Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Tigist Tadesse Shonte | -- | 1748 | 2024-02-27 09:59:12 | | | |
| 2 | Lindsay Dong | Meta information modification | 1748 | 2024-02-28 04:04:35 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Shonte, T.T.; Mulla, M.F.; Foley, L.; Pathania, S. Packaging Systems for Mushrooms. Encyclopedia. Available online: https://encyclopedia.pub/entry/55513 (accessed on 07 February 2026).
Shonte TT, Mulla MF, Foley L, Pathania S. Packaging Systems for Mushrooms. Encyclopedia. Available at: https://encyclopedia.pub/entry/55513. Accessed February 07, 2026.
Shonte, Tigist Tadesse, Mehraj Fatema Mulla, Lorraine Foley, Shivani Pathania. "Packaging Systems for Mushrooms" Encyclopedia, https://encyclopedia.pub/entry/55513 (accessed February 07, 2026).
Shonte, T.T., Mulla, M.F., Foley, L., & Pathania, S. (2024, February 27). Packaging Systems for Mushrooms. In Encyclopedia. https://encyclopedia.pub/entry/55513
Shonte, Tigist Tadesse, et al. "Packaging Systems for Mushrooms." Encyclopedia. Web. 27 February, 2024.
Copy Citation
Desirable techno-functional properties of packaging materials such as permeability and mechanical and thermal properties play a key role in maintaining quality by preventing off-flavour development, contamination, browning, and softening, thereby extending the shelf life of mushrooms. Regardless of the type of packaging system, they all play an important role in maintaining or improving the quality of mushrooms and extending their shelf life. Each packaging system has unique performance characteristics defining their strengths/benefits in terms of preserving edible mushrooms, safety, and circular economy impact.
mushrooms
nanopackaging
biodegradable packaging
edible coatings
1. Introduction
Agaricus bisporus, Lentinus edodes, Pleurotus ostreatus, Flammulina velutipes, and Pleurotus eryngii are common edible mushroom species found in mainstream foods [1]. Because of its great flavor and nutritional value, Agaricus bisporus, commonly referred to as “white button mushroom”, is the most widely grown and consumed edible mushroom in the world, making up 30% of all mushroom production [2]. It is a rich source of nutrients including protein, amino acids, and dietary fiber [3][4][5], antioxidants [5][6], terpenoids [7], lectins [8], phenolic compounds [5][9], polysaccharides [5][10], and ergosterols [11][12].
Edible mushroom production and trade are expanding globally, with a significant positive impact on human living standards [13]. According to the Global Mushroom Market (2023–2030) research report, the compound annual growth rate (CAGR) of the mushroom market increased globally by 9.2% from USD 57.18 billion in 2022 to USD 62.44 billion in 2023. At a CAGR of 9.8%, the mushroom market is projected to reach USD 90.88 billion in 2027. Teagasc Fact sheet Horticulture reported that around 68,000 tonnes of Agaricus bisporus are produced annually in Ireland, nearly all of which are exported to the UK, with 20% used to supply the domestic market [14]. The fact sheet states that the mushroom industry contributes to the Irish economy with a production value of approximately EUR 130 million in 2022. However, exporting to continental Europe is not feasible due to the short shelf life of mushrooms, as they typically only last three days at ambient conditions and five to eight days in a cold storage system [15], as well as to the narrow margins that the industry faces.
The main contributing factors to the short shelf life of mushrooms are their high moisture content and enzyme activity coupled with their lack of a cuticle [16], which aggravates the respiratory and metabolic rates of the seeds’ tender tissues. Additionally, these factors make mushrooms more vulnerable to mechanical damage and microbial contamination, which can result in browning and a decline in quality [17][18][19]. These effects involve physicochemical quality degradation, including cap opening, loss of essential phenolic compounds, proteins, and vitamins, water loss, cell membrane deterioration, loss of firmness, and increased microbial activity in the process of storage and transportation [20][21], in turn leading to loss of nutritional value, flavor, market acceptability value, and shelf life. Recent findings have reported a wide range of preservation techniques for fresh mushrooms, including irradiation [22], ultrasonication [22], pulsed eclectic field treatment [23][24], [25][26], 1-methylcyclopropene treatment [27], modified atmosphere packaging, active packaging, edible coatings and nanopackaging [1][15][28][29][30][31], and biodegradable packaging made from materials such as dextran/chitosan [32].
More specifically, edible coatings and biodegradable packaging are safe and environmentally friendly packaging systems; they are made from natural substances such as pectin, chitosan, or sodium alginate to maintain quality and extend the shelf life of mushrooms [32][33][34][35][36], and are mostly composed of active ingredients with antibacterial and antioxidant activities [37][38]. Studies have shown that the incorporation of essential oils, active ingredients, and nanoparticles in edible coatings and biodegradable film packaging can improve the techno-functional properties of the packaging materials [39]. Furthermore, tyrosine inhibitors such as plant extracts, fungus and bacterial extracts, and synthetic and natural phenolic compounds are utilized to control mushroom browning [40][41][42][43].
