Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jamir Pitton Rissardo | -- | 2518 | 2024-02-26 23:31:09 | | | |
| 2 | Fanny Huang | -13 word(s) | 2505 | 2024-02-27 06:08:26 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Pitton Rissardo, J.; Fornari Caprara, A.L. Stroke of Cortical Hand Knob Area. Encyclopedia. Available online: https://encyclopedia.pub/entry/55494 (accessed on 06 March 2026).
Pitton Rissardo J, Fornari Caprara AL. Stroke of Cortical Hand Knob Area. Encyclopedia. Available at: https://encyclopedia.pub/entry/55494. Accessed March 06, 2026.
Pitton Rissardo, Jamir, Ana Letícia Fornari Caprara. "Stroke of Cortical Hand Knob Area" Encyclopedia, https://encyclopedia.pub/entry/55494 (accessed March 06, 2026).
Pitton Rissardo, J., & Fornari Caprara, A.L. (2024, February 26). Stroke of Cortical Hand Knob Area. In Encyclopedia. https://encyclopedia.pub/entry/55494
Pitton Rissardo, Jamir and Ana Letícia Fornari Caprara. "Stroke of Cortical Hand Knob Area." Encyclopedia. Web. 26 February, 2024.
Copy Citation
The cortical hand knob region of the brain is a knob-like segment of the precentral gyrus, projecting into the middle genu of the central sulcus. This anatomic landmark is responsible for intricate control of hand motor movements and has often been implicated in motor weakness following stroke.
hand knob
precentral gyrus
1. Introduction
Stroke remains the world’s second-largest cause of death and contributes significantly to the global burden of diseases [1]. Approximately 90 percent of strokes have modifiable risk factors [2]. Data has shown that men are more affected than women when analyzing the incidence of ischemic strokes secondary to vascular factors, and women tend to present with atypical symptoms, resulting in delayed care [3].
The early recognition of symptoms suggestive of a cerebrovascular accident is important to initiate treatment and decrease disabilities. Management of stroke includes rehabilitation and treatment of post-stroke complications, which encompasses not only physical disabilities, but also higher levels of anxiety and depression. The time window of stroke presentation is essential and determines management and outcomes [4].
Stroke can present with focal and nonfocal symptoms. Focal deficits are neurological symptoms and signs that can be localized to a defined anatomical location of the brain [5]. Nonfocal symptoms cannot be pinpointed to a specific localization in the brain and can be harder to recognize. Generalized weakness and confusion are the most commonly reported nonfocal symptoms [6].
There should be a high index of suspicion for stroke in patients presenting with the aforementioned signs and symptoms, especially if they have comorbidities or genetic risk factors, such as cerebral arteriopathy, factor V Leiden mutation, and sickle cell anemia [7].
Another approach to classify strokes is based on the circulation area affected. Posterior circulation is involved in one out of five strokes, and diagnosis is often challenging. Patients can present with visual symptoms, loss of balance, confusion, vertigo, dysarthria, and dizziness [8]. Moreover, the National Institutes of Health Stroke Scale is the standardized assessment tool for stroke, but its sensitivity in posterior circulation strokes is low [9].
The most common stroke mimics include peripheral vertigo, accounting for twenty-five percent of stroke mimics, seizures with Todd’s paralysis as presenting signs, syncope, and functional disorders. Metabolic etiologies such as hypoglycemia, hypokalemia, and hyponatremia can present with confusion and lethargy and often trigger stroke code activation [9].
In this context, while the homunculus theory has existed since the 1930s, it was in 1995 that, for the first time, documentation of a distinct area responsible for hand function was established. An omega/epsilon-shaped region at the junction point between the precentral sulcus and central sulcus on the axial plane was noted to be involved in the motor function of the hand in functional brain MRI. Since then, it also served as a reliable landmark for neuroanatomical localization. Yousry et al. in 1997 reported the first patient that had a circumscribed infarct of the “hand knob area” who presented with isolated contralateral arm weakness without sensation changes [10]. Multiple case reports and series have been published since describing the same observation (Figure 1). It is noteworthy that the most frequent cause of isolated hand weakness is peripheral neuropathy and stroke is only rarely suspected in these patients. Hand knob stroke can present similarly to radial, median, or ulnar nerve injury and without the classic pyramidal or cortical signs. In this way, patients with hand knob stroke are at high risk of being misdiagnosed and receiving inadequate treatment. The lack of investigation of the underlying etiology of the stroke could contribute to improper management of modifiable risk factors such as diabetes, hyperlipidemia, obesity, and hypertension, which could increase the risk of future ischemic events and disabilities. Understanding the hand knob region, its implications, and its role in stroke can further guide rehabilitation efforts and future patient care.
