Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Martina Derme | -- | 2030 | 2024-02-27 10:21:07 | | | |
| 2 | Peter Tang | Meta information modification | 2030 | 2024-02-28 02:22:20 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Derme, M.; Briante, M.; Ceccanti, M.; Giannini, G.; Vitali, M.; Messina, M.P.; Piccioni, M.G.; Mattia, A.; Nicotera, S.; Crognale, A. Prenatal Alcohol Exposure and Metabolic Disorders in Pediatrics. Encyclopedia. Available online: https://encyclopedia.pub/entry/55519 (accessed on 07 February 2026).
Derme M, Briante M, Ceccanti M, Giannini G, Vitali M, Messina MP, et al. Prenatal Alcohol Exposure and Metabolic Disorders in Pediatrics. Encyclopedia. Available at: https://encyclopedia.pub/entry/55519. Accessed February 07, 2026.
Derme, Martina, Martina Briante, Mauro Ceccanti, Giuseppe Giannini, Mario Vitali, Marisa Patrizia Messina, Maria Grazia Piccioni, Alessandro Mattia, Simona Nicotera, Alba Crognale. "Prenatal Alcohol Exposure and Metabolic Disorders in Pediatrics" Encyclopedia, https://encyclopedia.pub/entry/55519 (accessed February 07, 2026).
Derme, M., Briante, M., Ceccanti, M., Giannini, G., Vitali, M., Messina, M.P., Piccioni, M.G., Mattia, A., Nicotera, S., & Crognale, A. (2024, February 27). Prenatal Alcohol Exposure and Metabolic Disorders in Pediatrics. In Encyclopedia. https://encyclopedia.pub/entry/55519
Derme, Martina, et al. "Prenatal Alcohol Exposure and Metabolic Disorders in Pediatrics." Encyclopedia. Web. 27 February, 2024.
Copy Citation
Prenatal alcohol exposure is responsible for increasing chronic disease risk in later life, including obesity and metabolic syndrome. Alcohol drinking may compromise endogenous antioxidant capacity, causing an increase in free radicals and reactive oxygen species in the newborn. Excessive reactive oxygen species could attack the cellular proteins, lipids, and nucleic acids, leading to cellular dysfunction. Moreover, oxidative stress could play a crucial role in the altered synthesis and release of neurotrophins and progressive mitochondrial modifications with uncontrolled apoptosis.
fetal alcohol spectrum disorder (FASD)
prenatal alcohol exposure (PAE)
oxidative stress
metabolic disorders
1. Introduction
Prenatal alcohol exposure (PAE) is the foremost avoidable reason for congenital abnormalities and developmental disabilities and affects 2.4–4.8/1000 children [1]. PAE may also raise, in later life, chronic disease risks such as obesity, metabolic syndrome [2], and liver disease [3][4].
Worldwide, almost 10% of pregnant women drink alcohol. The highest rate of alcoholism during pregnancy is in Europe 25.2%), followed by the American Region (11.2%), the Western Pacific Region (8.6%), the African Region (10.0%), and the South East Asia Region (1.8%). The lowest prevalence is present in the Eastern Mediterranean Region (0.2%) (Figure 1) [5]. Different Mediterranean studies measuring gestational alcohol drinking in women through the analysis of different ethanol metabolites or in the hair, meconium, or urine data showed high variability with values ranging from 3 to 4% up to more than 30% [6][7][8][9][10][11][12].
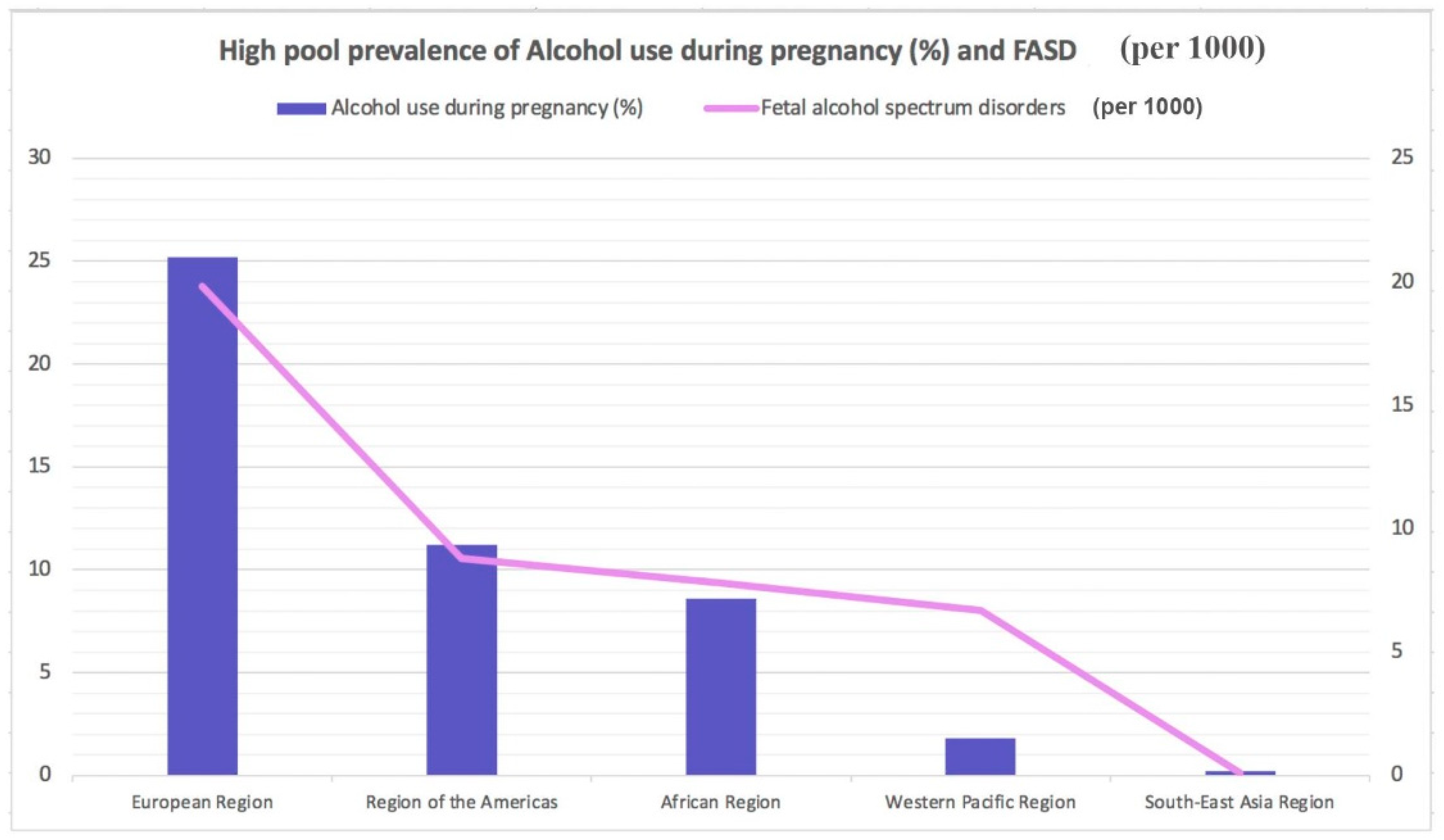
Figure 1. Highest pooled prevalence of alcohol use during pregnancy (%) and fetal alcohol spectrum disorders (per 1000).