2. Mechanisms of Action and Preservation Effects
2.1. Changes in Quality of Fresh Mushrooms
Fresh mushrooms are flavorful, have a moisture content ranging from 81.8% to 94.8%, and are rich sources of nutrients such as carbohydrates (50%–65%), protein (19%–35%), fat (2%–6%), minerals, dietary fiber, phenolic compounds, and vitamins [44]. A wide range of studies have shown change in quality of fresh mushrooms during storage, including texture [45][46][47], color [16][48][49][50], nutrients, and flavor [17][45][47][51][52][53]. These quality changes in mushrooms are attributed to the cumulative effects of respiratory, energy, membrane lipids, and reactive oxygen species metabolic reactions due to changes in enzymatic activity and microbial activity in response to intrinsic factors and the atmosphere surrounding the product. For example, the transport of electrons in mitochondria cells results in excessive reactive oxygen species accumulation such as H2O2 and O2− in mushroom [54]. These processes can lead to oxidative damage to nutrients such as membrane lipids, nucleic acids, and proteins as well as enzyme activity inhibition, leakage of electrolytes, and increased electrical conductivity, ultimately causing tissue aging, nutritional quality loss, and reduced shelf life of mushrooms [46][55].
Figure 1 provides an overview of changes in mushroom quality along with the mechanisms of action and preservation effects of five major packaging systems for the preservation of fresh edible mushrooms: edible coatings, modified atmosphere packaging, active packaging, biodegradable packaging, and nanopackaging. For instance, edible coatings and biodegradable packaging made with active ingredients can successfully delay or minimize browning reactions by inhibiting tyrosinase and polyphenol oxidase activities in fresh mushrooms [56][57][58][59].
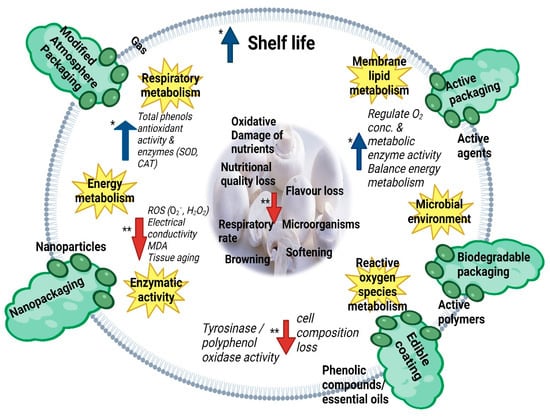
Figure 1. Mechanisms of action and preservation effects of active packaging, edible coating, biodegradable packaging, modified atmosphere packaging, and nanopackaging for the preservation of fresh edible mushrooms. CAT—catalase, H2O2—hydrogen peroxide, MDA—malondialdehyde, O2−—superoxide radical, ROS—reactive oxygen species, SOD—superoxide dismutase, * an increase, ** a decrease.
Nanopackaging minimizes tissue aging, electrical conductivity, and the accumulation of reactive oxygen species (ROS) by controlling the energy metabolism and enzymatic activity of mushrooms [29]. MAP lowers the rate of respiratory metabolism, thereby reducing the loss of cell wall components and cell swelling [60]. By regulating metabolic enzyme activity, oxygen concentration, and energy metabolism, active packaging can lessen membrane lipid metabolism and microbial growth, thereby preventing loss of nutrients and flavor from mushrooms [30][61]. The mechanisms of action of packaging systems can be explained by enzymatic, (such as tyrosinase and polyphenol oxidase activity), antimicrobial, antioxidant, and respiration activity [59][62][63]. The following sections highlight the mechanisms of action and preservation effects of edible coatings, MAP, active packaging, biodegradable packaging, and nanopackaging.
2.2. Edible Coatings, Essential Oils, and Tyrosinase Inhibitors
Edible coatings have moderate to excellent barrier, preservative, cosmetic, and aesthetic qualities, are biocompatible and environmentally friendly, and can usually be consumed with food [17]. Edible coatings combined with active ingredients can supply bioactive compounds to enhance the quality of edible mushrooms or prolong their shelf life, in addition to superior qualities of high air permeability and moisture permeability [64]. Chitosan, guar gum, sodium alginate, aloe vera, leek powder, pectin, carboxymethyl, and cellulose are commonly used as edible coating materials for edible mushrooms; they are based on natural biopolymers with essential oils, nanoparticles, and active ingredients such as cinnamon (Table 1). For example, the mixture of chitosan with guar gum can significantly increase antimicrobial activity, reduce cell wall and membrane destructive symptoms, and maintain higher firmness, protein, and ascorbic acid while increasing total soluble solids and reducing sugars of Lentinus edodes mushrooms [65]. Edible coatings made from a cinnamon nanoemulsion active ingredient in polymeric matrixes of alginate and glycerol significantly decreased the respiration rate, PPO activity, pseudomonas count, and weight loss while increasing antioxidant activity and maintaining firmness, color, and total polyphenols of Agaricus bisporus mushrooms [66].