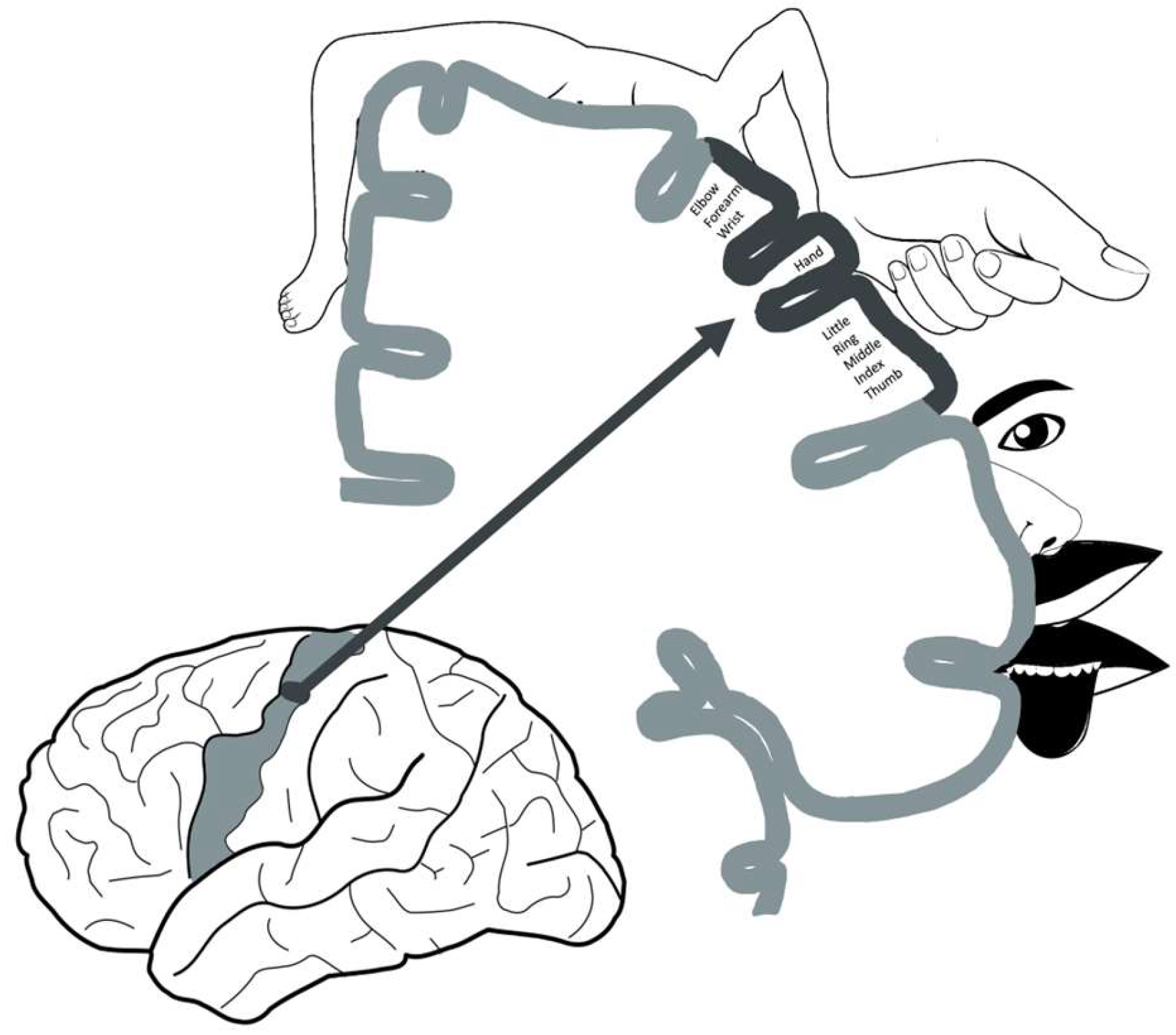
Figure 1. Schematic diagram of the homunculus, in detail, and the hand motor cortex. The hand motor cortex is located in the superior aspect of the precentral gyrus, in the “middle knee” region of the central sulcus.
