegas, M.; Roman, S. Genes, Emotions and Gut Microbiota: The next Frontier for the Gastroenterologist. World J. Gastroenterol. 2017, 23, 3030–3042.
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.-M.; Kennedy, S.; et al. Richness of Human Gut Microbiome Correlates with Metabolic Markers. Nature 2013, 500, 541–546.
- Cho, I.; Blaser, M.J. The Human Microbiome: At the Interface of Health and Disease. Nat. Rev. Genet. 2012, 13, 260–270.
- Hughes, R.L.; Holscher, H.D. Fueling Gut Microbes: A Review of the Interaction between Diet, Exercise, and the Gut Microbiota in Athletes. Adv. Nutr. 2021, 12, 2190–2215.
- Mills, S.; Stanton, C.; Lane, J.A.; Smith, G.J.; Ross, R.P. Precision Nutrition and the Microbiome, Part I: Current State of the Science. Nutrients 2019, 11, 923.
- Bashiardes, S.; Godneva, A.; Elinav, E.; Segal, E. Towards Utilization of the Human Genome and Microbiome for Personalized Nutrition. Curr. Opin. Biotechnol. 2018, 51, 57–63.
- Kolodziejczyk, A.A.; Zheng, D.; Elinav, E. Diet-Microbiota Interactions and Personalized Nutrition. Nat. Rev. Microbiol. 2019, 17, 742–753.
- Klimenko, N.S.; Odintsova, V.E.; Revel-Muroz, A.; Tyakht, A.V. The Hallmarks of Dietary Intervention-Resilient Gut Microbiome. npj Biofilms Microbiomes 2022, 8, 77.
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.I.; Godneva, A.; Kalka, I.N.; Bar, N.; et al. Environment Dominates over Host Genetics in Shaping Human Gut Microbiota. Nature 2018, 555, 210–215.
- Mardinoglu, A.; Wu, H.; Bjornson, E.; Zhang, C.; Hakkarainen, A.; Räsänen, S.M.; Lee, S.; Mancina, R.M.; Bergentall, M.; Pietiläinen, K.H.; et al. An Integrated Understanding of the Rapid Metabolic Benefits of a Carbohydrate-Restricted Diet on Hepatic Steatosis in Humans. Cell Metab. 2018, 27, 559–571.e5.
- Lazar, V.; Ditu, L.-M.; Pircalabioru, G.G.; Picu, A.; Petcu, L.; Cucu, N.; Chifiriuc, M.C. Gut Microbiota, Host Organism, and Diet Trialogue in Diabetes and Obesity. Front. Nutr. 2019, 6, 21.
- Menni, C.; Jackson, M.A.; Pallister, T.; Steves, C.J.; Spector, T.D.; Valdes, A.M. Gut Microbiome Diversity and High-Fibre Intake Are Related to Lower Long-Term Weight Gain. Int. J. Obes. 2017, 41, 1099–1105.
- Deschasaux, M.; Bouter, K.E.; Prodan, A.; Levin, E.; Groen, A.K.; Herrema, H.; Tremaroli, V.; Bakker, G.J.; Attaye, I.; Pinto-Sietsma, S.-J.; et al. Depicting the Composition of Gut Microbiota in a Population with Varied Ethnic Origins but Shared Geography. Nat. Med. 2018, 24, 1526–1531.
- Nettleton, J.E.; Reimer, R.A.; Shearer, J. Reshaping the Gut Microbiota: Impact of Low Calorie Sweeteners and the Link to Insulin Resistance? Physiol. Behav. 2016, 164, 488–493.
- Abou-Donia, M.B.; El-Masry, E.M.; Abdel-Rahman, A.A.; McLendon, R.E.; Schiffman, S.S. Splenda Alters Gut Microflora and Increases Intestinal P-Glycoprotein and Cytochrome p-450 in Male Rats. J. Toxicol. Environ. Health A 2008, 71, 1415–1429.
- Bian, X.; Chi, L.; Gao, B.; Tu, P.; Ru, H.; Lu, K. Gut Microbiome Response to Sucralose and Its Potential Role in Inducing Liver Inflammation in Mice. Front. Physiol. 2017, 8, 487.