Table 1. Mechanism of actions and preservation effects of edible coatings of fresh edible mushrooms.
| Edible Coating | Applicable | Mechanism of Actions a | Preservation Effects b | Reference |
|---|---|---|---|---|
| Chitosan-guar gum | Lentinus edodes | Significantly reduced cell wall and membrane destructive symptoms Increased antimicrobial activity |
Maintained higher firmness, protein, and ascorbic acid Increased total soluble solids and reducing sugars |
[65] |
| Alginate-glycerol-cinnamon nanoemulsions | Agaricus bisporus | Decreased respiration rate Reduced polyphenol oxidase activity Reduced Pseudomonas counts Increased antioxidant activity |
Decreased weight loss Maintained firmness Maintained colour and total polyphenols |
[66] |
| Pectin-chitosan-sodium alginate- carboxymethyl cellulose- N-acetyl cysteine |
Agaricus bisporus | Controlled lipid peroxidation Increased antioxidant activity |
Delayed weight loss and cap opening | [67] |
| Aloe vera-basil essential oil | Agaricus bisporus | Reduced polyphenol oxidase, respiration, and electrolyte leakage rate Increased phenylalanine ammonia-lyase and antioxidant activity |
Reduced weight loss and softening Increased total phenolic contents Delayed browning and colour change |
[68] |
| Leek powder sunflower oil-guar gum | Agaricus bisporus | Reduced the rate of respiration | Reduced weight loss Maintained colour |
[69] |
| Alginate-nanoAg-Silver nitrate-sodium Borohydride-polyvinylpyrrolidone |
Lentinus edodes | Reduced the rate of respiration and physiological activity | Extended shelf life Reduced weight loss softening, browning, and microbial counts. Increased total soluble solids |
[70] |
a respiration and energy metabolism, antimicrobial activity, antioxidant activity; b nutritional value, shelf life, and sensory quality.
Table 2 shows the effects incorporation of different sources of essential oils in edible coatings on the shelf life of fresh mushroom. A recent study showed that an edible coating with cajuput (Melaleuca cajuputi Powell.) essential oil extract minimized weight loss and respiration rate while maintaining firmness, color, and fungal antioxidant metabolites, and had a shelf life of 12 days [28]. Furthermore, edible coatings of mushroom with Citrus aurantium essential oil provided 20 days of shelf life [71], and Eucalyptus leaf essential oil provided 12 days of shelf life [61][72].
Table 2. The effects of different sources of essential oils in edible coatings on the shelf life of fresh mushrooms.
| Essential Oils | Shelf Life | References |
|---|---|---|
| Eucalyptus leaf | 12 | [61][72] |
| Lemon | 12 | [45] |
| Cinnamon | 5 | [73][74] |
| Tocopherol with zein | 12 | [75] |
| Cinnamaldehyde | 12 | [66] |
| Satureja khuzistanica | 16 | [76] |
| Citrus aurantium peel | 20 | [77] |
| Cumin seed | 20 | [77] |
| Cuminum cyminum | 20 | [77] |
| Citrus aurantium | 20 | [71] |
| Melaleuca cajuputi Powell. | 12 | [28] |
Steroids, alkaloids, and phenolic compounds make up the majority of the diverse range of tyrosinase inhibitors isolated from plant sources and fungi, and are frequently incorporated in polymeric matrixes of edible coatings. Phenolic compounds, which can range in size from simple to large and complex tannins and derived polyphenols, display strong tyrosinase inhibition because of their molecular weight and quantity of aromatic rings [42][56][59]. To identify new sources of anti-tyrosinase compounds, research has been performed on the tyrosinase inhibitory activity of several plant extracts [59][78], and all significantly inhibited tyrosinase activity.
A smaller class of alkaloids and polyphenols found in fungi, including Aspergillus sp., Paecilomyces sp., Trichoderma sp., Phellinus linteus, Daedalea dickinsii, and Dictyophora indusiata, have been reported to selectively block the enzyme and are a source of novel tyrosinase inhibitors [40][41]. Studies have shown that four distinct strains of lactic acid bacteria isolated from cow faeces exhibit tyrosinase inhibitory activity [79]. For instance, the most active compounds within the group of natural flavones, flavanols, isoflavones, and flavanones inhibited mushroom tyrosinase with an IC50 of 44–500 μM, while natural anthocyanidins, aurones, and chalcones had an IC50 ranging from 18 to 106.7 μM, which was in comparison to kojic acid (a potent inhibitor of tyrosinase) with a tyrosinase inhibitory activity of IC50 of 59–318 μM (Table 3).