2. Overview
A total of 24 reports containing 150 patients were found. The mean and median ages were 65 and 67 years, respectively (range = 30 to 106 years). A total of 62 percent (93 of 150) of the individuals were male. According to the TOAST criteria for the classification of the stroke, 59 individuals had a stroke due to large-artery atherosclerosis, 8 had small-vessel occlusion, 20 had cardioembolism, 25 were determined, and 38 were undetermined.
3. Epidemiology
Less than one percent of strokes reported occur in the hand knob region, the precentral gyrus of the motor cortex. Some of the risk factors reported include age, sex, and vascular pathologies. Also, the literature points to atheroembolism and cardioembolism as the most common causes, although hemorrhagic strokes in the hand knob areas have been reported. The majority of the cases of hand knob stroke have a good functional outcome secondary to neuronal plasticity, and the risk of recurrence is low. The rarity of the presentation poses challenges in terms of data acquisition regarding the clinical course [11].
The clinical features of hand knob stroke are mild and functional recovery is good. This delays the presentation of the patient, and, added to this, physicians might not be familiar with it and misdiagnose it as a peripheral neuropathy. Imaging findings might not be read accurately as the area of the stroke is small, and the pretest probability of stroke would be low. These factors contribute to the low prevalence of reported hand knob strokes, although the actual number of cases may be higher [12]. Additionally, lack of cortical, pyramidal, and cerebellar symptoms results in evaluating physicians favoring a peripheral source. The mean age of diagnosis of hand knob stroke is 56–71 years old, and data is inconclusive about gender distribution. The term pseudo-peripheral nerve palsy is used to describe this presentation. However, patients often have vascular risk factors favoring the thought process of an alternate diagnosis [13].
Wang et al. reported that hyperhomocysteinemia was the most common risk factor for hand knob infarction [13], while Orosz et al. considered that hypertension was the most common [14]. This last finding was also found by Zhang et al., who observed that hypertension was the most frequent risk factor in patients with hand knob stroke, followed by hyperlipidemia and hyperhomocysteinemia [12].
4. Neuroanatomy and Neuroimaging
The precentral knob is a knob-like segment of the precentral gyrus projecting to the middle genu of the central sulcus that is known to be a reliable anatomic landmark for the motor hand area. Injuries to this area can be caused by various reasons, including stroke, traumatic brain injuries, infections, or tumors. Damage to the precentral knob can result in significant motor impairment to the contralateral hands and fingers. The precentral knob is arranged in a somatotopic fashion where various parts of the hand can be mapped to specific locations, which in turn allows us to localize the area of damage resulting in the motor deficit [15].
The hand motor cortex area has been useful in targeting the treatment of multiple neurological conditions such as stroke, amyotrophic lateral sclerosis, brain tumors in adults, and spasticity secondary to cerebral palsy in children (using transcranial magnetic stimulation). The hand knob area has been described consistently as an omega-shaped structure in the axial plane, and this description remains widely accepted [16]. A hook-like structure in the sagittal plane is also accepted. Six variations of anatomical appearance, including epsilon, mediately asymmetric epsilon, laterally asymmetric epsilon, null, mature omega, and immature omega, have been reported in the literature (Figure 2). These variations describe adult brain imaging findings, and when developing brains are considered, immature omega variants are often reported [17].
