
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marta González-García | -- | 3020 | 2024-02-27 10:46:46 | | | |
| 2 | Peter Tang | + 2 word(s) | 3022 | 2024-02-28 02:31:53 | | |
Video Upload Options
In humans, speech is a complex process that requires the coordinated involvement of various components of the phonatory system, which are monitored by the central nervous system. The larynx in particular plays a crucial role, as it enables the vocal folds to meet and converts the exhaled air from our lungs into audible sounds. Voice production requires precise and sustained exhalation, which generates an air pressure/flow that creates the pressure in the glottis required for voice production. Voluntary vocal production begins in the laryngeal motor cortex (LMC), a structure found in all mammals, although the specific location in the cortex varies in humans. The LMC interfaces with various structures of the central autonomic network associated with cardiorespiratory regulation to allow the perfect coordination between breathing and vocalization. The main subcortical structure involved in this relationship is the mesencephalic periaqueductal grey matter (PAG). The PAG is the perfect link to the autonomic pontomedullary structures such as the parabrachial complex (PBc), the Kölliker–Fuse nucleus (KF), the nucleus tractus solitarius (NTS), and the nucleus retroambiguus (nRA), which modulate cardiovascular autonomic function activity in the vasomotor centers and respiratory activity at the level of the generators of the laryngeal-respiratory motor patterns that are essential for vocalization.
1. Introduction
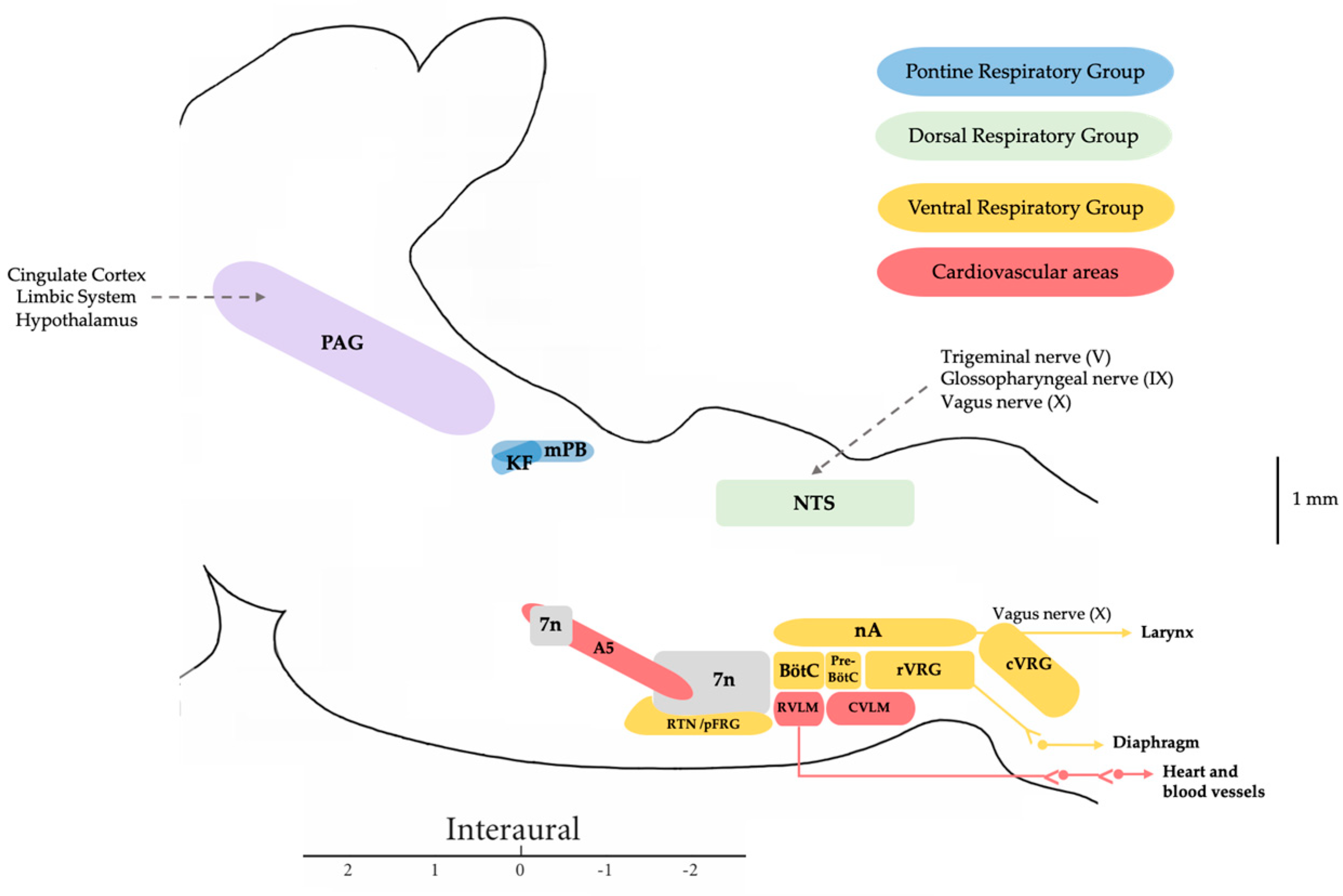
2. Central Autonomic Network Involved in the Control of Respiration and Vocal Emission
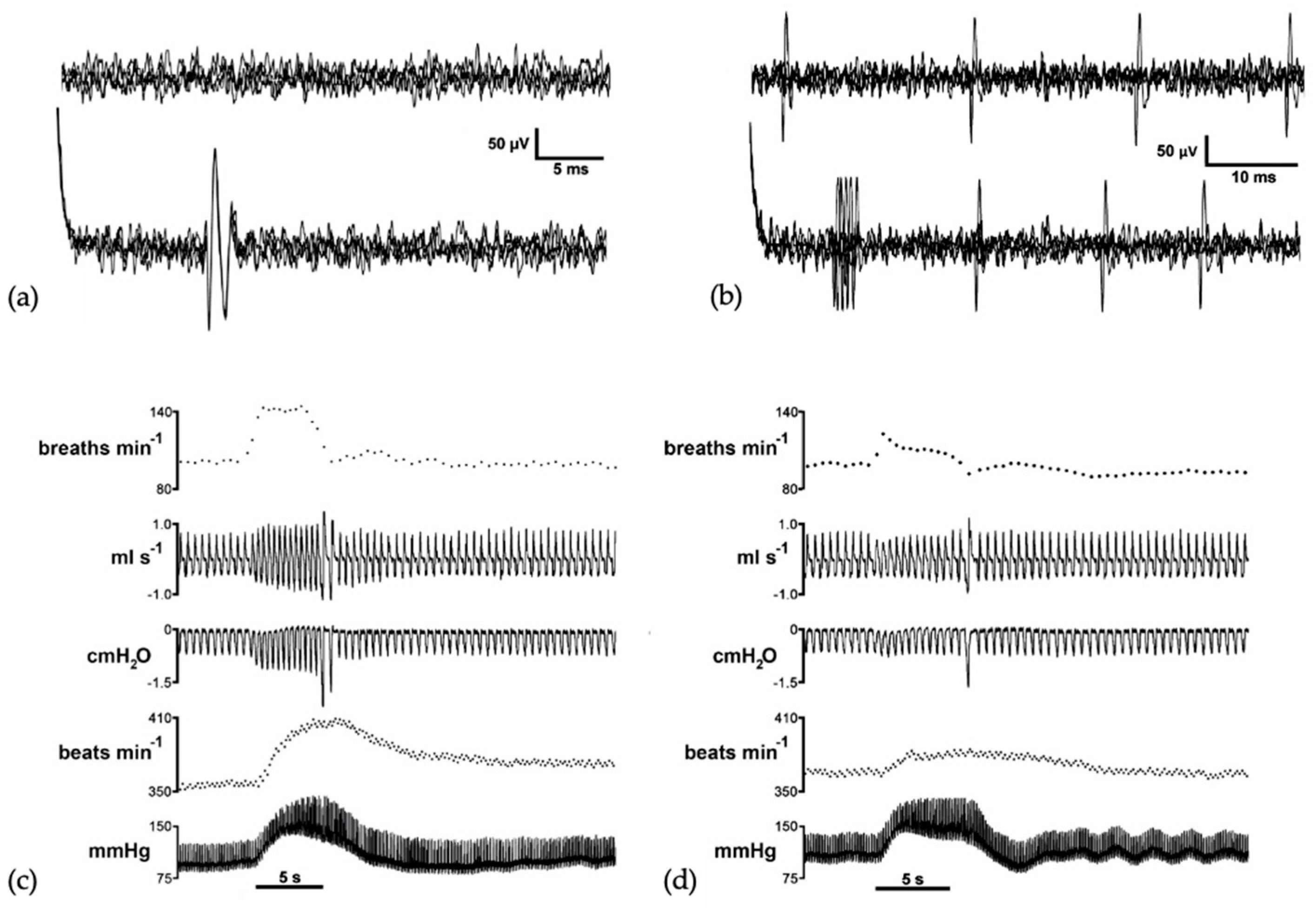
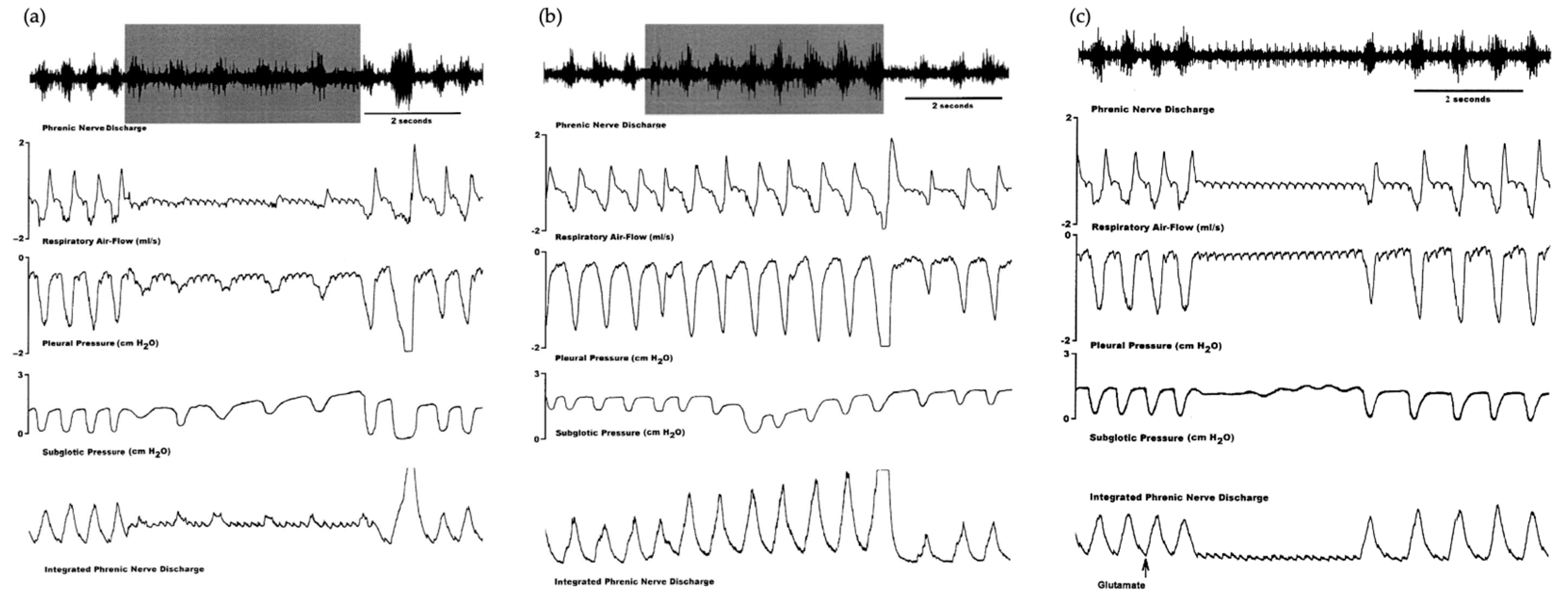
3. Vocalization in Apes: Connectivity between the PAG and the Laryngeal Motor Cortex
4. Clinical Implications
References
- Albersheim-Carter, J.; Blubaum, A.; Ballagh, I.H.; Missaghi, K.; Siuda, E.R.; McMurray, G.; Bass, A.H.; Dubuc, R.; Kelley, D.B.; Schmidt, M.F.; et al. Testing the evolutionary conservation of vocal motoneurons in vertebrates. Respir. Physiol. Neurobiol. 2016, 224, 2–10.