Table 3. Natural and synthetic phenolic compounds at half-maximal inhibitory concentration (IC50) values against white button mushroom tyrosinase.
| Compound Name | Tyrosinase Inhibition (IC50 Values (mM)) |
References |
|---|---|---|
| Natural anthocyanidins | 18–78 | [80][81] |
| Natural aurones | 31.7–98.5 | [81] |
| Synthetic aurones | 31–100 | [82] |
| Natural chalcones | 23–106.7 | [58] |
| Synthetic chalcones | 29.3–114.4 | [56][83] |
| Natural flavones | 110 | [56][57][58][59] |
| Natural flavanols | 55–300 | [56][57] |
| Natural isoflavones | 52–500 | [59] |
| Natural flavanones | 44–500 | [80][81] |
| Synthetic flavonols | 53–182 | [81] |
| Kojic acid | 59–318 | [56][57][58][59][84] |
References
- Huo, J.; Zhang, M.; Wang, D.S.; Mujumdar, A.; Bhandari, B.; Zhang, L. New preservation and detection technologies for edible mushrooms: A review. J. Sci. Food Agric. 2023, 103, 3230–3248.
- Muszyńska, B.; Kała, K.; Sułkowska-Ziaja, K.; Krakowska, A.; Opoka, W. Agaricus bisporus and its in vitro culture as a source of indole compounds released into artificial digestive juices. Food Chem. 2016, 199, 509–515.
- Guo, Y.; Chen, X.; Gong, P. Classification, structure and mechanism of antiviral polysaccharides derived from edible and medicinal fungus. Int. J. Biol. Macromol. 2021, 183, 1753–1773.
- Sun, B.; Chen, X.; Xin, G.; Qin, S.; Chen, M.; Jiang, F. Effect of 1-methylcyclopropene (1-MCP) on quality of button mushrooms (Agaricus bisporus) packaged in different packaging materials. Postharvest Biol. Technol. 2020, 159, 111023.
- Das, A.K.; Nanda, P.K.; Dandapat, P.; Bandyopadhyay, S.; Gullón, P.; Sivaraman, G.K.; McClements, D.J.; Gullón, B.; Lorenzo, J.M. Edible mushrooms as functional ingredients for development of healthier and more sustainable muscle foods: A flexitarian approach. Molecules 2021, 26, 2463.
- Taşkın, H.; Süfer, Ö.; Attar, Ş.H.; Bozok, F.; Baktemur, G.; Büyükalaca, S.; Kafkas, N.E. Total phenolics, antioxidant activities and fatty acid profiles of six Morchella species. J. Food Sci. Technol. 2021, 58, 692–700.
- Dasgupta, A.; Acharya, K. Mushrooms: An emerging resource for therapeutic terpenoids. 3 Biotech 2019, 9, 396.
- El-Maradny, Y.A.; El-Fakharany, E.M.; Abu-Serie, M.M.; Hashish, M.H.; Selim, H.S. Lectins purified from medicinal and edible mushrooms: Insights into their antiviral activity against pathogenic viruses. Int. J. Biol. Macromol. 2021, 179, 239–258.
- Acar, M.; Ayan, A.K.; Aytaç, S.; Arslanoglu, Ş.F. Morphological Characterization of Nettle Lines Collected in of International. In Proceedings of the International Biological, Agricultural and Life Science Congress, Lviv, Ukrain, 7–8 November 2019; pp. 493–499.
- Letizia, F.C.M.; Giuseppe, G.; Giuseppe, P.; Fortunato, V.; Ferraro, C.V.; Cateni, F.; Procida, Á.G.; Procida, G.; Gargano, M.L.; Venturella, G.; et al. Mycochemicals in wild and cultivated mushrooms: Nutrition and health. Phytochem. Rev. 2021, 21, 339–383.
- Saini, R.K.; Rauf, A.; Khalil, A.A.; Ko, E.Y.; Keum, Y.S.; Anwar, S.; Alamri, A.; Rengasamy, K.R.R. Edible mushrooms show significant differences in sterols and fatty acid compositions. S. Afr. J. Bot. 2021, 141, 344–356.
- Nowak, R.; Nowacka-Jechalke, N.; Pietrzak, W.; Gawlik-Dziki, U. A new look at edible and medicinal mushrooms as a source of ergosterol and ergosterol peroxide—UHPLC-MS/MS analysis. Food Chem. 2022, 369, 130927.
- Sun, Y.; Zhang, M.; Fang, Z. Efficient physical extraction of active constituents from edible fungi and their potential bioactivities: A review. Trends Food Sci. Technol. 2020, 105, 468–482.
- The Irish Mushroom Industry. Teagasc Fact Sheet Hortic 7-Mushroom Prod. 2020. (V1 2020). Available online: https://www.teagasc.ie/crops/horticulture/mushrooms/ (accessed on 26 January 2024).
- Castellanos-Reyes, K.; Villalobos-Carvajal, R.; Beldarrain-Iznaga, T. Fresh mushroom preservation techniques. Foods 2021, 10, 2126.
- Gholami, R.; Ahmadi, E.; Farris, S. Shelf life extension of white mushrooms (Agaricus bisporus) by low temperatures conditioning, modified atmosphere, and nanocomposite packaging material. Food Packag. Shelf Life 2017, 14, 88–95.