Numerous risk factors have been discovered for alcoholism in pregnancy: older age; higher socioeconomic status, salary, and educational levels; smoking; and unintended pregnancy [6][7][13][14].
Fetal alcohol spectrum disorders (FASD) is a “container” word that implies the type of circumstances resulting from PAE. FASD includes disorders such as partial fetal alcohol syndrome (pFAS), fetal alcohol syndrome (FAS), alcohol-related birth defects (ARBD), and alcohol-related neurological developmental disorders (ARND) [15][16][17][18][19][20][21][22][23][24]. Several FASD analytic guidelines have been proposed; among the most recent, Hoyme’s guidelines are quite useful [25]. These guidelines were made by a panel of expert authors who analyzed more than 10,000 children with potential FASD. They elaborated a diagnostic process that requires a multidisciplinary approach and a collaboration between pediatricians, geneticists, maternal-fetal specialists, psychiatrists, speech pathologists, physical therapists, audiologists, and ophthalmologists. The advantage of these guidelines is the possibility of elaborating a diagnosis in the prenatal period.
Oxidative stress seems to play a key role in the pathogenesis of both neuropsychiatric and metabolic disorders in pediatrics [26][27]. Ethanol (EtOH) can alter the endogenous antioxidant ability by depleting the levels of glutathione peroxidase and producing free radicals. Free radicals and reactive oxygen species (ROS), such as hydroxide (HO−) and superoxide (O2−) ions, are derived by O2 partial reduction. They can affect a cell’s structure by damaging nucleic acids, carbohydrates, proteins, and lipids. These molecules are responsible for inducing uninhibited apoptosis of fetal brain damage in children with FASD [28][29]. The FASD neuropsychiatric effects may be justified by EtOH drinking, inducing the apoptosis of serotoninergic neurons, as shown in rodent models [30].
2. Mechanism of Oxidative Stress in Metabolic Disorders
EtOH consumption in pregnancy results in an alteration of oxidative status. A recent case report [31] described increased oxidative stress in a mother abusing ethanol drinking during gestation and in her infant a few days after delivery. The FORT (free oxygen radicals test) was used, indeed, to measure the oxidative stress in the mother and her child [32]. The FORT is a colorimetric assay based on the ability of transition metals such as iron to catalyze, in the presence of hydroperoxides (ROOH), the formation of free radicals (reactions 1–2), which are then entrapped by an amine derivative, CrNH2. The amine reacts with free radicals, creating a colored, fairly long-lived radical cation, measurable at 505 nm (reaction 3). The color intensity correlates directly to the radical compounds and the hydroperoxide concentrations and, consequently, to the oxidative status of the sample according to the Lambert–Beer law [32]. Values superior to 330 U indicate a situation of progressing oxidative stress.
Oxidase enzymes (Nox), the mitochondria, and nicotinamide adenine dinucleotide phosphate (NADPH) are the two main apparatuses of ROS production inside the cell [33]. The Nox enzymes (Nox1, Nox2, Nox3, Nox4, Nox5, DUOX1, and DUOX2) are cell membrane proteins and Nox2-Nox3 are involved in different pathological circumstances [33]. ROS are produced in the mitochondria during oxidative phosphorylation by converting nicotinamide adenine dinucleotide (NADH) to NAD+ [34][35]. The superoxide anion and Nox2 are quickly converted by the superoxide dismutase enzyme into hydrogen peroxide (H2O2), an important signaling molecule [36][37]. Indeed, H2O2 is a potent oxidizing agent, and based on these considerations, cells are forced to secrete antioxidant peptides that convert H2O2 to water, including catalase, peroxiredoxin, thioredoxin, and glutathione (GSH) [35][38]. It is important that H2O2 production is equal to its reduction [39].
Pathological diseases such as insulin resistance, obesity, chronic inflammation, hyperglycemia, and dyslipidemia can cause overproduction of ROS [40][41]. The excessive ROS presence may elicit cellular damage, in particular, peroxidizing lipids and altering DNA [42]. Lipid peroxides, lipid peroxidation end products, may be toxic to the cell and should be removed by glutathione throughout a specific mechanism [43]. Indeed, previous investigations revealed that patients metabolically affected by the syndrome displayed greater biomarkers of oxidative damage and lower plasma antioxidant enzyme activity than healthy people [44]. Peroxidation and nitrosylation can alter nuclear acids and proteins [35]. These end products do not typically directly harm the cell [35]. However, the increase in inactive proteins may alter the cell’s capability to metabolize them, determining the activation of apoptosis and DNA damage [43]. In addition, such elevation in modified proteins reduces their function, leading to severe impairment of regular cell action [36][43]. The ROS overproduction leads to oxidative stress elevation, which also disrupts redox control and signaling, determining gene expression alteration and increasing stress response elements and growth factors by activating the apoptosis path [39][45]. Furthermore, oxidative stress may elicit profibrotic and proinflammatory pathways, which alter endothelial dysfunction and insulin metabolic signaling by promoting renal and cardiovascular fibrosis [39][46].