- Chassaing, B.; Koren, O.; Goodrich, J.K.; Poole, A.C.; Srinivasan, S.; Ley, R.E.; Gewirtz, A.T. Dietary Emulsifiers Impact the Mouse Gut Microbiota Promoting Colitis and Metabolic Syndrome. Nature 2015, 519, 92–96.
- Hemami, R.M.; Shakarami, A.; Ardekani, A.M.; Aghaii, S.; Makarem, D.; Nikrad, N.; Farhangi, M.A.; Pour Abbasi, M.S. Investigation of the Association between Habitual Dietary FODMAP Intake, Metabolic Parameters, Glycemic Status, and Anthropometric Features among Apparently Healthy Overweight and Obese Individuals. BMC Endocr. Disord. 2023, 23, 206.
- Halmos, E.P. When the Low FODMAP Diet Does Not Work. J. Gastroenterol. Hepatol. 2017, 32 (Suppl. S1), 69–72.
- Gibson, P.R. The Evidence Base for Efficacy of the Low FODMAP Diet in Irritable Bowel Syndrome: Is It Ready for Prime Time as a First-Line Therapy? J. Gastroenterol. Hepatol. 2017, 32 (Suppl. S1), 32–35.
- Mohan, M.; Chow, C.-E.T.; Ryan, C.N.; Chan, L.S.; Dufour, J.; Aye, P.P.; Blanchard, J.; Moehs, C.P.; Sestak, K. Dietary Gluten-Induced Gut Dysbiosis Is Accompanied by Selective Upregulation of microRNAs with Intestinal Tight Junction and Bacteria-Binding Motifs in Rhesus Macaque Model of Celiac Disease. Nutrients 2016, 8, 684.
- Bevilacqua, A.; Costabile, A.; Bergillos-Meca, T.; Gonzalez, I.; Landriscina, L.; Ciuffreda, E.; D’Agnello, P.; Corbo, M.R.; Sinigaglia, M.; Lamacchia, C. Impact of Gluten-Friendly Bread on the Metabolism and Function of In Vitro Gut Microbiota in Healthy Human and Coeliac Subjects. PLoS ONE 2016, 11, e0162770.
- Bonder, M.J.; Tigchelaar, E.F.; Cai, X.; Trynka, G.; Cenit, M.C.; Hrdlickova, B.; Zhong, H.; Vatanen, T.; Gevers, D.; Wijmenga, C.; et al. The Influence of a Short-Term Gluten-Free Diet on the Human Gut Microbiome. Genome Med. 2016, 8, 45.
- De Palma, G.; Nadal, I.; Collado, M.C.; Sanz, Y. Effects of a Gluten-Free Diet on Gut Microbiota and Immune Function in Healthy Adult Human Subjects. Br. J. Nutr. 2009, 102, 1154–1160.
- Hansen, L.B.S.; Roager, H.M.; Søndertoft, N.B.; Gøbel, R.J.; Kristensen, M.; Vallès-Colomer, M.; Vieira-Silva, S.; Ibrügger, S.; Lind, M.V.; Mærkedahl, R.B.; et al. A Low-Gluten Diet Induces Changes in the Intestinal Microbiome of Healthy Danish Adults. Nat. Commun. 2018, 9, 4630.
- Lebwohl, B.; Cao, Y.; Zong, G.; Hu, F.B.; Green, P.H.R.; Neugut, A.I.; Rimm, E.B.; Sampson, L.; Dougherty, L.W.; Giovannucci, E.; et al. Long Term Gluten Consumption in Adults without Celiac Disease and Risk of Coronary Heart Disease: Prospective Cohort Study. BMJ 2017, 357, j1892.
- Grimm, E.R.; Steinle, N.I. Genetics of Eating Behavior: Established and Emerging Concepts. Nutr. Rev. 2011, 69, 52–60.