- Marçal, S.; Sousa, A.S.; Taofiq, O.; Antunes, F.; Morais, A.M.M.B.; Freitas, A.C.; Barros, L.; Ferreira, I.C.F.R.; Pintado, M. Impact of postharvest preservation methods on nutritional value and bioactive properties of mushrooms. Trends Food Sci. Technol. 2021, 110, 418–431.
- Wang, Q.; Chen, W.; Zhu, W.; McClements, D.J.; Liu, X.; Liu, F. A review of multilayer and composite films and coatings for active biodegradable packaging. NPJ Sci. Food 2022, 6, 18.
- Zhang, Y.; Venkitasamy, C.; Pan, Z.; Wang, W. Recent developments on umami ingredients of edible mushrooms—A review. Trends Food Sci. Technol. 2013, 33, 78–92.
- Xue, Z.; Hao, J.; Yu, W.; Kou, X. Effects of Processing and Storage Preservation Technologies on Nutritional Quality and Biological Activities of Edible Fungi: A Review. J. Food Process Eng. 2017, 40, e12437.
- Zhang, K.; Pu, Y.Y.; Sun, D.W. Recent advances in quality preservation of postharvest mushrooms (Agaricus bisporus): A review. Trends Food Sci. Technol. 2018, 78, 72–82.
- Shi, D.; Yin, C.; Fan, X.; Yao, F.; Qiao, Y.; Xue, S.; Lu, Q.; Feng, C.; Meng, J.; Gao, H. Effects of ultrasound and gamma irradiation on quality maintenance of fresh Lentinula edodes during cold storage. Food Chem. 2022, 373, 131478.
- Lagnika, C.; Zhang, M.; Mothibe, K.J. Effects of ultrasound and high pressure argon on physico-chemical properties of white mushrooms (Agaricus bisporus) during postharvest storage. Postharvest Biol. Technol. 2013, 82, 87–94.
- Yan, M.; Yuan, B.; Xie, Y.; Cheng, S.; Huang, H.; Zhang, W.; Chen, J.; Cao, C. Improvement of postharvest quality, enzymes activity and polyphenoloxidase structure of postharvest Agaricus bisporus in response to high voltage electric field. Postharvest Biol. Technol. 2020, 166, 111230.
- Huang, H.W.; Hsu, C.P.; Yang, B.B.; Wang, C.Y. Advances in the extraction of natural ingredients by high pressure extraction technology. Trends Food Sci. Technol. 2013, 33, 54–62.
- Zhang, J.; Yu, X.; Xu, B.; Yagoub, A.E.A.; Mustapha, A.T.; Zhou, C. Effect of intensive pulsed light on the activity, structure, physico-chemical properties and surface topography of polyphenol oxidase from mushroom. Innov. Food Sci. Emerg. Technol. 2021, 72, 102741.
- Xu, F.; Liu, Y.; Shan, X.; Wang, S. Evaluation of 1-methylcyclopropene (1-MCP) treatment combined with nano-packaging on quality of pleurotus eryngii. J. Food Sci. Technol. 2018, 55, 4424–4431.
- Chaudhari, A.K.; Das, S.; Singh, B.K.; Kishore Dubey, N. Green facile synthesis of cajuput (Melaleuca cajuputi Powell.) essential oil loaded chitosan film and evaluation of its effectiveness on shelf-life extension of white button mushroom. Food Chem. 2023, 401, 134114.
- Feng, Y.; Xu, H.; Sun, Y.; Xia, R.; Hou, Z.; Li, Y.; Wang, Y.; Pan, S.; Fan, Y.; Zhu, J.; et al. Review of packaging for improving storage quality of fresh edible mushrooms. Packag. Technol. Sci. 2023, 36, 629–646.
- Guo, Y.; Chen, X.; Gong, P.; Wang, R.; Qi, Z.; Deng, Z.; Han, A.; Long, H.; Wang, J.; Yao, W.; et al. Advances in Postharvest Storage and Preservation Strategies for Pleurotus eryngii. Foods 2023, 12, 1046.
- Liu, K.; Chen, Y.Y.; Pan, L.H.; Li, Q.M.; Luo, J.P.; Zha, X.Q. Co-encapsulation systems for delivery of bioactive ingredients. Food Res. Int. 2022, 155, 111073.
- Díaz-Montes, E.; Castro-Muñoz, R. Edible films and coatings as food-quality preservers: An overview. Foods 2021, 10, 249.
- Kumar, N.; Pratibha; Prasad, J.; Yadav, A.; Upadhyay, A.; Neeraj; Shukla, S.; Petkoska, A.T.; Heena; Suri, S.; et al. Recent Trends in Edible Packaging for Food Applications—Perspective for the Future. Food Eng. Rev. 2023, 15, 718–747.