3. Oxidative Stress in Pediatrics after Fetal Alcohol Exposure
FASD is an umbrella expression defining all the circumstances resulting from PAE: partial fetal alcohol syndrome (pFAS), fetal alcohol syndrome (FAS), alcohol-related neurodevelopmental disorder (ARND), and alcohol-related birth defects (ARBD) [47].
The vulnerability to ethanol strongly depends on the genetic background of each individual [48], and particularly for gestation, it is not possible to establish a consumption-safe level. Indeed, the only practicable recommendation for pregnant women is to avoid alcohol use completely. Damage due to PAE can be long-lasting with no cure [49], so early management and correct identification may support prevention and alleviate the metabolic and neurological consequences affecting the FASD person later in life. FASD severity depends on the amount and drinking frequency, as well as the gestational age at which the ethanol was assumed by the pregnant woman [50][51]. Intervention services, prevention and sensibilization for the mothers could moderate the FASD incidence [52].
The fetus has inadequate or null aptitudes in alcohol metabolization and removal [53]. Indeed, the several enzymes aimed at ethanol degradation gradually elevate their actions during the various steps of gestation [32].
Figure 2 summarizes the mechanisms through which oxidative stress has a major role in the pathogenesis of neurological and metabolic diseases of patients exposed to alcohol in the prenatal period.
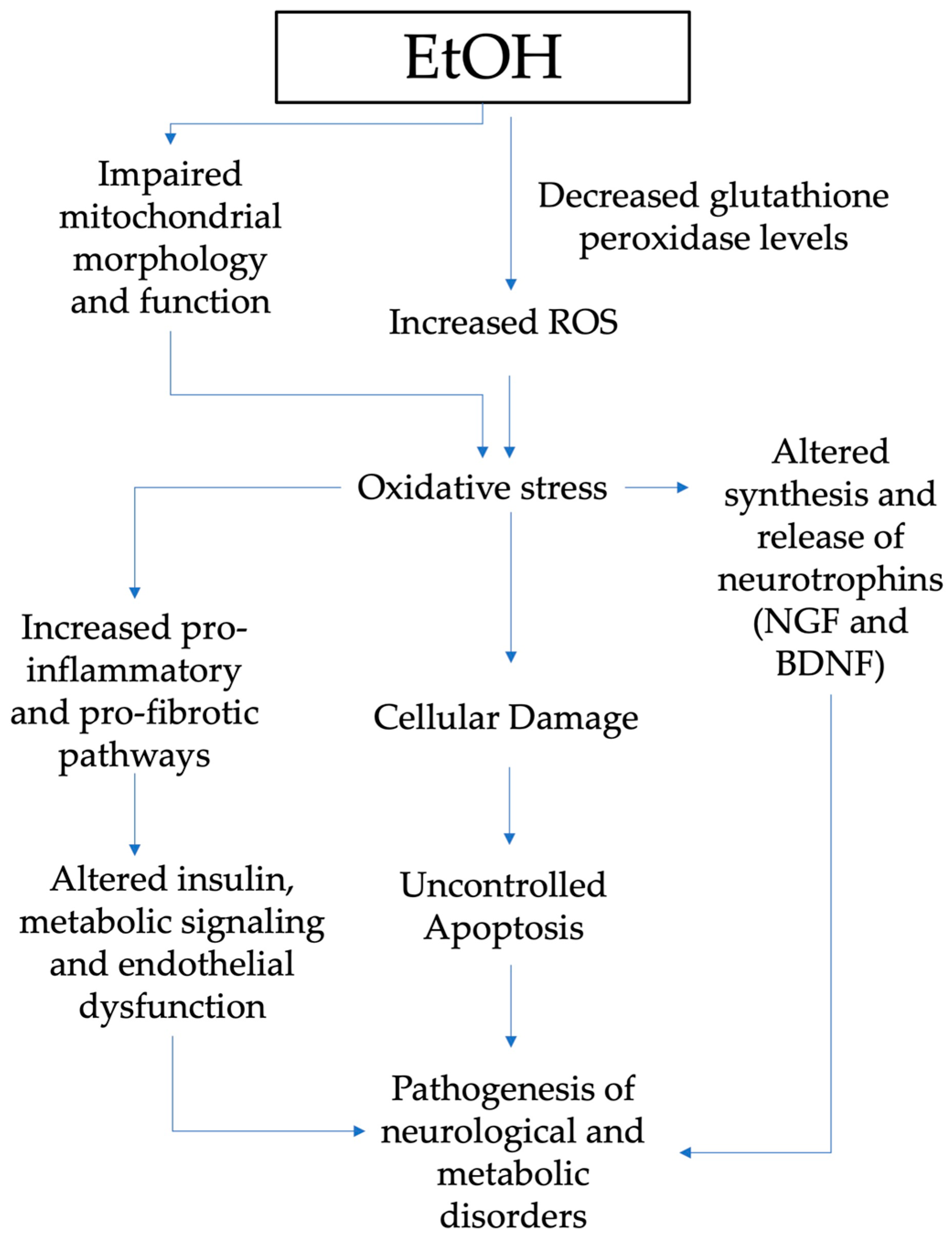
Figure 2. Pathogenesis of neurological and metabolic diseases and role of oxidative stress in patients exposed to alcohol in the prenatal period (EtOH, ethanol; ROS, reactive oxygen species; NGF, nerve growth factor; BDNF, brain-derived neurotrophic factor.
EtOH can alter the endogenous antioxidant ability by reducing GSH and generating free radicals, which are considered to be responsible for uncontrolled apoptosis [54][55]. Chronic and acute alcohol drinking during prenatal development also impacts mitochondrial function and morphology, another crucial cell oxidative stress source [56]. Depleted mitochondrial activity is discovered in the early postnatal stage in liver and brain tissues, including the cerebellar brain cells of prenatally exposed rats [56].