- Ramos-Lopez, O.; Milagro, F.I.; Allayee, H.; Chmurzynska, A.; Choi, M.S.; Curi, R.; De Caterina, R.; Ferguson, L.R.; Goni, L.; Kang, J.X.; et al. Guide for Current Nutrigenetic, Nutrigenomic, and Nutriepigenetic Approaches for Precision Nutrition Involving the Prevention and Management of Chronic Diseases Associated with Obesity. Lifestyle Genom. 2017, 10, 43–62.
- Paoloni-Giacobino, A.; Grimble, R.; Pichard, C. Genetics and Nutrition. Clin. Nutr. 2003, 22, 429–435.
- Fulton, J.L.; Dinas, P.C.; Carrillo, A.E.; Edsall, J.R.; Ryan, E.J.; Ryan, E.J. Impact of Genetic Variability on Physiological Responses to Caffeine in Humans: A Systematic Review. Nutrients 2018, 10, 1373.
- Sachse, C.; Brockmöller, J.; Bauer, S.; Roots, I. Functional Significance of a C→A Polymorphism in Intron 1 of the Cytochrome P450 CYP1A2 Gene Tested with Caffeine. Br. J. Clin. Pharmacol. 1999, 47, 445–449.
- Corella, D.; Peloso, G.; Arnett, D.K.; Demissie, S.; Cupples, L.A.; Tucker, K.; Lai, C.-Q.; Parnell, L.D.; Coltell, O.; Lee, Y.-C.; et al. APOA2, Dietary Fat, and Body Mass Index: Replication of a Gene-Diet Interaction in 3 Independent Populations. Arch. Intern. Med. 2009, 169, 1897–1906.
- Zeisel, S.H. A Conceptual Framework for Studying and Investing in Precision Nutrition. Front. Genet. 2019, 10, 200.
- LeMieux, M.; Al-Jawadi, A.; Wang, S.; Moustaid-Moussa, N. Metabolic Profiling in Nutrition and Metabolic Disorders. Adv. Nutr. 2013, 4, 548–550.
- Trouwborst, I.; Gijbels, A.; Jardon, K.M.; Siebelink, E.; Hul, G.B.; Wanders, L.; Erdos, B.; Péter, S.; Singh-Povel, C.M.; de Vogel-van den Bosch, J.; et al. Cardiometabolic Health Improvements upon Dietary Intervention Are Driven by Tissue-Specific Insulin Resistance Phenotype: A Precision Nutrition Trial. Cell Metab. 2023, 35, 71–83.e5.
- Krishnan, S.; Newman, J.W.; Hembrooke, T.A.; Keim, N.L. Variation in Metabolic Responses to Meal Challenges Differing in Glycemic Index in Healthy Women: Is It Meaningful? Nutr. Metab. 2012, 9, 26.
- Ferguson, L.R.; De Caterina, R.; Görman, U.; Allayee, H.; Kohlmeier, M.; Prasad, C.; Choi, M.S.; Curi, R.; De Luis, D.A.; Gil, Á.; et al. Guide and Position of the International Society of Nutrigenetics/Nutrigenomics on Personalised Nutrition: Part 1—Fields of Precision Nutrition. J. Nutr. Nutr. 2016, 9, 12–27.
- Sarwer, D.B.; Polonsky, H.M. The Psychosocial Burden of Obesity. Endocrinol. Metab. Clin. N. Am. 2016, 45, 677–688.
- Rizzo, A.; Sitibondo, A. Obesity and Life History: The Hypothesis of Psychological Phenotypes. Psych 2023, 5, 866–875.
- Grossniklaus, D.A.; Dunbar, S.B.; Tohill, B.C.; Gary, R.; Higgins, M.K.; Frediani, J. Psychological Factors Are Important Correlates of Dietary Pattern in Overweight Adults. J. Cardiovasc. Nurs. 2010, 25, 450–460.
- Butler, M.J.; Eckel, L.A. Eating as a Motivated Behavior: Modulatory Effect of High Fat Diets on Energy Homeostasis, Reward Processing, and Neuroinflammation. Integr. Zool. 2018, 13, 673–686.
- Vahia, V.N. Diagnostic and Statistical Manual of Mental Disorders 5: A Quick Glance. Indian J. Psychiatry 2013, 55, 220–223.