- Matloob, A.; Ayub, H.; Mohsin, M.; Ambreen, S.; Khan, F.A.; Oranab, S.; Rahim, M.A.; Khalid, W.; Nayik, G.A.; Ramniwas, S.; et al. A Review on Edible Coatings and Films: Advances, Composition, Production Methods, and Safety Concerns. ACS Omega 2023, 8, 28932–28944.
- Liyanapathiranage, A.; Dassanayake, R.S.; Gamage, A.; Karri, R.R.; Manamperi, A.; Evon, P.; Jayakodi, Y.; Madhujith, T.; Merah, O. Recent Developments in Edible Films and Coatings for Fruits and Vegetables. Coatings 2023, 13, 1177.
- Ribeiro, I.S.; Maciel, G.M.; Bortolini, D.G.; de Andrade Arruda Fernande, I.; Maroldi, W.V.; Pedro, A.C.; Rubio, F.T.V.; Haminiuk, C.W.I. Sustainable innovations in edible films and coatings: An overview. Trends Food Sci. Technol. 2024, 143, 104272.
- Guimarães, J.E.R.; de la Fuente, B.; Pérez-Gago, M.B.; Andradas, C.; Carbó, R.; Mattiuz, B.H.; Palou, L. Antifungal activity of GRAS salts against Lasiodiplodia theobromae in vitro and as ingredients of hydroxypropyl methylcellulose-lipid composite edible coatings to control Diplodia stem-end rot and maintain postharvest quality of citrus fruit. Int. J. Food Microbiol. 2019, 301, 9–18.
- Tahir, H.E.; Xiaobo, Z.; Mahunu, G.K.; Arslan, M.; Abdalhai, M.; Zhihua, L. Recent developments in gum edible coating applications for fruits and vegetables preservation: A review. Carbohydr. Polym. 2019, 224, 115141.
- Dierings de Souza, E.J.; Kringel, D.H.; Guerra Dias, A.R.; da Rosa Zavareze, E. Polysaccharides as wall material for the encapsulation of essential oils by electrospun technique. Carbohydr. Polym. 2021, 265, 118068.
- He, Y.; Suyama, T.L.; Kim, H.; Glukhov, E.; Gerwick, W.H. Discovery of Novel Tyrosinase Inhibitors from Marine Cyanobacteria. Front. Microbiol. 2022, 13, 912621.
- Hwang, C.Y.; Halim, Y.; Sugata, M.; Rosa, D.; Wijaya, S.P.; Steven, E. Assessment of Agaricus bisporus mushroom as protective agent against ultraviolet exposure. bioRxiv 2021.
- Nazir, Y.; Rafique, H.; Roshan, S.; Shamas, S.; Ashraf, Z.; Rafiq, M.; Tahir, T.; Qureshi, Z.U.R.; Aslam, A.; Asad, M.H.H. Bin Molecular Docking, Synthesis, and Tyrosinase Inhibition Activity of Acetophenone Amide: Potential Inhibitor of Melanogenesis. Biomed Res. Int. 2022, 2022, 1040693.
- Obaid, R.J.; Mughal, E.U.; Naeem, N.; Sadiq, A.; Alsantali, R.I.; Jassas, R.S.; Moussa, Z.; Ahmed, S.A. Natural and synthetic flavonoid derivatives as new potential tyrosinase inhibitors: A systematic review. RSC Adv. 2021, 11, 22159–22198.
- Rathore, H.; Prasad, S.; Sharma, S. Mushroom nutraceuticals for improved nutrition and better human health: A review. PharmaNutrition 2017, 5, 35–46.
- Wang, L.; Zhou, Y.; Wang, Y.Y.; Bu, H.; Dong, T. Changes in cell wall metabolism and flavor qualities of mushrooms (Agaricus bernardii) under EMAP treatments during storage. Food Packag. Shelf Life 2021, 29, 100732.
- Li, Y.; Ding, S.; Kitazawa, H.; Wang, Y. Storage temperature effect on quality related with cell wall metabolism of shiitake mushrooms (Lentinula edodes) and its modeling. Food Packag. Shelf Life 2022, 32, 100865.
- Liu, Q.; Cui, X.; Song, Z.; Kong, W.; Kang, Y.; Kong, W.; Ng, T.B. Coating shiitake mushrooms (Lentinus edodes) with a polysaccharide from Oudemansiella radicata improves product quality and flavor during postharvest storage. Food Chem. 2021, 352, 129357.
- Dokhanieh, A.Y.; Aghdam, M.S. Postharvest browning alleviation of Agaricus bisporus using salicylic acid treatment. Sci. Hortic. 2016, 207, 146–151.
- Sun, B.; Ren, H.; Chen, X.; Ma, F.; Yu, G.; Chen, M.; Jiang, F. Short-term anaerobic treatment combined with perforation mediated MAP on the quality of Agaricus bisporus mushroom. Postharvest Biol. Technol. 2021, 176, 111518.