Oxidative stress has a key role in the altered synthesis and release of growth factors such as the nerve growth factor—NGF and brain-derived neurotrophic factor—BDNF [57]. Animal model studies disclosed many findings on gestational alcohol exposure’s effects on neurotrophins [58][59]. Indeed, maternal alcohol exposure during gestation affects the neurotrophins’ brain signaling pathways, as well as in target tissues for ethanol intoxication. NGF and BDNF are peptides that not only play a pivotal role in the development, survival, and function of the central and peripheral nervous systems but also regulate the pathogenesis of other problems induced by alcohol exposure [58][60][61].
The supplementation with natural compounds with antioxidant properties, such as the polyphenols extracted from vegetables, might, of course, counteract the toxic prooxidant effect of alcohol abuse during pregnancy [62][63][64][65][66][67]. Furthermore, a healthy diet during pregnancy containing proper amounts of fresh vegetables containing polyphenols, as evidenced by the Mediterranean diet, might reduce the oxidative stress induced by gestational alcohol drinking [68][69][70][71][72][73][74][75].
4. FASD and Metabolic Disorders
PAE and subsequent FASD can cause lifelong alterations in affected offspring, including a permanent imbalance in metabolic homeostasis. These effects of alcohol consumption during pregnancy could be linked to an increased risk of intrauterine growth restriction (IUGR) [76] due to blood flow impairment [77] and abnormal placentation process [78], with following catch-up growth [79]. This phenomenon is strongly correlated with the development of some features of metabolic syndrome, such as central obesity, glucose intolerance, and dyslipidemia [80].
Several studies conducted both in humans and in animal models try to underline the effects of PAE on metabolism. In 2020, Weeks et al. [81] demonstrated, with a retrospective cross-sectional study in adults with any form of FASD diagnosis, that alcohol exposure in utero increases the incidence of hypertriglyceridemia, type 2 diabetes mellitus, and lower HDL cholesterol, independently of BMI in the case of the male cohort. Female patients had, instead, an increased risk of being overweight and obese. They confirmed these data with zebrafish, a particularly suitable model considering its flexibility and anatomical similarities to humans, the presence of many evolutionary conserved pathways, and the simplicity of alcohol administration in an aqueous environment [82].
The relationship between PAE and HFD was also analyzed by Shen and colleagues [83] in 2014 when they evaluated the susceptibility of female adult offspring to HFD-induced nonalcoholic fatty liver disease (NAFLD), which is considered a liver indicator of metabolic syndrome [84]. In the group exposed to PAE and HFD, they found a decrease in serum corticosterone and an increase in serum IGF-1, glucose, and triglyceride with notable catch-up growth, higher metabolic status, and NAFLD formation. The authors suggest a “two-programming” hypothesis for the augmented risk of NAFLD in the case of prenatal alcohol exposure, in which the “first programming” consists of the intrauterine programming of liver glucose and lipid metabolic function and the “second programming” is led by postnatal adaptive catch-up growth triggered by intrauterine programming of glucocorticoid-IGF1 axis.
5. FASD and Cardiovascular Disease
Congenital heart defects (CHDs) are the most common congenital anomaly, with a worldwide prevalence of 9.1 in 1000 live births [85][86]. The etiology of CHDs is still unknown; most of them are due to genetic anomalies and aneuploidies. The Maternal Heart Association established that prenatal exposure to therapeutic drugs and substances of abuse, like alcohol and cigarettes, are important risk factors [87]. PAE has also been shown to be related to the occurrence of CHDs. Alcohol has severe effects on the cardiovascular system, leading to various disease states such as arrhythmias [88] and dilated cardiomyopathy [89].
The cardiotoxicity of alcohol does not interest only adult consumers. According to the Centers for Disease Control and Prevention (CDCP), about 10% of pregnant women mentioned drinking fluently, and approximately 50% of them mentioned binge drinking, which increases the risk of FASD [90]. The proportion of children with CHDs among children with FASD is almost 67% [91]. The molecular mechanisms can explain the illness, but the American teratogenic effects of PAE are still poorly understood because of the complexity of alcohol effects and the correlation with timing, amount, and duration of exposure, as well as genetic susceptibility [92].
By modeling chronic alcohol exposure-induced cardiotoxicity in hiPSC-CMs, they showed that EtOH causes decreased mitochondrial membrane potential and mitochondrial content, decreased mitochondrial function, and altered expression of related genes [93]. EtOH also modified the glycolytic process and carbohydrate metabolic process as well as a reply to hypoxia, increased glycolysis, decreased mitochondrial function, and increased oxidative stress [93]. Therefore, an upregulation of T-cell chemotaxis has been shown as a potential causal link to proinflammatory response [93]. Further studies are needed to better understand the mechanisms of alcohol cardiotoxicity and teratogenicity in order to prevent the dramatic effect of PAE on the offspring of mothers consuming alcohol.
References
- May, P.A.; Baete, A.; Russo, J.; Elliott, A.J.; Blankenship, J.; Kalberg, W.O.; Buckley, D.; Brooks, M.; Hasken, J.; Abdul-Rahman, O.; et al. Prevalence and Characteristics of Fetal Alcohol Spectrum Disorders. Pediatrics 2014, 134, 855–866.
- Moore, E.M.; Riley, E.P. What Happens When Children with Fetal Alcohol Spectrum Disorders Become Adults? Curr. Dev. Disord Rep. 2015, 2, 219–227.
- Asiedu, B.; Nyakudya, T.T.; Lembede, B.W.; Chivandi, E. Early-life exposure to alcohol and the risk of alcohol-induced liver disease in adulthood. Birth Defects Res. 2021, 113, 451–468.
- Liu, Q.; Gao, F.; Liu, X.; Li, J.; Wang, Y.; Han, J.; Wang, X. Prenatal alcohol exposure and offspring liver dysfunction: A systematic review and meta-analysis. Arch. Gynecol. Obstet. 2016, 294, 225–231.