- Srp, F.; Steiger, E.; Gulledge, A.D.; Matarese, L.E.; Paysinger, J.; Roncagli, T.; Stebbins, J.; Sullivan, M. Psychosocial Issues of Nutritional Support. A Multidisciplinary Interpretation. Nurs. Clin. N. Am. 1989, 24, 447–459.
- Steptoe, A.; Frank, P. Obesity and Psychological Distress. Philos. Trans. R. Soc. B Biol. Sci. 2023, 378, 20220225.
- Reiss, F.; Meyrose, A.-K.; Otto, C.; Lampert, T.; Klasen, F.; Ravens-Sieberer, U. Socioeconomic Status, Stressful Life Situations and Mental Health Problems in Children and Adolescents: Results of the German BELLA Cohort-Study. PLoS ONE 2019, 14, e0213700.
- James, W.P.; Nelson, M.; Ralph, A.; Leather, S. Socioeconomic Determinants of Health. The Contribution of Nutrition to Inequalities in Health. BMJ Br. Med. J. 1997, 314, 1545.
- Foroozanfar, Z.; Moghadami, M.; Mohsenpour, M.A.; Houshiarrad, A.; Farmani, A.; Akbarpoor, M.A.; Shenavar, R. Socioeconomic Determinants of Nutritional Behaviors of Households in Fars Province, Iran, 2018. Front. Nutr. 2022, 9, 956293.
- French, S.A.; Tangney, C.C.; Crane, M.M.; Wang, Y.; Appelhans, B.M. Nutrition Quality of Food Purchases Varies by Household Income: The SHoPPER Study. BMC Public Health 2019, 19, 231.
- Voruganti, V.S. Precision Nutrition: Recent Advances in Obesity. Physiology 2023, 38, 42–50.
- San-Cristobal, R.; Navas-Carretero, S.; Martínez-González, M.Á.; Ordovas, J.M.; Martínez, J.A. Contribution of Macronutrients to Obesity: Implications for Precision Nutrition. Nat. Rev. Endocrinol. 2020, 16, 305–320.
- Goni, L.; Cuervo, M.; Milagro, F.I.; Martínez, J.A. Future Perspectives of Personalized Weight Loss Interventions Based on Nutrigenetic, Epigenetic, and Metagenomic Data. J. Nutr. 2015, 146, 905S–912S.
- Wassel, C.L.; Pankow, J.S.; Rasmussen-Torvik, L.J.; Li, N.; Taylor, K.D.; Guo, X.; Goodarzi, M.O.; Palmas, W.R.; Post, W.S. Associations of SNPs in ADIPOQ and Subclinical Cardiovascular Disease in the Multi-Ethnic Study of Atherosclerosis (MESA). Obesity 2011, 19, 840–847.
- Cuevas-Sierra, A.; Ramos-Lopez, O.; Riezu-Boj, J.I.; Milagro, F.I.; Martinez, J.A. Diet, Gut Microbiota, and Obesity: Links with Host Genetics and Epigenetics and Potential Applications. Adv. Nutr. 2019, 10, S17–S30.
- Fall, T.; Mendelson, M.; Speliotes, E.K. Recent Advances in Human Genetics and Epigenetics of Adiposity: Pathway to Precision Medicine? Gastroenterology 2017, 152, 1695–1706.
- Yang, J.; Zhang, Y. Protein Structure and Function Prediction Using I-TASSER. Curr. Protoc. Bioinform. 2015, 52, 5.8.1–5.8.15.
- Locke, A.E.; Kahali, B.; Berndt, S.I.; Justice, A.E.; Pers, T.H.; Day, F.R.; Powell, C.; Vedantam, S.; Buchkovich, M.L.; Yang, J.; et al. Genetic Studies of Body Mass Index Yield New Insights for Obesity Biology. Nature 2015, 518, 197–206.
- Haro, D.; Marrero, P.F.; Relat, J. Nutritional Regulation of Gene Expression: Carbohydrate-, Fat- and Amino Acid-Dependent Modulation of Transcriptional Activity. Int. J. Mol. Sci. 2019, 20, 1386.
