
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Lu Chen | -- | 1873 | 2024-02-27 02:39:15 | | | |
| 2 | Peter Tang | Meta information modification | 1873 | 2024-02-27 03:31:07 | | |
Video Upload Options
Pyrrolizidine alkaloids (PAs) are naturally occurring secondary metabolites of plants. More than 660 types of PAs have been identified from an estimated 6000 plants, and approximately 120 of these PAs are hepatotoxic. As a result of PAs being found in spices, herbal teas, honey, and milk, PAs are considered contaminants in foods, posing a potential risk to human health.
1. Introduction
2. Chemical Structure and Toxicity of PAs
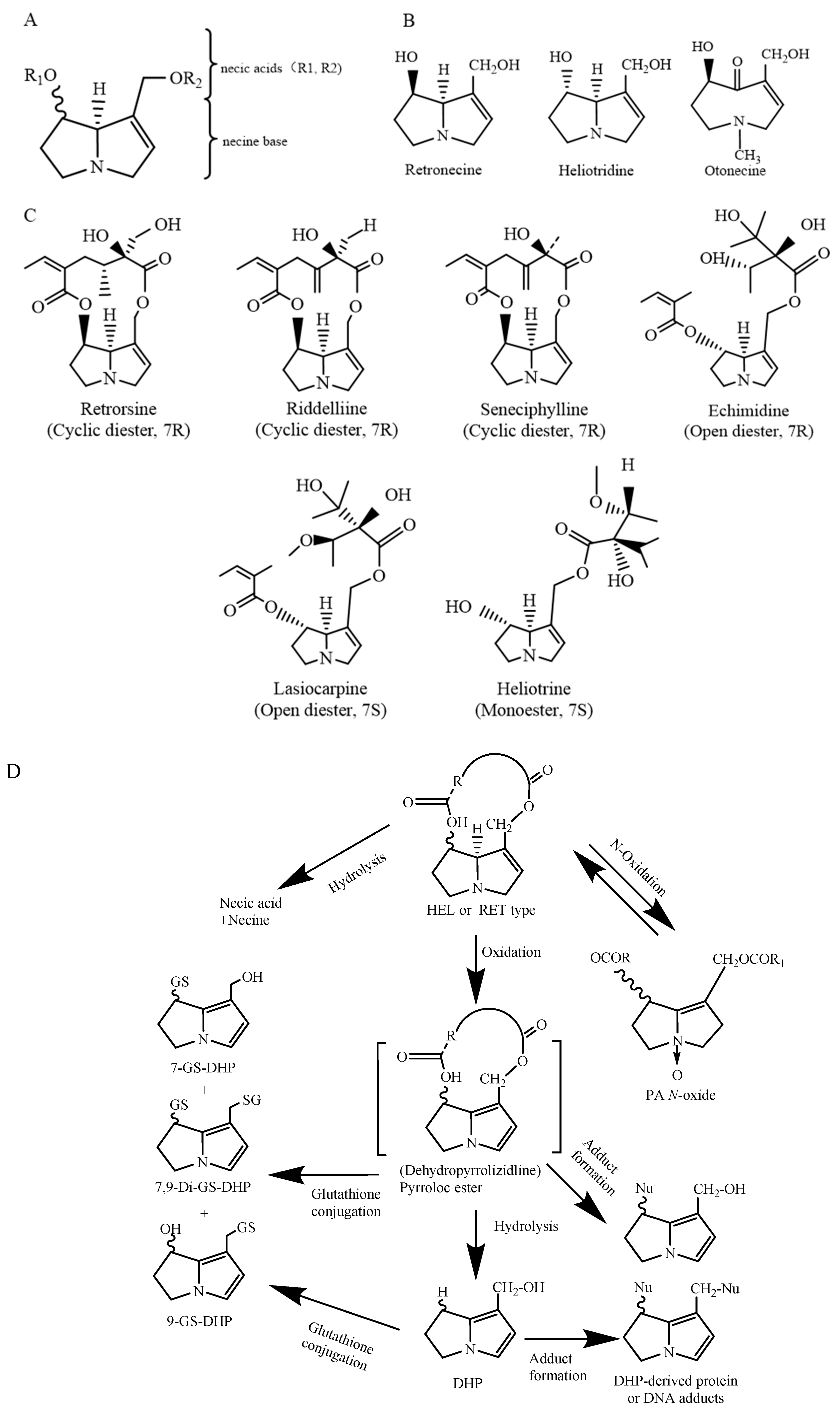
3. Toxic Effects of PAs
3.1. Acute Toxicity
3.2. Cytotoxicity
|
PAs |
Cell Line |
Exposure Dose (μM) |
Exposure Time (h) |
IC50/IC20 (μM) |
References |
|---|---|---|---|---|---|
|
Seneciphylline |
HepG2 |
62.5, 125, 250, 500, 1000 |
24 |
660 a |
[24] |
|
Clivorine |
130 a |
||||
|
Retrorsine |
270 a |
||||
|
Platyphylline |
850 a |
||||
|
Senecionine |
340 a |
||||
|
Lasiocarpine |
CLR-2118 |
19, 38, 75, 300 |
24 |
14 b |
[25] |
|
Senecionine |
mouse primary hepatocytes |
1, 3, 10, 30, 100 |
48 |
5.41 b |
[32] |
|
Adonifoline |
49.91 b |
a IC20 b IC50; IC20 values refer to the calculated 20% inhibitory concentrations on cell viability; IC50 values refer to half-maximal inhibitory concentrations on cell viability.
3.3. Genotoxicity and Carcinogenicity
References
- Dusemund, B.; Nowak, N.; Sommerfeld, C.; Lindtner, O.; Schafer, B.; Lampen, A. Risk assessment of pyrrolizidine alkaloids in food of plant and animal origin. Food Chem. Toxicol. 2018, 115, 63–72.
- Smith, L.W.; Culvenor, C.C. Plant sources of hepatotoxic pyrrolizidine alkaloids. J. Nat. Prod. 1981, 44, 129–152.
- European Food Safety Authority (EFSA). Dietary Exposure Assessment to Pyrrolizidine Alkaloids in the European Population; European Food Safety Authority: Parma, Italy, 2016.