- Inagi, K.; Schultz, E.; Ford, C.N. An Anatomic Study of the Rat Larynx: Establishing the Rat Model for Neuromuscular Function. Otolaryngol. Head Neck Surg. 1998, 118, 74–81.
- Fernandes, A.C.N.; Ferreira, M.V.N.; Serra, L.S.M.; Kuckelhaus, S.A.S.; da Silva, E.M.; Sampaio, A.L.L. Methodological Approaches for Vocal Folds Experiments in Laryngology: A Scoping Review. J. Voice 2022, in press.
- Uludag, M.; Aygun, N.; Kartal, K.; Besler, E.; Isgor, A. Innervation of the human posterior cricoarytenoid muscle by the external branch of the superior laryngeal nerve. Head Neck 2017, 39, 2200–2207.
- Allen, E.; Minutello, K.; Murcek, B.W. Anatomy, Head and Neck, Larynx Recurrent Laryngeal Nerve; StatPearls Publishing: Treasure Island, FL, USA, 2023.
- Jürgens, U. The neural control of vocalization in mammals: A review. J. Voice 2009, 23, 1–10.
- Simonyan, K. The laryngeal motor cortex: Its organization and connectivity. Curr. Opin. Neurobiol. 2014, 28, 15–21.
- Tschida, K.; Michael, V.; Takatoh, J.; Han, B.X.; Zhao, S.; Sakurai, K.; Mooney, R.; Wang, F. A Specialized Neural Circuit Gates Social Vocalizations in the Mouse. Neuron 2019, 103, 459–472.e4.
- Del Negro, C.A.; Funk, G.D.; Feldman, J.L. Breathing matters. Nat. Rev. Neurosci. 2018, 19, 351–367.
- De Troyer, A.; Kirkwood, P.A.; Wilson, T.A. Respiratory action of the intercostal muscles. Physiol. Rev. 2005, 85, 717–756.
- Torres-Tamayo, N.; García-Martínez, D.; Lois Zlolniski, S.; Torres-Sánchez, I.; García-Río, F.; Bastir, M. 3D analysis of sexual dimorphism in size, shape and breathing kinematics of human lungs. J. Anat. 2018, 232, 227–237.
- Welch, J.F.; Kipp, S.; Sheel, A.W. Respiratory muscles during exercise: Mechanics, energetics, and fatigue. Curr. Opin. Physiol. 2019, 10, 102–109.
- Mortola, J.P. Lung viscoelasticity: Implications on breathing and forced expiration. Clin. Pulm. Med. 2013, 20, 144–148.
- Wertheimer, E. Recherches expérimentales sur les centres respiratoires de la moelle épinière. J. Anat. Physiol. Norm. Pathol. Homme Animaux 1886, 22, 458–507.
- Hollinshead, W.H.; Keswani, N.H. Localization of the phrenic nucleus in the spinal cord of man. Anat. Rec. 1956, 125, 683–699.
- Wu, J.; Capelli, P.; Bouvier, J.; Goulding, M.; Arber, S.; Fortin, G. A V0 core neuronal circuit for inspiration. Nat. Commun. 2017, 8, 544.
- Dutschmann, M.; Jones, S.E.; Subramanian, H.H.; Stanic, D.; Bautista, T.G. The physiological significance of postinspiration in respiratory control. Prog. Brain Res. 2014, 212, 113–130.
- Shinozaki, Y.; Yokota, S.; Miwakeichi, F.; Pokorski, M.; Aoyama, R.; Fukuda, K.; Yoshida, H.; Toyama, Y.; Nakamura, M.; Okada, Y. Structural and functional identification of two distinct inspiratory neuronal populations at the level of the phrenic nucleus in the rat cervical spinal cord. Brain Struct. Funct. 2019, 224, 57–72.
- Nishimura, T.; Tokuda, I.T.; Miyachi, S.; Dunn, J.C.; Herbst, C.T.; Ishimura, K.; Kaneko, A.; Kinoshita, Y.; Koda, H.; Saers, J.P.P.; et al. Evolutionary loss of complexity in human vocal anatomy as an adaptation for speech. Science 2022, 377, 760–763.