- Bravo-Ruiz, I.; Medina, M.Á.; Martínez-Poveda, B. From Food to Genes: Transcriptional Regulation of Metabolism by Lipids and Carbohydrates. Nutrients 2021, 13, 1513.
- Yang, C.; Liu, J.; Wu, X.; Bao, P.; Long, R.; Guo, X.; Ding, X.; Yan, P. The Response of Gene Expression Associated with Lipid Metabolism, Fat Deposition and Fatty Acid Profile in the Longissimus Dorsi Muscle of Gannan Yaks to Different Energy Levels of Diets. PLoS ONE 2017, 12, e0187604.
- Lenard, N.R.; Berthoud, H.-R. Central and Peripheral Regulation of Food Intake and Physical Activity: Pathways and Genes. Obesity 2008, 16, S11–S22.
- de Wouters d’Oplinter, A.; Huwart, S.J.P.; Cani, P.D.; Everard, A. Gut Microbes and Food Reward: From the Gut to the Brain. Front. Neurosci. 2022, 16, 947240.
- Venkatapoorna, C.M.K.; Ayine, P.; Parra, E.P.; Koenigs, T.; Phillips, M.; Babu, J.R.; Sandey, M.; Geetha, T. Association of Salivary Amylase (AMY1) Gene Copy Number with Obesity in Alabama Elementary School Children. Nutrients 2019, 11, 1379.
- Acosta, A.; Camilleri, M.; Abu Dayyeh, B.; Calderon, G.; Gonzalez, D.; McRae, A.; Rossini, W.; Singh, S.; Burton, D.; Clark, M.M. Selection of Antiobesity Medications Based on Phenotypes Enhances Weight Loss: A Pragmatic Trial in an Obesity Clinic. Obesity 2021, 29, 662–671.
- Cordero, P.; Li, J.; Oben, J.A. Epigenetics of Obesity: Beyond the Genome Sequence. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 361.
- Rudkowska, I. Genomics and Personalized Nutrition. Nutrients 2021, 13, 1128.
- Jenzer, H.; Sadeghi-Reeves, L. Nutrigenomics-Associated Impacts of Nutrients on Genes and Enzymes With Special Consideration of Aromatase. Front. Nutr. 2020, 7, 37.
- Arkadianos, I.; Valdes, A.M.; Marinos, E.; Florou, A.; Gill, R.D.; Grimaldi, K.A. Improved Weight Management Using Genetic Information to Personalize a Calorie Controlled Diet. Nutr. J. 2007, 6, 29.
- Gardner, C.D.; Trepanowski, J.F.; Del Gobbo, L.C.; Hauser, M.E.; Rigdon, J.; Ioannidis, J.P.A.; Desai, M.; King, A.C. Effect of Low-Fat vs Low-Carbohydrate Diet on 12-Month Weight Loss in Overweight Adults and the Association With Genotype Pattern or Insulin Secretion: The DIETFITS Randomized Clinical Trial. JAMA 2018, 319, 667–679.
- Roden, D.M.; McLeod, H.L.; Relling, M.V.; Williams, M.S.; Mensah, G.A.; Peterson, J.F.; Van Driest, S.L. Pharmacogenomics. Lancet 2019, 394, 521–532.
- O’Toole, P.W.; Marchesi, J.R.; Hill, C. Next-Generation Probiotics: The Spectrum from Probiotics to Live Biotherapeutics. Nat. Microbiol. 2017, 2, 1–6.
- Brusaferro, A.; Cozzali, R.; Orabona, C.; Biscarini, A.; Farinelli, E.; Cavalli, E.; Grohmann, U.; Principi, N.; Esposito, S. Is It Time to Use Probiotics to Prevent or Treat Obesity? Nutrients 2018, 10, 1613.
- Cunningham, M.; Azcarate-Peril, M.A.; Barnard, A.; Benoit, V.; Grimaldi, R.; Guyonnet, D.; Holscher, H.D.; Hunter, K.; Manurung, S.; Obis, D.; et al. Shaping the Future of Probiotics and Prebiotics. Trends Microbiol. 2021, 29, 667–685.