- Popova, S.; Lange, S.; Probst, C.; Gmel, G.; Rehm, J. Estimation of national, regional, and global prevalence of alcohol use during pregnancy and fetal alcohol syndrome: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e290–e299.
- Joya, X.; Pacifici, R.; Salat-Batlle, J.; Garciá-Algar, O.; Pichini, S. Maternal and neonatal hair and breast milk in the assessment of perinatal exposure to drugs of abuse. Bioanalysis 2015, 7, 1273–1297.
- Morini, L.; Groppi, A.; Marchei, E.; Vagnarelli, F.; Algar, O.G.; Zuccari, P.; Pichini, S. Population Baseline of Meconium Ethyl Glucuronide and Ethyl Sulfate Concentrations in Newborns of Nondrinking Women in 2 Mediterranean Cohorts. Ther. Drug Monit. 2010, 32, 359–363.
- Morini, L.; Marchei, E.; Vagnarelli, F.; Garcia Algar, O.; Groppi, A.; Mastrobattista, L.; Pichini, S. Ethyl glucuronide and ethyl sulfate in meconium and hair-potential biomarkers of intrauterine exposure to ethanol. Forensic. Sci. Int. 2010, 196, 74–77.
- Pichini, S.; Morini, L.; Marchei, E.; Palmi, I.; Rotolo, M.C.; Vagnarelli, F.; Garcia-Algar, O.; Vall, O.; Zuccaro, P. Ethylglucuronide and ethylsulfate in meconium to assess gestational ethanol exposure: Preliminary results in two Mediterranean cohorts. Can. J. Clin. Pharmacol. 2009, 16, e370–e375.
- Pichini, S.; Marchei, E.; Vagnarelli, F.; Tarani, L.; Raimondi, F.; Maffucci, R.; Sacher, B.; Bisceglia, M.; Rapisardi, G.; Elicio, M.R.; et al. Assessment of Prenatal Exposure to Ethanol by Meconium Analysis: Results of an Italian Multicenter Study. Alcohol. Clin. Exp. Res. 2012, 36, 417–424.
- Ferraguti, G.; Ciolli, P.; Carito, V.; Battagliese, G.; Mancinelli, R.; Ciafrè, S.; Tirassa, P.; Ciccarelli, R.; Cipriani, A.; Messina, M.P.; et al. Ethylglucuronide in the urine as a marker of alcohol consumption during pregnancy: Comparison with four alcohol screening questionnaires. Toxicol. Lett. 2017, 275, 49–56.
- Ceci, F.M.; Fiore, M.; Agostinelli, E.; Tahara, T.; Greco, A.; Ralli, M.; Polimeni, A.; Lucarelli, M.; Colletti, R.; Angeloni, A.; et al. Urinary Ethyl Glucuronide for the Assessment of Alcohol Consumption During Pregnancy: Comparison between Biochemical Data and Screening Questionnaires. Curr. Med. Chem. 2021, 29, 3125–3141.
- McCormack, C.; Hutchinson, D.; Burns, L.; Wilson, J.; Elliott, E.; Allsop, S.; Najman, J.; Jacobs, S.; Rossen, L.; Olsson, C.; et al. Prenatal Alcohol Consumption Between Conception and Recognition of Pregnancy. Alcohol. Clin. Exp. Res. 2017, 41, 369–378.
- Tsang, T.W.; Kingsland, M.; Doherty, E.; Anderson, A.E.; Tully, B.; Crooks, K.; Symonds, I.; Tremain, D.; Dunlop, A.J.; Wiggers, J.; et al. Predictors of alcohol use during pregnancy in Australian women. Drug Alcohol. Rev. 2022, 41, 171–181.
- Burd, L. FASD and ADHD: Are they related and How? BMC Psychiatry 2016, 16, 325.
- Goulden, K.J. Are FASD guidelines: Practical and sustainable? (multiple letters). CMAJ Can. Med. Assoc. J. 2005, 173, 1070–1071.
- Denys, K.; Rasmussen, C.; Henneveld, D. The Effectiveness of a Community-Based Intervention for Parents with FASD. Community Ment. Health J. 2009, 47, 209–219.
- Tunc-Ozcan, E.; Sittig, L.J.; Harper, K.M.; Graf, E.N.; Redei, E.E. Hypothesis: Genetic and epigenetic risk factors interact to modulate vulnerability and resilience to FASD. Front. Genet. 2014, 5, 261.
- Aragón, A.S.; Kalberg, W.O.; Buckley, D.; Barela-Scott, L.M.; Tabachnick, B.G.; May, P.A. Neuropsychological study of FASD in a sample of American Indian children: Processing simple versus complex information. Alcohol. Clin. Exp. Res. 2008, 32, 2136–2148.
- May, P.A.; Gossage, J.P.; Kalberg, W.O.; Robinson, L.K.; Buckley, D.; Manning, M.; Hoyme, H.E. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Dev. Disabil. Res. Rev. 2009, 15, 176–192.
- Premji, S.S.; Semenic, S. Do Canadian prenatal record forms integrate evidence-based guidelines for the diagnosis of a FASD? Can. J. Public Health 2009, 100, 274–280.
- Kalberg, W.O.; Buckley, D. FASD: What types of intervention and rehabilitation are useful? Neurosci. Biobehav. Rev. 2007, 31, 278–285.
- Chang, G. Reducing Prenatal Alcohol Exposure and the Incidence of FASD: Is the Past Prologue? Alcohol. Res. 2023, 43, 2.
- Riley, E.P.; Infante, M.A.; Warren, K.R. Fetal Alcohol Spectrum Disorders: An Overview. Neuropsychol. Rev. 2011, 21, 73–80.
- Hoyme, H.E.; Kalberg, W.O.; Elliott, A.J.; Blankenship, J.; Buckley, D.; Marais, A.-S.; Manning, M.A.; Robinson, L.K.; Adam, M.P.; Abdul-Rahman, O.; et al. Updated Clinical Guidelines for Diagnosing Fetal Alcohol Spectrum Disorders. Pediatrics 2016, 138, e20154256.