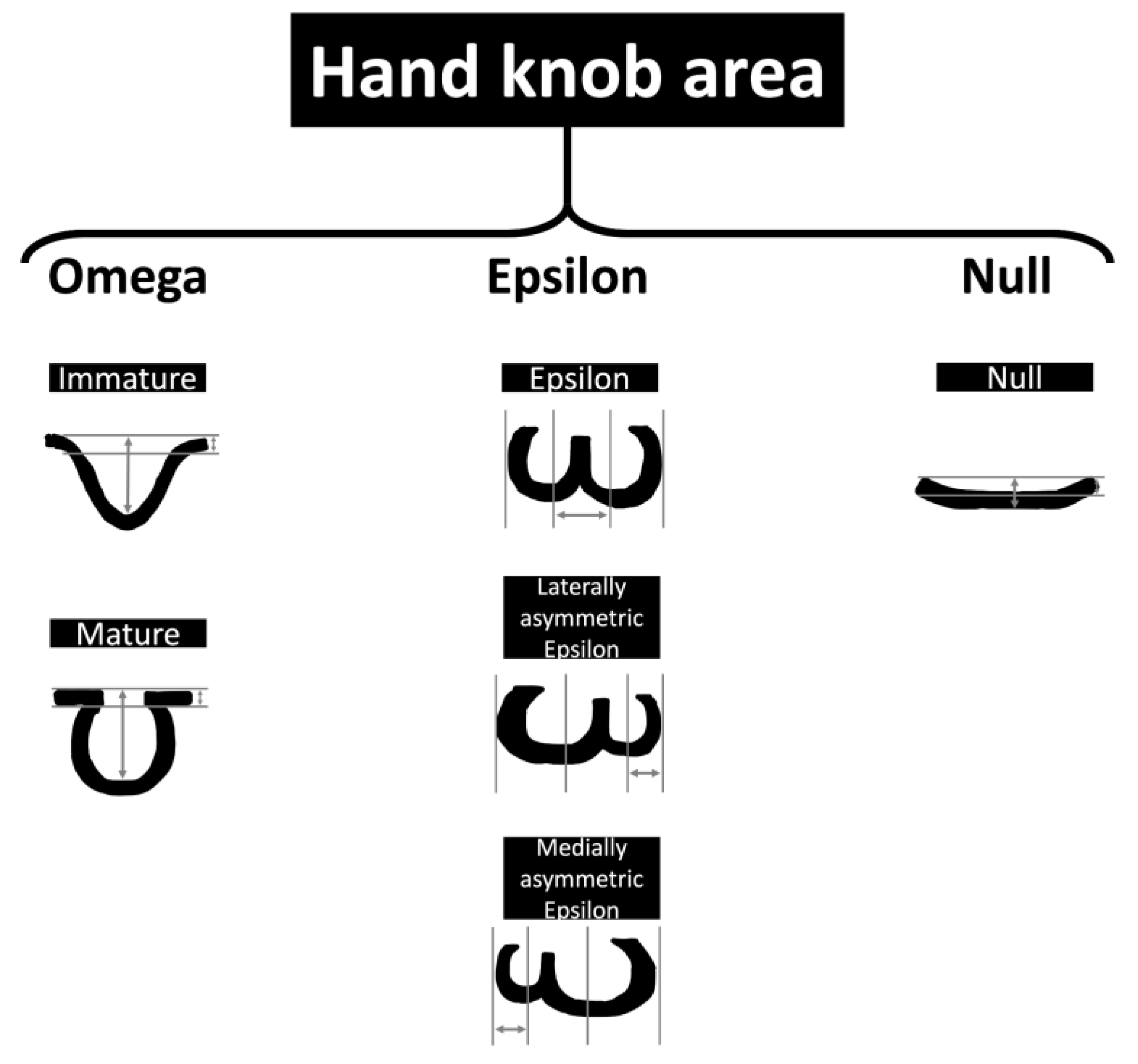
Figure 2. Schematic representation of the hand motor cortex variances. The measurements represent the lengths used in neuroimaging studies to assess the hand knob area.
The hand knob area of the premotor cortex has been studied by Francis et al. using microelectrode recordings, and it was found that face, arm, leg, and head movements are intermixed in the hand knob area. These findings facilitate the decoding of movements using brain-computer interfaces and essentially provide a compositional code of brain motor function. Clinically, this can be helpful for the transfer of motor skills across limbs (for example, analogous movements of hand grasping and toe curling), as was studied in tetraplegic patients. This study represents the ongoing importance of the hand knob area in functional neuroscience [18]. Animal studies have also demonstrated that the caudal region of the hand knob area controls simple movements, and the rostral region is involved in choosing movements through different corticospinal connections and networks, demonstrating the complex architecture of the hand knob area [19].
4.1. Neuroanatomy Development
Hand weakness, in the absence of other deficits, can be due to central or peripheral lesions. It is oftentimes misdiagnosed as damage to peripheral nerves, notably the median or ulnar nerve. Motor control and movement planning can be further localized to specific regions within the frontal lobe and are subdivided into M1 (primary motor cortex located in the precentral gyrus of the frontal lobe), M2 (supplementary motor area responsible for coordination and planning of movements, primarily bilateral movements), and M3 (premotor cortex, responsible for preparing the body for movements). Most hand weakness due to central lesions is localized to the M1 knob area. The knob area is particularly associated with movements of the fingers and hand with distinct regions that correspond to individual fingers or specific hand functions. In a study conducted by Chen et al. in 2006, the clinical and radiological profiles of patients were studied to better understand the location of lesions resulting in isolated motor hand weakness in those with cerebral infarction. The results of the study demonstrated that isolated hand weakness in cerebral infarctions can be localized to areas other than the M1 knob area, which has been the area most implicated in these deficits. Other locations emphasized in this study include infarctions of the angular gyri due to stenosis of the internal carotid artery [20].