- Nicolucci, A.C.; Hume, M.P.; Martínez, I.; Mayengbam, S.; Walter, J.; Reimer, R.A. Prebiotics Reduce Body Fat and Alter Intestinal Microbiota in Children Who Are Overweight or With Obesity. Gastroenterology 2017, 153, 711–722.
- Sivamaruthi, B.S.; Kesika, P.; Suganthy, N.; Chaiyasut, C. A Review on Role of Microbiome in Obesity and Antiobesity Properties of Probiotic Supplements. BioMed Res. Int. 2019, 2019, e3291367.
- Hartstra, A.V.; Bouter, K.E.C.; Bäckhed, F.; Nieuwdorp, M. Insights into the Role of the Microbiome in Obesity and Type 2 Diabetes. Diabetes Care 2015, 38, 159–165.
- Dominguez, L.J.; Veronese, N.; Di Bella, G.; Cusumano, C.; Parisi, A.; Tagliaferri, F.; Ciriminna, S.; Barbagallo, M. Mediterranean Diet in the Management and Prevention of Obesity. Exp. Gerontol. 2023, 174, 112121.
- Fogelholm, M.; Anderssen, S.; Gunnarsdottir, I.; Lahti-Koski, M. Dietary Macronutrients and Food Consumption as Determinants of Long-Term Weight Change in Adult Populations: A Systematic Literature Review. Food Nutr. Res. 2012, 56, 19103.
- Smith, J.D.; Hou, T.; Ludwig, D.S.; Rimm, E.B.; Willett, W.; Hu, F.B.; Mozaffarian, D. Changes in Intake of Protein Foods, Carbohydrate Amount and Quality, and Long-Term Weight Change: Results from 3 Prospective Cohorts1234. Am. J. Clin. Nutr. 2015, 101, 1216–1224.
- Mozaffarian, D. Dietary and Policy Priorities for Cardiovascular Disease, Diabetes, and Obesity—A Comprehensive Review. Circulation 2016, 133, 187–225.
- Wang, L.; Wang, H.; Zhang, B.; Popkin, B.M.; Du, S. Elevated Fat Intake Increases Body Weight and the Risk of Overweight and Obesity among Chinese Adults: 1991–2015 Trends. Nutrients 2020, 12, 3272.
- Collins, C.E.; Young, A.F.; Hodge, A. Diet Quality Is Associated with Higher Nutrient Intake and Self-Rated Health in Mid-Aged Women. J. Am. Coll. Nutr. 2008, 27, 146–157.
- Rolls, E.T. Taste, Olfactory and Food Texture Reward Processing in the Brain and the Control of Appetite. Proc. Nutr. Soc. 2012, 71, 488–501.
- Popp, C.J.; Hu, L.; Kharmats, A.Y.; Curran, M.; Berube, L.; Wang, C.; Pompeii, M.L.; Illiano, P.; St-Jules, D.E.; Mottern, M.; et al. Effect of a Personalized Diet to Reduce Postprandial Glycemic Response vs a Low-Fat Diet on Weight Loss in Adults With Abnormal Glucose Metabolism and Obesity. JAMA Netw. Open 2022, 5, e2233760.
- Macleod, M.; Gregor, A.; Barnett, C.; Magee, E.; Thompson, J.; Anderson, A.S. Provision of Weight Management Advice for Obese Women during Pregnancy: A Survey of Current Practice and Midwives’ Views on Future Approaches. Matern. Child. Nutr. 2012, 9, 467–472.
- Celis-Morales, C.; Livingstone, K.M.; Marsaux, C.F.; Macready, A.L.; Fallaize, R.; O’Donovan, C.B.; Woolhead, C.; Forster, H.; Walsh, M.C.; Navas-Carretero, S.; et al. Effect of Personalized Nutrition on Health-Related Behaviour Change: Evidence from the Food4Me European Randomized Controlled Trial. Int. J. Epidemiol. 2017, 46, 578–588.
- Zenun Franco, R.; Fallaize, R.; Weech, M.; Hwang, F.; Lovegrove, J.A. Effectiveness of Web-Based Personalized Nutrition Advice for Adults Using the eNutri Web App: Evidence From the EatWellUK Randomized Controlled Trial. J. Med. Internet Res. 2022, 24, e29088.