- Faienza, M.F.; Francavilla, R.; Goffredo, R.; Ventura, A.; Marzano, F.; Panzarino, G.; Marinelli, G.; Cavallo, L.; Di Bitonto, G. Oxidative Stress in Obesity and Metabolic Syndrome in Children and Adolescents. Horm. Res. Paediatr. 2012, 78, 158–164.
- Fuglestad, A.J.; Fink, B.A.; Eckerle, J.K.; Boys, C.J.; Hoecker, H.L.; Kroupina, M.G.; Zeisel, S.H.; Georgieff, M.K.; Wozniak, J.R. Inadequate intake of nutrients essential for neurodevelopment in children with fetal alcohol spectrum disorders (FASD). Neurotoxicol. Teratol. 2013, 39, 128–132.
- Nguyen, V.T.; Chong, S.; Tieng, Q.M.; Mardon, K.; Galloway, G.J.; Kurniawan, N.D. Radiological studies of fetal alcohol spectrum disorders in humans and animal models: An updated comprehensive review. Magn. Reson. Imaging 2017, 43, 10–26.
- Donald, K.A.; Eastman, E.; Howells, F.M.; Adnams, C.; Riley, E.P.; Woods, R.P.; Narr, K.L.; Stein, D.J. Neuroimaging effects of prenatal alcohol exposure on the developing human brain: A magnetic resonance imaging review. Acta Neuropsychiatr. 2015, 27, 251–269.
- Sari, Y.; Zhou, F.C. Prenatal Alcohol Exposure Causes Long-Term Serotonin Neuron Deficit in Mice. Alcohol. Clin. Exp. Res. 2004, 28, 941–948.
- Derme, M.; Piccioni, M.G.; Brunelli, R.; Crognale, A.; Denotti, M.; Ciolli, P.; Scomparin, D.; Tarani, L.; Paparella, R.; Terrin, G.; et al. Oxidative Stress in a Mother Consuming Alcohol during Pregnancy and in Her Newborn: A Case Report. Antioxidants 2023, 12, 1216.
- Denny, L.; Coles, S.; Blitz, R. Fetal Alcohol Syndrome and Fetal Alcohol Spectrum Disorders. Am. Fam. Physician 2017, 96, 515–522.
- DeVallance, E.; Li, Y.; Jurczak, M.J.; Cifuentes-Pagano, E.; Pagano, P.J. The Role of NADPH Oxidases in the Etiology of Obesity and Metabolic Syndrome: Contribution of Individual Isoforms and Cell Biology. Antioxid. Redox Signal. 2019, 31, 687–709.
- Ray, P.D.; Huang, B.-W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 2012, 24, 981–990.
- Zhu, J.; Wu, F.; Yue, S.; Chen, C.; Song, S.; Wang, H.; Zhao, M. Functions of reactive oxygen species in apoptosis and ganoderic acid biosynthesis in Ganoderma lucidum. FEMS Microbiol. Lett. 2019, 366, fnaa015.
- Veith, A.; Moorthy, B. Role of cytochrome P450s in the generation and metabolism of reactive oxygen species. Curr. Opin. Toxicol. 2018, 7, 44–51.
- Nolfi-Donegan, D.; Braganza, A.; Shiva, S. Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020, 37, 101674.
- Beckhauser, T.F.; Francis-Oliveira, J.; De Pasquale, R. Reactive Oxygen Species: Physiological and Physiopathological Effects on Synaptic Plasticity. J. Exp. Neurosci. 2016, 10s1, JEN.S39887.
- Touyz, R.M.; Rios, F.J.; Alves-Lopes, R.; Neves, K.B.; Camargo, L.L.; Montezano, A.C. Oxidative Stress: A Unifying Paradigm in Hypertension. Can. J. Cardiol. 2020, 36, 659–670.
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902.
- Čolak, E.; Pap, D. The role of oxidative stress in the development of obesity and obesity-related metabolic disorders. J. Med. Biochem. 2021, 40, 1–9.
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642.
- Gaschler, M.M.; Stockwell, B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425.
- Bekkouche, L.; Bouchenak, M.; Malaisse, W.; Yahia, D. The Mediterranean Diet Adoption Improves Metabolic, Oxidative, and Inflammatory Abnormalities in Algerian Metabolic Syndrome Patients. Horm. Metab. Res. 2014, 46, 274–282.
- Samson, S.L.; Garber, A.J. Metabolic Syndrome. Endocrinol. Metab. Clin. N. Am. 2014, 43, 1–23.
- Scioli, M.G.; Storti, G.; D’Amico, F.; Rodríguez Guzmán, R.; Centofanti, F.; Doldo, E.; Miranda, E.M.C.; Orlandi, A. Oxidative Stress and New Pathogenetic Mechanisms in Endothelial Dysfunction: Potential Diagnostic Biomarkers and Therapeutic Targets. J. Clin. Med. 2020, 9, 1995.
- Brems, C.; Johnson, M.E.; Metzger, J.S.; Dewane, S.L. College students’ knowledge about fetal alcohol spectrum disorder. J. Popul. Ther. Clin. Pharmacol. 2014, 21, e159–e166.
- Ferraguti, G.; Pascale, E.; Lucarelli, M. Alcohol Addiction: A Molecular Biology Perspective. Curr. Med. Chem. 2015, 22, 670–684.
- Wilhoit, L.F.; Scott, D.A.; Simecka, B.A. Fetal Alcohol Spectrum Disorders: Characteristics, Complications, and Treatment. Community Ment. Health J. 2017, 53, 711–718.
- McCormack, J.C.; Chu, J.T.W.; Marsh, S.; Bullen, C. Knowledge, attitudes, and practices of fetal alcohol spectrum disorder in health, justice, and education professionals: A systematic review. Res. Dev. Disabil. 2022, 131, 104354.
- Popova, S.; Charness, M.E.; Burd, L.; Crawford, A.; Hoyme, H.E.; Mukherjee, R.A.S.; Riley, E.P.; Elliott, E.J. Fetal alcohol spectrum disorders. Nat. Rev. Dis. Primers 2023, 9, 11.