An interesting observation has been made by Hanakawa et al. in 2005. While contralateral precentral gyrus activity was known to be associated with movements of the arm, the ipsilateral precentral gyrus played a part in complex movements and stroke recovery. Previous reports described activity in the right precentral gyrus during movement of the left arm, whereas the left precentral gyrus activity was observed during ipsilateral and contralateral hand movements. Overall, contralateral precentral gyrus activity is reported more commonly. The M1 segment of the ipsilateral brain region and ventral premotor cortex are involved, although further studies are required to have a definitive conclusion [21].
A case study written by Ahdab et al. in 2020 [22] assessed the somatotopy within the primary motor cortex (M1). In this case, a 29-year-old man with recurrent strokes affecting finger muscles was studied. Motor mapping after the first cerebral attack revealed overlapping representations within M1 without evidence of distinct somatotopic organization. The hand knob region was spared in this incident. However, a subsequent stroke localized to the hand knob region resulted in significant selective weakness to the first dorsal interosseus muscle while sparing the abductor digiti minimi. The significance of this finding contradicts the initial mapping. Also, it emphasizes the nuanced organization of M1, where each muscle correlates to a unique area with limited ability for alternate pathways to compensate. Moreover, it is understood that the medial fingers and proximal joints are connected to the medial aspect of the hand knob region, whereas the lateral fingers are represented laterally. Understanding these organizations is crucial in addressing motor deficits and formulating the best treatment plan for patients [22].
4.2. Changes in Motor Function after Stroke
Hamzei et al. studied cortical reorganization after constraint-induced movement therapy, a type of motor rehabilitation, in stroke patients using functional MRI and transcranial magnetic stimulation. Patients with intact M1 regions and descending motor fibers had decreased activation of the sensory-motor cortex ipsilateral to the lesion, but intracortical excitability was higher. The converse was also true, indicating reorganization involving other brain regions [23]. A “recruitment activation pattern” of the bilateral sensory-motor cortices, premotor cortex, and supplementary motor area has been observed in patients with M1 lesions in previous studies [24].
Motor rehabilitation is key after a stroke to improve the chances of functional recovery. Functional imaging techniques have been used to demonstrate that changes in cortical activity occur with clinical motor improvement. A study by Jang et al. found a correlation between motor recovery with increased sensory-motor cortex activity of the ipsilateral hemisphere along with increased size in individuals with hand knob infarct [25].
Jang et al., 2005 demonstrated an improvement in hand motor function associated with sensory cortex involvement using functional MRI. The hand knob area was infarcted in both subjects studied, and recovery was over several months, which was attributed to neuroplasticity. The reorganization of the cortex surrounding an infarcted region is a valid explanation for plasticity. An intact cortical spinal tract originating in the premotor cortex, the supplementary motor area, and the parietal lobe is required for the above-described reorganization [26].
Reorganization of both motor and sensory function into a perilesional area following a stroke has been described for the first time by Jang et al. A patient with a right-sided hand knob area infarct recovered function 6 months later. Also, the functional MRI demonstrated activation of the lateral area of the infarct when a special apparatus was used to elicit active and passive movements. Previous studies have also demonstrated the reorganization of motor function into posterior and lateral regions of the infarcted area. Literature reports that cortical strokes had better functional recovery than strokes affecting the corona radiata and posterior limb of the internal capsule, suggesting a lack of perilesional reorganization in these regions [27].
Connectivity of specific regions of the brain, such as the ipsilateral (to the lesion) basal ganglia, thalamus, medullary pyramid, and contralateral cerebellum, have been linked to the improvement of motor function in patients with intracerebral hemorrhage resulting in hemiparesis [28]. Diffusion tensor imaging using probabilistic tracking was utilized to establish connections between these brain regions and improve hand motor function. The corticospinal tract and the extramedullary pyramids play key roles in motor function [29].