- Nielsen, D.E.; Shih, S.; El-Sohemy, A. Perceptions of Genetic Testing for Personalized Nutrition: A Randomized Trial of DNA-Based Dietary Advice. J. Nutr. Nutr. 2014, 7, 94–104.
- Horne, J.; Madill, J.; Gilliland, J. Incorporating the “Theory of Planned Behavior” into Personalized Healthcare Behavior Change Research: A Call to Action. Pers. Med. 2017, 14, 521–529.
- Drabsch, T.; Holzapfel, C. A Scientific Perspective of Personalised Gene-Based Dietary Recommendations for Weight Management. Nutrients 2019, 11, 617.
- Horne, J.; Gilliland, J.; O’Connor, C.; Seabrook, J.; Madill, J. Enhanced Long-Term Dietary Change and Adherence in a Nutrigenomics-Guided Lifestyle Intervention Compared to a Population-Based (GLB/DPP) Lifestyle Intervention for Weight Management: Results from the NOW Randomised Controlled Trial. BMJ Nutr. Prev. Health 2020, 3, 49–59.
- Popkin, B.M.; Adair, L.S.; Ng, S.W. Global Nutrition Transition and the Pandemic of Obesity in Developing Countries. Nutr. Rev. 2012, 70, 3–21.
- Smith, A.D.; Crippa, A.; Woodcock, J.; Brage, S. Physical Activity and Incident Type 2 Diabetes Mellitus: A Systematic Review and Dose–Response Meta-Analysis of Prospective Cohort Studies. Diabetologia 2016, 59, 2527–2545.
- Adams, M.A.; Hurley, J.C.; Todd, M.; Bhuiyan, N.; Jarrett, C.L.; Tucker, W.J.; Hollingshead, K.E.; Angadi, S.S. Adaptive Goal Setting and Financial Incentives: A 2 × 2 Factorial Randomized Controlled Trial to Increase Adults’ Physical Activity. BMC Public Health 2017, 17, 286.
- Jakicic, J.M.; Davis, K.K.; Rogers, R.J.; King, W.C.; Marcus, M.D.; Helsel, D.; Rickman, A.D.; Wahed, A.S.; Belle, S.H. Effect of Wearable Technology Combined with a Lifestyle Intervention on Long-Term Weight Loss: The IDEA Randomized Clinical Trial. JAMA 2016, 316, 1161–1171.
- Joseph, R.P.; Keller, C.; Adams, M.A.; Ainsworth, B.E. Print versus a Culturally-Relevant Facebook and Text Message Delivered Intervention to Promote Physical Activity in African American Women: A Randomized Pilot Trial. BMC Womens Health 2015, 15, 30.
- Marsaux, C.F.; Celis-Morales, C.; Fallaize, R.; Macready, A.L.; Kolossa, S.; Woolhead, C.; O’Donovan, C.B.; Forster, H.; Navas-Carretero, S.; San-Cristobal, R.; et al. Effects of a Web-Based Personalized Intervention on Physical Activity in European Adults: A Randomized Controlled Trial. J. Med. Internet Res. 2015, 17, e231.
- Godino, J.G.; van Sluijs, E.M.F.; Marteau, T.M.; Sutton, S.; Sharp, S.J.; Griffin, S.J. Lifestyle Advice Combined with Personalized Estimates of Genetic or Phenotypic Risk of Type 2 Diabetes, and Objectively Measured Physical Activity: A Randomized Controlled Trial. PLoS Med. 2016, 13, e1002185.
- Nielsen, D.E.; Carere, D.A.; Wang, C.; Roberts, J.S.; Green, R.C.; Green, R.C.; Krier, J.B.; Kalia, S.S.; Christensen, K.D.; Nielsen, D.E.; et al. Diet and Exercise Changes Following Direct-to-Consumer Personal Genomic Testing. BMC Med. Genom. 2017, 10, 24.
- Anhê, F.F.; Varin, T.V.; Schertzer, J.D.; Marette, A. The Gut Microbiota as a Mediator of Metabolic Benefits after Bariatric Surgery. Can. J. Diabetes 2017, 41, 439–447.