- Fu, Y.; Yu, Y.; Tan, H.; Wang, B.; Peng, W.; Sun, Q. Metabolomics reveals dopa melanin involved in the enzymatic browning of the yellow cultivars of East Asian golden needle mushroom (Flammulina filiformis). Food Chem. 2022, 370, 131295.
- Wang, T.; Yun, J.; Zhang, Y.; Bi, Y.; Zhao, F.; Niu, Y. Effects of ozone fumigation combined with nano-film packaging on the postharvest storage quality and antioxidant capacity of button mushrooms (Agaricus bisporus). Postharvest Biol. Technol. 2021, 176, 111501.
- Donglu, F.; Wenjian, Y.; Kimatu, B.M.; Liyan, Z.; Xinxin, A.; Qiuhui, H. Comparison of flavour qualities of mushrooms (Flammulina velutipes) packed with different packaging materials. Food Chem. 2017, 232, 1–9.
- Sun, L.B.; Zhang, Z.Y.; Xin, G.; Sun, B.X.; Bao, X.J.; Wei, Y.Y.; Zhao, X.M.; Xu, H.R. Advances in umami taste and aroma of edible mushrooms. Trends Food Sci. Technol. 2020, 96, 176–187.
- Wang, Y.; Mo, Y.; Li, D.; Xiang, C.; Jiang, Z.; Wang, J. The main factors inducing postharvest lignification in king oyster mushrooms (Pleurotus eryngii): Wounding and ROS-mediated senescence. Food Chem. 2019, 301, 125224.
- Wang, Z.; Chen, L.; Yang, H.; Wang, A. Effect of exogenous glycine betaine on qualities of button mushrooms (Agaricus bisporus) during postharvest storage. Eur. Food Res. Technol. 2015, 240, 41–48.
- Chang, T.S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475.
- Hu, K.; Dars, A.G.; Liu, Q.; Xie, B.; Sun, Z. Phytochemical profiling of the ripening of Chinese mango (Mangifera indica L.) cultivars by real-time monitoring using UPLC-ESI-QTOF-MS and its potential benefits as prebiotic ingredients. Food Chem. 2018, 256, 171–180.
- Guo, N.; Wang, C.; Shang, C.; You, X.; Zhang, L.; Liu, W. Integrated study of the mechanism of tyrosinase inhibition by baicalein using kinetic, multispectroscopic and computational simulation analyses. Int. J. Biol. Macromol. 2018, 118, 57–68.
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2019, 34, 279–309.
- Primožič, M.; Knez, Ž.; Leitgeb, M. (Bio)nanotechnology in food science—Food packaging. Nanomaterials 2021, 11, 292.
- Chen, M.; Yan, X.; Cheng, M.; Zhao, P.; Wang, Y.; Zhang, R.; Wang, X.; Wang, J.; Chen, M. Preparation, characterization and application of poly(lactic acid)/corn starch/eucalyptus leaf essential oil microencapsulated active bilayer degradable film. Int. J. Biol. Macromol. 2022, 195, 264–273.
- Jafarzadeh, S.; Mahdi, S.; Salehabadi, A.; Mohammadi, A.; Uthaya, U.S.; Khalil, H.P.S.A. Trends in Food Science & Technology Biodegradable green packaging with antimicrobial functions based on the bioactive compounds from tropical plants and their by-products. Trends Food Sci. Technol. 2020, 100, 262–277.
- Correa, M.G.; Martínez, F.B.; Vidal, C.P.; Streitt, C.; Escrig, J.; de Dicastillo, C.L. Antimicrobial metal-based nanoparticles: A review on their synthesis, types and antimicrobial action. Beilstein J. Nanotechnol. 2020, 11, 1450–1469.
- Amin, U.; Khan, M.U.; Majeed, Y.; Rebezov, M.; Khayrullin, M.; Bobkova, E.; Shariati, M.A.; Chung, I.M.; Thiruvengadam, M. Potentials of polysaccharides, lipids and proteins in biodegradable food packaging applications. Int. J. Biol. Macromol. 2021, 183, 2184–2198.
- Huang, Q.; Qian, X.; Jiang, T.; Zheng, X. Effect of chitosan and guar gum based composite edible coating on quality of mushroom (Lentinus edodes) during postharvest storage. Sci. Hortic. 2019, 253, 382–389.
- Louis, E.; Villalobos-Carvajal, R.; Reyes-Parra, J.; Jara-Quijada, E.; Ruiz, C.; Andrades, P.; Gacitúa, J.; Beldarraín-Iznaga, T. Preservation of mushrooms (Agaricus bisporus) by an alginate-based-coating containing a cinnamaldehyde essential oil nanoemulsion. Food Packag. Shelf Life 2021, 28, 100662.
- Pleșoianu, A.M.; Nour, V. Effect of Some Polysaccharide-Based Edible Coatings on Fresh White Button Mushroom (Agaricus bisporus) Quality during Cold Storage. Agriculture 2022, 12, 1491.