- Esposito, A.; Demeurisse, G.; Alberti, B.; Fabbro, F. Complete mutism after midbrain periaqueductal gray lesion. Neuroreport 1999, 10, 681–685.
- Jürgens, U. The role of the periaqueductal grey in vocal behaviour. Behav. Brain Res. 1994, 62, 107–117.
- Kittelberger, J.M.; Land, B.R.; Bass, A.H. Midbrain periaqueductal gray and vocal patterning in a teleost fish. J. Neurophysiol. 2006, 96, 71–85.
- Farley, G.R.; Barlow, S.M.; Netsell, R. Factors influencing neural activity in parabrachial regions during cat vocalizations. Exp. Brain Res. 1992, 89, 341–351.
- Lu, C.L.; Jürgens, U. Effects of chemical stimulation in the periaqueductal gray on vocalization in the squirrel monkey. Brain Res. Bull. 1993, 32, 143–151.
- Díaz-Casares, A.; López-González, M.V.; Peinado-Aragonés, C.A.; González-Barón, S.; Dawid-Milner, M.S. Parabrachial complex glutamate receptors modulate the cardiorespiratory response evoked from hypothalamic defense area. Auton. Neurosci. Basic Clin. 2012, 169, 124–134.
- López-González, M.V.; Díaz-Casares, A.; González-García, M.; Peinado-Aragonés, C.A.; Barbancho, M.A.; Carrillo de Albornoz, M.; Dawid-Milner, M.S. Glutamate receptors of the A5 region modulate cardiovascular responses evoked from the dorsomedial hypothalamic nucleus and perifornical area. J. Physiol. Biochem. 2018, 74, 325–334.
- González-García, M.; Carrillo-Franco, L.; Peinado-Aragonés, C.A.; Díaz-Casares, A.; Gago, B.; López-González, M.V.; Dawid-Milner, M.S. Impact of the glutamatergic neurotransmission within the A5 region on the cardiorespiratory response evoked from the midbrain dlPAG. Pflügers Arch. Eur. J. Physiol. 2023, 475, 505–516.
- Díaz-Casares, A.; López-González, M.V.; Peinado-Aragonés, C.A.; Lara, J.P.; González-Barón, S.; Dawid-Milner, M.S. Role of the parabrachial complex in the cardiorespiratory response evoked from hypothalamic defense area stimulation in the anesthetized rat. Brain Res. 2009, 1279, 58–70.
- López-González, M.V.; Díaz-Casares, A.; Peinado-Aragonés, C.A.; Lara, J.P.; Barbancho, M.A.; Dawid-Milner, M.S. Neurons of the A5 region are required for the tachycardia evoked by electrical stimulation of the hypothalamic defence area in anaesthetized rats. Exp. Physiol. 2013, 98, 1279–1294.
- López-González, M.V.; González-García, M.; Peinado-Aragonés, C.A.; Barbancho, M.Á.; Díaz-Casares, A.; Dawid-Milner, M.S. Pontine A5 region modulation of the cardiorespiratory response evoked from the midbrain dorsolateral periaqueductal grey. J. Physiol. Biochem. 2020, 76, 561–572.
- Lara, J.P.; Dawid-Milner, M.S.; López, M.V.; Montes, C.; Spyer, K.M.; González-Barón, S. Laryngeal effects of stimulation of rostral and ventral pons in the anaesthetized rat. Brain Res. 2002, 934, 97–106.
- Dutschmann, M.; Dick, T.E. Pontine mechanisms of respiratory control. Compr. Physiol. 2012, 2, 2443–2469.
- Dhingra, R.R.; Dick, T.E.; Furuya, W.I.; Galán, R.F.; Dutschmann, M. Volumetric mapping of the functional neuroanatomy of the respiratory network in the perfused brainstem preparation of rats. J. Physiol. 2020, 598, 2061–2079.
- Yang, C.F.; Feldman, J.L. Efferent projections of excitatory and inhibitory preBötzinger Complex neurons. J. Comp. Neurol. 2018, 526, 1389–1402.
- Anderson, T.M.; Garcia, A.J., 3rd; Baertsch, N.A.; Pollak, J.; Bloom, J.C.; Wei, A.D.; Rai, K.G.; Ramirez, J.M. A novel excitatory network for the control of breathing. Nature 2016, 536, 76–80.