- Thomas, J.D.; Warren, K.R.; Hewitt, B.G. Fetal alcohol spectrum disorders: From research to policy. Alcohol. Res. Health 2010, 33, 118–126.
- Burd, L.; Blair, J.; Dropps, K. Prenatal alcohol exposure, blood alcohol concentrations and alcohol elimination rates for the mother, fetus and newborn. J. Perinatol. 2012, 32, 652–659.
- Micangeli, G.; Menghi, M.; Profeta, G.; Tarani, F.; Mariani, A.; Petrella, C.; Barbato, C.; Ferraguti, G.; Ceccanti, M.; Tarani, L.; et al. The Impact of Oxidative Stress on Pediatrics Syndromes. Antioxidants 2022, 11, 1983.
- Fiore, M.; Petrella, C.; Coriale, G.; Rosso, P.; Fico, E.; Ralli, M.; Greco, A.; De Vincentiis, M.; Minni, A.; Polimeni, A.; et al. Markers of Neuroinflammation in the Serum of Prepubertal Children with Fetal Alcohol Spectrum Disorders. CNS Neurol. Disord. Drug Targets 2022, 21, 854–868.
- Carvalho, C.; Moreira, P.I. Oxidative Stress: A Major Player in Cerebrovascular Alterations Associated to Neurodegenerative Events. Front. Physiol. 2018, 9, 806.
- Boschen, K.E.; Klintsova, A.Y. Neurotrophins in the Brain: Interaction With Alcohol Exposure During Development. Vitam. Horm. 2017, 104, 197–242.
- Carito, V.; Ceccanti, M.; Ferraguti, G.; Coccurello, R.; Ciafrè, S.; Tirassa, P.; Fiore, M. NGF and BDNF Alterations by Prenatal Alcohol Exposure. Curr. Neuropharmacol. 2019, 17, 308–317.
- Xia, L.P.; Shen, L.; Kou, H.; Zhang, B.J.; Zhang, L.; Wu, Y.; Li, X.J.; Xiong, J.; Yu, Y.; Wang, H. Prenatal ethanol exposure enhances the susceptibility to metabolic syndrome in offspring rats by HPA axis-associated neuroendocrine metabolic programming. Toxicol. Lett. 2014, 226, 98–105.
- Fiore, M.; Laviola, G.; Aloe, L.; di Fausto, V.; Mancinelli, R.; Ceccanti, M. Early exposure to ethanol but not red wine at the same alcohol concentration induces behavioral and brain neurotrophin alterations in young and adult mice. Neurotoxicology 2009, 30, 59–71.
- Aloe, L.; Tirassa, P. The effect of long-term alcohol intake on brain NGF-targe cells of aged rats. Alcohol 1992, 9, 299–304.
- Gupta, K.K.; Gupta, V.K.; Shirasaka, T. An Update on Fetal Alcohol Syndrome—Pathogenesis, Risks, and Treatment. Alcohol. Clin. Exp. Res. 2016, 40, 1594–1602.
- Joya, X.; Garcia-Algar, O.; Salat-Batlle, J.; Pujades, C.; Vall, O. Advances in the development of novel antioxidant therapies as an approach for fetal alcohol syndrome prevention. Birth Defects Res. A Clin. Mol. Teratol. 2015, 103, 163–177.
- Young, J.K.; Giesbrecht, H.E.; Eskin, M.N.; Aliani, M.; Suh, M. Nutrition implications for fetal alcohol spectrum disorder. Adv. Nutr. 2014, 5, 675–692.
- Petrella, C.; Carito, V.; Carere, C.; Ferraguti, G.; Ciafrè, S.; Natella, F.; Bello, C.; Greco, A.; Ralli, M.; Mancinelli, R.; et al. Oxidative stress inhibition by resveratrol in alcohol-dependent mice. Nutrition 2020, 79–80, 110783.
- Carito, V.; Ceccanti, M.; Cestari, V.; Natella, F.; Bello, C.; Coccurello, R.; Mancinelli, R.; Fiore, M. Olive polyphenol effects in a mouse model of chronic ethanol addiction. Nutrition 2017, 33, 65–69.
- Kumar, A.; Singh, C.K.; LaVoie, H.A.; DiPette, D.J.; Singh, U.S. Resveratrol restores Nrf2 Level and prevents ethanol-induced toxic effects in the cerebellum of a rodent model of fetal alcohol spectrum disorders. Mol. Pharmacol. 2011, 80, 446–457.
- Gouveri, E.; Diamantopoulos, E.J. The Mediterranean Diet and Metabolic Syndrome. In The Mediterranean Diet: An Evidence-Based Approach; Academic Press: Cambridge, MA, USA, 2015; pp. 313–323.
- Trichopoulou, A.; Lagiou, P.; Kuper, H.; Trichopoulos, D. Cancer and Mediterranean dietary traditions. Cancer Epidemiol. Biomark. Prev. 2000, 9, 869–873.
- Giacosa, A.; Barale, R.; Bavaresco, L.; Faliva, M.A.; Gerbi, V.; La Vecchia, C.; Negri, E.; Opizzi, A.; Perna, S.; Pezzotti, M.; et al. Mediterranean Way of Drinking and Longevity. Crit. Rev. Food Sci. Nutr. 2016, 56, 635–640.
- del Carmen Ramírez-Tortose, M.; Pulido-Moran, M.; Granados, S.; Gaforio, J.J.; Quiles, J.L. Hydroxytyrosol as a Component of the Mediterranean Diet and Its Role in Disease Prevention. In The Mediterranean Diet: An Evidence-Based Approach; Elsevier: Amsterdam, The Netherlands, 2015; pp. 205–215.
- Hornedo-Ortega, R.; Cerezo, A.B.; de Pablos, R.M.; Krisa, S.; Richard, T.; García-Parrilla, M.C.; Troncoso, A.M. Phenolic compounds characteristic of the mediterranean diet in mitigating microglia-mediated neuroinflammation. Front. Cell Neurosci. 2018, 12, 373.