- Qin, Q.; Yan, S.; Yang, Y.; Chen, J.; Li, T.; Gao, X.; Yan, H.; Wang, Y.; Wang, J.; Wang, S.; et al. A Metagenome-Wide Association Study of the Gut Microbiome and Metabolic Syndrome. Front. Microbiol. 2021, 12, 682721.
- Davis, C.D. The Gut Microbiome and Its Role in Obesity. Nutr. Today 2016, 51, 167–174.
- Fan, Y.; Pedersen, O. Gut Microbiota in Human Metabolic Health and Disease. Nat. Rev. Microbiol. 2021, 19, 55–71.
- Wiciński, M.; Gębalski, J.; Gołębiewski, J.; Malinowski, B. Probiotics for the Treatment of Overweight and Obesity in Humans—A Review of Clinical Trials. Microorganisms 2020, 8, 1148.
- Shirvani-Rad, S.; Tabatabaei-Malazy, O.; Mohseni, S.; Hasani-Ranjbar, S.; Soroush, A.-R.; Hoseini-Tavassol, Z.; Ejtahed, H.-S.; Larijani, B. Probiotics as a Complementary Therapy for Management of Obesity: A Systematic Review. Evid. Based Complement. Alternat. Med. 2021, 2021, 6688450.
- Hijová, E. Synbiotic Supplements in the Prevention of Obesity and Obesity-Related Diseases. Metabolites 2022, 12, 313.
- Zhang, Z.; Mocanu, V.; Cai, C.; Dang, J.; Slater, L.; Deehan, E.C.; Walter, J.; Madsen, K.L. Impact of Fecal Microbiota Transplantation on Obesity and Metabolic Syndrome—A Systematic Review. Nutrients 2019, 11, 2291.
- Puljiz, Z.; Kumric, M.; Vrdoljak, J.; Martinovic, D.; Ticinovic Kurir, T.; Krnic, M.O.; Urlic, H.; Puljiz, Z.; Zucko, J.; Dumanic, P.; et al. Obesity, Gut Microbiota, and Metabolome: From Pathophysiology to Nutritional Interventions. Nutrients 2023, 15, 2236.
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity Alters Gut Microbial Ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075.
- Jardon, K.M.; Canfora, E.E.; Goossens, G.H.; Blaak, E.E. Dietary Macronutrients and the Gut Microbiome: A Precision Nutrition Approach to Improve Cardiometabolic Health. Gut 2022, 71, 1214–1226.
- Tremaroli, V.; Karlsson, F.; Werling, M.; Ståhlman, M.; Kovatcheva-Datchary, P.; Olbers, T.; Fändriks, L.; le Roux, C.W.; Nielsen, J.; Bäckhed, F. Roux-En-Y Gastric Bypass and Vertical Banded Gastroplasty Induce Long-Term Changes on the Human Gut Microbiome Contributing to Fat Mass Regulation. Cell Metab. 2015, 22, 228–238.
- Zhang, H.; DiBaise, J.K.; Zuccolo, A.; Kudrna, D.; Braidotti, M.; Yu, Y.; Parameswaran, P.; Crowell, M.D.; Wing, R.; Rittmann, B.E.; et al. Human Gut Microbiota in Obesity and after Gastric Bypass. Proc. Natl. Acad. Sci. USA 2009, 106, 2365–2370.
- Oliphant, K.; Allen-Vercoe, E. Macronutrient Metabolism by the Human Gut Microbiome: Major Fermentation by-Products and Their Impact on Host Health. Microbiome 2019, 7, 91.
- Zeevi, D.; Korem, T.; Zmora, N.; Israeli, D.; Rothschild, D.; Weinberger, A.; Ben-Yacov, O.; Lador, D.; Avnit-Sagi, T.; Lotan-Pompan, M.; et al. Personalized Nutrition by Prediction of Glycemic Responses. Cell 2015, 163, 1079–1094.
More
Information
Subjects:
Nutrition & Dietetics
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
515
Revisions:
2 times
(View History)
Update Date:
28 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