- Fu, P.P.; Xia, Q.S.; Lin, G.; Chou, M.W. Pyrrolizidine alkaloids—Genotoxicity, metabolism enzymes, metabolic activation, and mechanisms. Drug Metab. Rev. 2004, 36, 1–55.
- Zhuge, Y.; Liu, Y.; Xie, W.; Zou, X.; Xu, J.; Wang, J.; Chinese Society of Gastroenterology Committee of Hepatobiliary Disease. Expert consensus on the clinical management of pyrrolizidine alkaloid-induced hepatic sinusoidal obstruction syndrome. J. Gastroenterol. Hepatol. 2019, 34, 634–642.
- Casado, N.; Casado-Hidalgo, G.; González-Gómez, L.; Morante-Zarcero, S.; Sierra, I. Insight into the Impact of Food Processing and Culinary Preparations on the Stability and Content of Plant Alkaloids Considered as Natural Food Contaminants. Appl. Sci. 2023, 13, 1704.
- Casado, N.; Morante-Zarcero, S.; Sierra, I. The concerning food safety issue of pyrrolizidine alkaloids: An overview. Trends Food Sci. Technol. 2022, 120, 123–139.
- European Food Safety Authority (EFSA). Risks for Human Health Related to the Presence of Pyrrolizidine Alkaloids in Honey, Tea, Herbal Infusions and Food Supplements; European Food Safety Authority: Parma, Italy, 2017; p. e04908.
- Merz, K.H.; Schrenk, D. Interim relative potency factors for the toxicological risk assessment of pyrrolizidine alkaloids in food and herbal medicines. Toxicol. Lett. 2016, 263, 44–57.
- Madge, I.; Gehling, M.; Schone, C.; Winterhalter, P.; These, A. Pyrrolizidine alkaloid profiling of four Boraginaceae species from Northern Germany and implications for the analytical scope proposed for monitoring of maximum levels. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2020, 37, 1339–1358.
- Xia, Q.; Zhao, Y.; Lin, G.; Beland, F.A.; Cai, L.; Fu, P.P. Pyrrolizidine Alkaloid-Protein Adducts: Potential Non-invasive Biomarkers of Pyrrolizidine Alkaloid-Induced Liver Toxicity and Exposure. Chem. Res. Toxicol. 2016, 29, 1282–1292.