- Holstege, G. The somatic motor system. Prog. Brain Res. 1996, 107, 9–26.
- Subramanian, H.H.; Balnave, R.J.; Holstege, G. Microstimulation in Different Parts of the Periaqueductal Gray Generates Different Types of Vocalizations in the Cat. J. Voice 2021, 35, 804.e9–804.e25.
- Holstege, G. The emotional motor system. Eur. J. Morphol. 1992, 30, 67–79.
- Jürgens, U.; Hage, S.R. On the role of the reticular formation in vocal pattern generation. Behav. Brain Res. 2007, 182, 308–314.
- Brown, S.; Ngan, E.; Liotti, M. A larynx area in the human motor cortex. Cereb. Cortex 2008, 18, 837–845.
- Ludlow, C.L. Central nervous system control of the laryngeal muscles in humans. Respir. Physiol. Neurobiol. 2005, 147, 205–222.
- Loucks, T.M.; Poletto, C.J.; Simonyan, K.; Reynolds, C.L.; Ludlow, C.L. Human brain activation during phonation and exhalation: Common volitional control for two upper airway functions. Neuroimage 2007, 36, 131–143.
- Jones, B.E.; Yang, T.Z. The efferent projections from the reticular formation and the locus coeruleus studied by anterograde and retrograde axonal transport in the rat. Comp. Neurol. 1985, 242, 56–92.
- Krohn, F.; Novello, M.; van der Giessen, R.S.; De Zeeuw, C.I.; Pel, J.J.M.; Bosman, L.W.J. The integrated brain network that controls respiration. Elife 2023, 12, e83654.
- Holstege, G.; Subramanian, H.H. Two different motor systems are needed to generate human speech. J. Comp. Neurol. 2016, 524, 1558–1577.
- Jürgens, U.; Pratt, R. Role of the periaqueductal grey in vocal expression of emotion. Brain Res. 1979, 167, 367–378.
- Kirzinger, A.; Jürgens, U. Cortical lesion effects and vocalization in the squirrel monkey. Brain Res. 1982, 233, 299–315.
- Ludlow, C.L. Central nervous system control of interactions between vocalization and respiration in mammals. Head Neck 2011, 33, 121–125.
- Subramanian, H.H.; Holstege, G. The nucleus retroambiguus control of respiration. J. Neurosci. 2009, 29, 3824–3832.
- Schadt, C.R.; Cox, K.L.; Tramontana, M.G.; Byrne, D.W.; Davis, T.L.; Fang, J.Y.; Konrad, P.E.; Padaliya, B.; Mutter, R.W.; Gill, C.E.; et al. Depression and intelligence in patients with Parkinson’s disease and deep-brain stimulation. J. Natl. Med. Assoc. 2006, 98, 1121–1125.
- Peterson, J.R.; Watts, C.R.; Morris, J.A.; Shelton, J.M.; Cooper, B.G. Laryngeal aging and acoustic changes in male rat ultrasonic vocalizations. Dev. Psychobiol. 2013, 55, 818–828.
- Drager, L.F.; Genta, P.R.; Pedrosa, R.P.; Nerbass, F.B.; Gonzaga, C.C.; Krieger, E.M.; Lorenzi-Filho, G. Characteristics and predictors of obstructive sleep apnea in patients with systemic hypertension. Am. J. Cardiol. 2010, 105, 1135–1139.
- Wang, X.; Guo, R.; Zhao, W.; Pilowsky, P.M. Medullary mediation of the laryngeal adductor reflex: A possible role in sudden infant death syndrome. Respir. Physiol. Neurobiol. 2016, 226, 121–127.
- Mor, N.; Simonyan, K.; Blitzer, A. Central voice production and pathophysiology of spasmodic dysphonia. Laryngoscope 2018, 128, 177–183.
- Christopher, K.L.; Morris, M.J. Vocal cord dysfunction, paradoxic vocal fold motion, or laryngomalacia? Our understanding requires an interdisciplinary approach. Otolaryngol. Clin. N. Am. 2010, 43, 43–66.
- Van Houtte, E.; Van Lierde, K.; Claeys, S. Pathophysiology and treatment of muscle tension dysphonia: A review of the current knowledge. J. Voice 2011, 25, 202–207.