A meta-analysis performed by Rehme et al. provided a comprehensive understanding of neuronal activity post-stroke. Prior studies had limitations, including limited power, low reliability of imaging techniques, and potential confounding factors blurring interpretation. The authors described increased activation of bilateral premotor cortices and contralateral (to the lesion) motor area post-stroke. This activity depends on several factors, including the degree of motor impairment and the time after stroke [30].
In 2016, Jang et al. investigated injury to the corticofugal tract through diffusion tensor tractography (DTT) in limb-kinetic apraxia among subjects with a history of corona radiata infarct. Using DTT, researchers could reconstruct the corticospinal tract and the corticofugal tract. These tracts are neural pathways responsible for voluntary motor control. Fractional anisotropy, mean diffusivity, and tract volume were measured for both tracts. The authors found that there were significant decreases in fractional anisotropy and tract volumes in the hemisphere affected by the infarct when compared to the unaffected hemisphere. Also, the findings were notable within the precentral hand knob distribution of the corticospinal tract, with the study concluding that limb-kinetic apraxia as a result of damage to the corticofugal tract may also be joined by damage to the corticospinal tract as well [31].
References
- Tei, H. Monoparesis of the right hand following a localised infarct in the left “precentral knob”. Neuroradiology 1999, 41, 269–270.
- Back, T.; Mrowka, M. Infarction of the “hand knob” area. Neurology 2001, 57, 1143.
- Gass, A.; Szabo, K.; Behrens, S.; Rossmanith, C.; Hennerici, M. A diffusion-weighted MRI study of acute ischemic distal arm paresis. Neurology 2001, 57, 1589–1594.
- Granziera, C.; Kuntzer, T.; Vingerhoets, F.; Cereda, C. Small cortical stroke in the" hand knob" mimics anterior interosseous syndrome. J. Neurol. 2008, 255, 1423–1424.
- Hall, J.; Flint, A. “Hand knob” infarction. J. Neurol. Neurosurg. Psych. 2008, 79, 406.
- Peters, N.; Müller-Schunk, S.; Freilinger, T.; Düring, M.; Pfefferkorn, T.; Dichgans, M. Ischemic stroke of the cortical “hand knob” area: Stroke mechanisms and prognosis. J. Neurol. 2009, 256, 1146–1151.
- Kitamura, E.; Hamada, J.; Suzuki, K.; Akutsu, T.; Kan, S.; Mochizuki, H. A case of pure motor isolated finger palsy due to cerebral infarction. Rinsho Shinkeigaku 2010, 50, 572–577.
- Alstadhaug, K.B.; Sjulstad, A. Isolated hand paresis: A case series. Cerebrovasc. Dis. Extra 2013, 3, 65–73.
- Dekeyzer, S.; Acou, M.; Hemelsoet, D. Right hand knob infarction. Acta Neurol. Bel. 2014, 114, 135–136.
- Jusufovic, M.; Lygren, A.; Aamodt, A.H.; Nedregaard, B.; Kerty, E. Pseudoperipheral palsy: A case of subcortical infarction imitating peripheral neuropathy. BMC Neurol. 2015, 15, 151.
- Finkelsteyn, A.M.; Saucedo, M.A.; Miquelini, L.A.; Chertcoff, A.; Bandeo, L.; Pacha, S.; Cejas, L.L.; Roca, C.U.; Pardal, M.F.; Reisin, R. Ischemic stroke of the “hand knob area”: A case series and literature review. J. Clin. Neurosci. 2019, 65, 100–105.
- Zhang, Z.; Sun, X.; Liu, X.; Wang, L.; Zhu, R. Clinical features, etiology, and prognosis of hand knob stroke: A case series. BMC Neurol. 2022, 22, 331.
- Wang, Y.; Dong, Q.; Li, S.-j.; Hu, W.-l. New clinical characteristics and risk factors of hand knob infarction. Neurol. Sci. 2018, 39, 857–862.
- Orosz, P.; Szőcs, I.; Rudas, G.; Folyovich, A.; Bereczki, D.; Vastagh, I. Cortical hand knob stroke: Report of 25 cases. J. Stroke Cerebrovasc. Dis. 2018, 27, 1949–1955.
- Yousry, T.; Schmid, U.; Alkadhi, H.; Schmidt, D.; Peraud, A.; Buettner, A.; Winkler, P. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain 1997, 120, 141–157.
- Kaneko, O.; Fischbein, N.; Rosenberg, J.; Wintermark, M.; Zeineh, M. The “white gray sign” identifies the central sulcus on 3T high-resolution T1-weighted images. Am. J. Neurorad. 2017, 38, 276–280.