- Schrenk, D.; Gao, L.; Lin, G.; Mahony, C.; Mulder, P.P.J.; Peijnenburg, A.; Pfuhler, S.; Rietjens, I.; Rutz, L.; Steinhoff, B.; et al. Pyrrolizidine alkaloids in food and phytomedicine: Occurrence, exposure, toxicity, mechanisms, and risk assessment—A review. Food Chem. Toxicol. 2020, 136, 111107.
- Allemang, A.; Mahony, C.; Lester, C.; Pfuhler, S. Relative potency of fifteen pyrrolizidine alkaloids to induce DNA damage as measured by micronucleus induction in HepaRG human liver cells. Food Chem. Toxicol. 2018, 121, 72–81.
- Chou, M.W.; Wang, Y.P.; Yan, J.; Yang, Y.C.; Beger, R.D.; Williams, L.D.; Doerge, D.R.; Fu, P.P. Riddelliine N-oxide is a phytochemical and mammalian metabolite with genotoxic activity that is comparable to the parent pyrrolizidine alkaloid riddelliine. Toxicol. Lett. 2003, 145, 239–247.
- Li, N.; Xia, Q.; Ruan, J.; Fu, P.P.; Lin, G. Hepatotoxicity and tumorigenicity induced by metabolic activation of pyrrolizidine alkaloids in herbs. Curr. Drug Metab. 2011, 12, 823–834.
- Ruan, J.; Yang, M.; Fu, P.; Ye, Y.; Lin, G. Metabolic activation of pyrrolizidine alkaloids: Insights into the structural and enzymatic basis. Chem. Res. Toxicol. 2014, 27, 1030–1039.
- Stegelmeier, B.L.; James, L.F.; Panter, K.E.; Ralphs, M.H.; Gardner, D.R.; Molyneux, R.J.; Pfister, J.A. The pathogenesis and toxicokinetics of locoweed (Astragalus and Oxytropis spp.) poisoning in livestock. J. Nat. Toxins 1999, 8, 35–45.
- Woolford, L.; Fletcher, M.T.; Boardman, W.S. Suspected pyrrolizidine alkaloid hepatotoxicosis in wild southern hairy-nosed wombats (Lasiorhinus latifrons). J. Agric. Food Chem. 2014, 62, 7413–7418.
- Stillman, A.E.; Huxtable, R.; Consroe, P.; Kohnen, P.; Smith, S. Hepatic Veno-Occlusive Disease Due to Pyrrolizidine (Senecio) Poisoning in Arizona. Gastroenterology 1977, 73, 349–352.
- Bull, L.B.; Dick, A.T.; Mc, K.J. The acute toxic effects of heliotrine and lasiocarpine, and their N-oxides, on the rat. J. Pathol. Bacteriol. 1958, 75, 17–25.
- Litvinchuk, M.D.; Gaiduk, R.I.; Kit, V.I. Spasmolytic properties of pyrrolizidine alkaloids. Farmakol. Toksikol. 1979, 42, 509–511.
- Schoental, R. Hepatotoxic activity of retrorsine, senkirkine and hydroxysenkirkine in newborn rats, and the role of epoxides in carcinogenesis by pyrrolizidine alkaloids and aflatoxins. Nature 1970, 227, 401–402.
- Culvenor, C.C.J.; Downing, D.T.; Edgar, J.A.; Jago, M.V. Pyrrolizidine Alkaloids as Alkylating and Antimitotic Agents. Ann. N. Y. Acad. Sci. 1969, 163, 837–847.
- Li, Y.H.; Kan, W.L.T.; Li, N.; Lin, G. Assessment of pyrrolizidine alkaloid-induced toxicity in an in vitro screening model. J. Ethnopharmacol. 2013, 150, 560–567.
- Field, R.A.; Stegelmeier, B.L.; Colegate, S.M.; Brown, A.W.; Green, B.T. An in vitro comparison of the cytotoxic potential of selected dehydropyrrolizidine alkaloids and some N-oxides. Toxicon 2015, 97, 36–45.