- Mohammadi, L.; Hassanzadeh Khankahdani, H.; Tanaka, F.; Tanaka, F. Postharvest shelf-life extension of button mushroom (Agaricus bisporus L.) by aloe vera gel coating enriched with basil essential oil. Environ. Control Biol. 2021, 59, 87–98.
- Yazıcıoğlu, N. Effects of Leek Powder and Sunflower Oil in Guar Gum Edible Coating on the Preservation of Mushrooms (Agaricus bisporus). Turk. J. Agric. Food Sci. Technol. 2023, 11, 2533–2539.
- Gao, M.; Feng, L.; Jiang, T. Browning inhibition and quality preservation of button mushroom (Agaricus bisporus) by essential oils fumigation treatment. Food Chem. 2014, 149, 107–113.
- Karimirad, R.; Behnamian, M.; Dezhsetan, S. Bitter orange oil incorporated into chitosan nanoparticles: Preparation, characterization and their potential application on antioxidant and antimicrobial characteristics of white button mushroom. Food Hydrocoll. 2020, 100, 105387.
- Guo, Y.; Chen, X.; Gong, P.; Wang, R.; Han, A.; Deng, Z.; Qi, Z.; Long, H.; Wang, J.; Yao, W.; et al. Advances in the Role and Mechanisms of Essential Oils and Plant Extracts as Natural Preservatives to Extend the Postharvest Shelf Life of Edible Mushrooms. Foods 2023, 12, 801.
- Niu, Y.; Yun, J.; Bi, Y.; Wang, T.; Zhang, Y.; Liu, H.; Zhao, F. Predicting the shelf life of postharvest Flammulina velutipes at various temperatures based on mushroom quality and specific spoilage organisms. Postharvest Biol Technol. 2020, 167, 111235.
- Shao, P.; Yu, J.; Chen, H.; Gao, H. Development of microcapsule bioactive paper loaded with cinnamon essential oil to improve the quality of edible fungi. Food Packag. Shelf. Life 2021, 27, 100617.
- Zhang, R.; Belwal, T.; Li, L.; Lin, X.; Xu, Y.; Luo, Z. Recent advances in polysaccharides stabilized emulsions for encapsulation and delivery of bioactive food ingredients: A review. Carbohydr. Polym. 2020, 242, 116388.
- Nasiri, M.; Barzegar, M.; Sahari, M.A.; Niakousari, M. Tragacanth gum containing Zataria multiflora Boiss. essential oil as a natural preservative for storage of button mushrooms (Agaricus bisporus). Food Hydrocoll. 2017, 72, 202–209.
- Karimirad, R.; Behnamian, M.; Dezhsetan, S. Application of chitosan nanoparticles containing Cuminum cyminum oil as a delivery system for shelf life extension of Agaricus bisporus. LWT 2019, 106, 218–228.
- Huang, W.-Y.; Cai, Y.-Z.; Zhang, Y. Natural phenolic compounds from medicinal herbs and dietary plants: Potential use for cancer prevention. Nutr. Cancer 2010, 62, 1–20.
- Ji, K.; Cho, Y.S.; Kim, Y.T. Tyrosinase Inhibitory and Anti-oxidative Effects of Lactic Acid Bacteria Isolated from Dairy Cow Feces. Probiotics Antimicrob. Proteins 2018, 10, 43–55.
- Loizzo, M.R.; Tundis, R.; Menichini, F. Natural and Synthetic Tyrosinase Inhibitors as Antibrowning Agents: An Update. Compr. Rev. Food Sci. Food Saf. 2012, 11, 378–398.
- Haudecoeur, R.; Carotti, M.; Gouron, A.; Maresca, M.; Buitrago, E.; Hardre, R.; Bergantino, E.; Jamet, H.N.; Belle, C.; Reglier, M.; et al. 2-Hydroxypyridine- N-oxide-Embedded Aurones as Potent Human Tyrosinase Inhibitors. ACS Med. Chem. Lett. 2016, 8, 55–60.
- Lee, H.; Yildiz, G.; dos Santos, L.C.; Jiang, S.; Andrade, J.E.; Engeseth, N.J.; Feng, H. Soy protein nano-aggregates with improved functional properties prepared by sequential pH treatment and ultrasonication. Food Hydrocoll. 2016, 55, 200–209.
- Chen, K.; Zhao, D.Y.; Chen, Y.L.; Wei, X.Y.; Li, Y.T.; Kong, L.M.; Hider, R.C.; Zhou, T. A Novel Inhibitor Against Mushroom Tyrosinase with a Double Action Mode and Its Application in Controlling the Browning of Potato. Food Bioprocess Technol. 2017, 10, 2146–2155.
- Deshmukh, B.S.; Waghmode, A. Role of wild edible fruits as a food resource: Traditional knowledge. Int. J. Pharm. Life Sci. 2011, 2, 919–924.
More
Information
Subjects:
Food Science & Technology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
831
Revisions:
2 times
(View History)
Update Date:
28 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