- Bloomfield, H.E.; Koeller, E.; Greer, N.; MacDonald, R.; Kane, R.; Wilt, T.J. Effects on health outcomes of a mediterranean diet with no restriction on fat intake: A systematic review and meta-analysis. Ann. Intern. Med. 2016, 165, 491–500.
- Petrella, C.; Di Certo, M.G.; Gabanella, F.; Barbato, C.; Ceci, F.M.; Greco, A.; Ralli, M.; Polimeni, A.; Angeloni, A.; Severini, C.; et al. Mediterranean Diet, Brain and Muscle: Olive Polyphenols and Resveratrol Protection in Neurodegenerative and Neuromuscular Disorders. Curr. Med. Chem. 2021, 28, 7595–7613.
- Fiore, M.; Messina, M.P.; Petrella, C.; D’Angelo, A.; Greco, A.; Ralli, M.; Ferraguti, G.; Tarani, L.; Vitali, M.; Ceccanti, M. Antioxidant properties of plant polyphenols in the counteraction of alcohol-abuse induced damage: Impact on the Mediterranean diet. J. Funct. Foods 2020, 71, 104012.
- Sebastiani, G.; Borrás-Novell, C.; Casanova, M.A.; Pascual Tutusaus, M.; Ferrero Martínez, S.; Gómez Roig, M.D.; García-Algar, O. The Effects of Alcohol and Drugs of Abuse on Maternal Nutritional Profile during Pregnancy. Nutrients 2018, 10, 1008.
- Bosco, C.; Diaz, E. Placental hypoxia and foetal development versus alcohol exposure in pregnancy. Alcohol Alcohol. 2012, 47, 109–117.
- Gundogan, F.; Elwood, G.; Longato, L.; Tong, M.; Feijoo, A.; Carlson, R.I.; Wands, J.R.; de la Monte, S.M. Impaired placentation in fetal alcohol syndrome. Placenta 2008, 29, 148–157.
- Morrison, J.L.; Duffield, J.A.; Muhlhausler, B.S.; Gentili, S.; McMillen, I.C. Fetal growth restriction, catch-up growth and the early origins of insulin resistance and visceral obesity. Pediatr. Nephrol. 2010, 25, 669–677.
- Kesavan, K.; Devaskar, S.U. Intrauterine Growth Restriction: Postnatal Monitoring and Outcomes. Pediatr. Clin. N. Am. 2019, 66, 403–423.
- Weeks, O.; Bossé, G.D.; Oderberg, I.M.; Akle, S.; Houvras, Y.; Wrighton, P.J.; LaBella, K.; Iversen, I.; Tavakoli, S.; Adatto, I.; et al. Fetal alcohol spectrum disorder predisposes to metabolic abnormalities in adulthood. J. Clin. Investig. 2020, 130, 2252–2269.
- Cararo, J.H.; Rico, E.P. Long-lasting implications of embryonic exposure to alcohol: Insights from zebrafish research. Dev. Neurobiol. 2022, 82, 29–40.
- Shen, L.; Liu, Z.; Gong, J.; Zhang, L.; Wang, L.; Magdalou, J.; Chen, L.; Wang, H. Prenatal ethanol exposure programs an increased susceptibility of non-alcoholic fatty liver disease in female adult offspring rats. Toxicol. Appl. Pharmacol. 2014, 274, 263–273.
- Greenfield, V.; Cheung, O.; Sanyal, A.J. Recent advances in nonalcholic fatty liver disease. Curr. Opin. Gastroenterol. 2008, 24, 320–327.
- van der Linde, D.; Konings, E.E.M.; Slager, M.A.; Witsenburg, M.; Helbing, W.A.; Takkenberg, J.J.M.; Roos-Hesselink, J.W. Birth Prevalence of Congenital Heart Disease Worldwide. J. Am. Coll. Cardiol. 2011, 58, 2241–2247.
- Dolk, H.; Loane, M.; Garne, E. Congenital Heart Defects in Europe. Circulation 2011, 123, 841–849.
- Jenkins, K.J.; Correa, A.; Feinstein, J.A.; Botto, L.; Britt, A.E.; Daniels, S.R.; Elixson, M.; Warnes, C.A.; Webb, C.L.; American Heart Association Council on Cardiovascular Disease in the Young. Noninherited Risk Factors and Congenital Cardiovascular Defects: Current Knowledge. Circulation 2007, 115, 2995–3014.
- Voskoboinik, A.; Wong, G.; Lee, G.; Nalliah, C.; Hawson, J.; Prabhu, S.; Sugumar, H.; Ling, L.-H.; McLellan, A.; Morton, J.; et al. Moderate alcohol consumption is associated with atrial electrical and structural changes: Insights from high-density left atrial electroanatomic mapping. Heart Rhythm. 2019, 16, 251–259.
- Glymour, M.M. Alcohol and cardiovascular disease. BMJ 2014, 349, g4334.
- England, L.J.; Bennett, C.; Denny, C.H.; Honein, M.A.; Gilboa, S.M.; Kim, S.Y.; Guy, G.P., Jr.; Tran, E.L.; Rose, C.E.; Bohm, M.K.; et al. Alcohol Use and Co-Use of Other Substances Among Pregnant Females Aged 12–44 Years—United States, 2015–2018. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1009–1014.
- Burd, L.; Deal, E.; Rios, R.; Adickes, E.; Wynne, J.; Klug, M.G. Congenital Heart Defects and Fetal Alcohol Spectrum Disorders. Congenit. Heart Dis. 2007, 2, 250–255.
- Ungerer, M.; Knezovich, J.; Ramsay, M. In utero alcohol exposure, epigenetic changes, and their consequences. Alcohol. Res. 2013, 35, 37–46.
- Hwang, H.; Liu, R.; Eldridge, R.; Hu, X.; Forghani, P.; Jones, D.P.; Xu, C. Chronic ethanol exposure induces mitochondrial dysfunction and alters gene expression and metabolism in human cardiac spheroids. Alcohol Clin. Exp. Res. 2023, 47, 643–658.
More
Information
Subjects:
Obstetrics & Gynaecology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
534
Revisions:
2 times
(View History)
Update Date:
28 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