- Wu, F.; Zhao, H.; Zhang, Y.; Wang, M.; Liu, C.; Wang, X.; Cheng, Y.; Jin, C.; Yang, J.; Li, X. Morphologic variants of the hand motor cortex in developing brains from neonates through childhood assessed by MR imaging. Am. J. Neurorad. 2022, 43, 292–298.
- Willett, F.R.; Deo, D.R.; Avansino, D.T.; Rezaii, P.; Hochberg, L.R.; Henderson, J.M.; Shenoy, K.V. Hand knob area of premotor cortex represents the whole body in a compositional way. Cell 2020, 181, 396–409.e326.
- Viganò, L.; Fornia, L.; Rossi, M.; Howells, H.; Leonetti, A.; Puglisi, G.; Nibali, M.C.; Bellacicca, A.; Grimaldi, M.; Bello, L. Anatomo-functional characterisation of the human “hand-knob”: A direct electrophysiological study. Cortex 2019, 113, 239–254.
- Chen, P.-L.; Hsu, H.-Y.; Wang, P.-Y. Isolated hand weakness in cortical infarctions. J. Formos. Med. Assoc. 2006, 105, 861–865.
- Hanakawa, T.; Parikh, S.; Bruno, M.K.; Hallett, M. Finger and face representations in the ipsilateral precentral motor areas in humans. J. Neurophysiol. 2005, 93, 2950–2958.
- Ahdab, R.; Ayache, S.S.; Hosseini, H.; Mansour, A.G.; Kerschen, P.; Farhat, W.H.; Chalah, M.A.; Lefaucheur, J.P. Precise finger somatotopy revealed by focal motor cortex injury. Neurophysiol. Clin. 2020, 50, 27–31.
- Hamzei, F.; Liepert, J.; Dettmers, C.; Weiller, C.; Rijntjes, M. Two different reorganization patterns after rehabilitative therapy: An exploratory study with fMRI and TMS. Neuroimage 2006, 31, 710–720.
- Strick, P.L.; Dum, R.P.; Rathelot, J.A. The cortical motor areas and the emergence of motor skills: A neuroanatomical perspective. Annu. Rev. Neurosci. 2021, 44, 425–447.
- Feydy, A.; Carlier, R.; Roby-Brami, A.; Bussel, B.; Cazalis, F.; Pierot, L.; Burnod, Y.; Maier, M.A. Longitudinal study of motor recovery after stroke: Recruitment and focusing of brain activation. Stroke 2002, 33, 1610–1617.
- Jang, S.H.; Cho, S.H.; Kim, Y.H.; Kwon, Y.H.; Byun, W.M.; Lee, S.J.; Park, S.M.; Chang, C.H. Cortical activation changes associated with motor recovery in patients with precentral knob infarct. Neuroreport 2004, 15, 395–399.
- Jang, S.H.; Ahn, S.H.; Yang, D.S.; Lee, D.K.; Kim, D.K.; Son, S.M. Cortical reorganization of hand motor function to primary sensory cortex in hemiparetic patients with a primary motor cortex infarct. Arch. Phys. Med. Rehabil. 2005, 86, 1706–1708.
- Jang, S.H.; Ahn, S.H.; Lee, J.; Cho, Y.W.; Son, S.M. Cortical reorganization of sensori-motor function in a patient with cortical infarct. NeuroRehabilitation 2010, 26, 163–166.
- Jang, S.H.; Kwon, Y.H.; Lee, M.Y.; Lee, D.Y.; Hong, J.H. Difference of neural connectivity for motor function in chronic hemiparetic stroke patients with intracerebral hemorrhage. Neurosci. Lett. 2012, 531, 80–85.
- Rehme, A.K.; Eickhoff, S.B.; Rottschy, C.; Fink, G.R.; Grefkes, C. Activation likelihood estimation meta-analysis of motor-related neural activity after stroke. Neuroimage 2012, 59, 2771–2782.
- Jang, S.H.; Seo, J.P. Limb-kinetic apraxia due to injury of corticofugal tracts from secondary motor area in patients with corona radiata infarct. Acta Neurol. Belg. 2016, 116, 467–472.
More
Information
Subjects:
Neurosciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Entry Collection:
Neurodegeneration
Revisions:
2 times
(View History)
Update Date:
27 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