- Ji, L.; Liu, T.; Wang, Z. Pyrrolizidine alkaloid clivorine induced oxidative injury on primary cultured rat hepatocytes. Hum. Exp. Toxicol. 2010, 29, 303–309.
- Nuringtyas, T.R.; Verpoorte, R.; Klinkhamer, P.G.; van Oers, M.M.; Leiss, K.A. Toxicity of pyrrolizidine alkaloids to Spodoptera exigua using insect cell lines and injection bioassays. J. Chem. Ecol. 2014, 40, 609–616.
- Gluck, J.; Waizenegger, J.; Braeuning, A.; Hessel-Pras, S. Pyrrolizidine Alkaloids Induce Cell Death in Human HepaRG Cells in a Structure-Dependent Manner. Int. J. Mol. Sci. 2020, 22, 202.
- Ji, L.; Chen, Y.; Liu, T.; Wang, Z. Involvement of Bcl-xL degradation and mitochondrial-mediated apoptotic pathway in pyrrolizidine alkaloids-induced apoptosis in hepatocytes. Toxicol. Appl. Pharmacol. 2008, 231, 393–400.
- Ji, L.L.; Zhang, M.; Sheng, Y.C.; Wang, Z.T. Pyrrolizidine alkaloid clivorine induces apoptosis in human normal liver L-02 cells and reduces the expression of p53 protein. Toxicol. In Vitro 2005, 19, 41–46.
- Ebmeyer, J.; Franz, L.; Lim, R.; Niemann, B.; Glatt, H.; Braeuning, A.; Lampen, A.; Hessel-Pras, S. Sensitization of Human Liver Cells Toward Fas-Mediated Apoptosis by the Metabolically Activated Pyrrolizidine Alkaloid Lasiocarpine. Mol. Nutr. Food Res. 2019, 63, e1801206.
- Xiong, A.Z.; Yang, L.; Ji, L.L.; Wang, Z.Y.; Yang, X.J.; Chen, Y.; Wang, X.L.; Wang, C.H.; Wang, Z.T. UPLC-MS based metabolomics study on Senecio scandens and S-vulgaris: An approach for the differentiation of two Senecio herbs with similar morphology but different toxicity. Metabolomics 2012, 8, 614–623.
- Chan, P.C.; Haseman, J.K.; Prejean, J.D.; Nyska, A. Toxicity and carcinogenicity of riddelliine in rats and mice. Toxicol. Lett. 2003, 144, 295–311.
- Rao, M.S.; Reddy, J.K. Malignant neoplasms in rats fed lasiocarpine. Br. J. Cancer 1978, 37, 289–293.
- Chen, T.; Mei, N.; Fu, P.P. Genotoxicity of pyrrolizidine alkaloids. J. Appl. Toxicol. 2010, 30, 183–196.
- Williams, G.M.; Mori, H.; Hirono, I.; Nagao, M. Genotoxicity of pyrrolizidine alkaloids in the hepatocyte primary culture/DNA-repair test. Mutat. Res. 1980, 79, 1–5.
- Berry, D.L.; Schoofs, G.M.; Schwass, D.E.; Molyneux, R.J. Genotoxic activity of a series of pyrrolizidine alkaloids in primary hepatocyte-mediated V79 cell mutagenesis and DNA repair assay. J. Nat. Toxins 1996, 5, 7–24.
- Xia, Q.S.; Zhao, Y.W.; Von Tungeln, L.S.; Doerge, D.R.; Lin, G.; Cai, L.N.; Fu, P.P. Pyrrolizidine Alkaloid-Derived DNA Adducts as a Common Biological Biomarker of Pyrrolizidine Alkaloid-Induced Tumorigenicity. Chem. Res. Toxicol. 2013, 26, 1384–1396.
- Louisse, J.; Rijkers, D.; Stoopen, G.; Holleboom, W.J.; Delagrange, M.; Molthof, E.; Mulder, P.P.J.; Hoogenboom, R.; Audebert, M.; Peijnenburg, A. Determination of genotoxic potencies of pyrrolizidine alkaloids in HepaRG cells using the gammaH2AX assay. Food Chem. Toxicol. 2019, 131, 110532.




